Abstract
Background
Elastase-1 is a proteolytic enzyme secreted by pancreatic acinar cells, and measurements of the concentration this enzyme are used to evaluate pancreatic exocrine function. We aimed to determine whether pancreatic exocrine function declines due to chronic hypercalcemia by measuring fecal elastase levels.
Methods
75 patients with primary hyperparathyroidism (18 men and 47 women) and 30 healthy subjects (11 men and 19 women) participated in this study. Renal function tests, lipid parameters, bone mineral density, and serum calcium, phosphorus, vitamin D, parathormone, glucose, and thyroid stimulating hormone levels as well as fecal elastase concentrations, were determined in these patients and controls.
Results
The mean fecal elastase level was 335.3 ± 181.4 μg/g in the PHPT group and 317.4 ± 157.3 μg/g in the control group. There was no significant difference in fecal elastase levels between the two groups (p = 0.5).
Conclusions
Chronic hypercalcemia in primary hyperparathyroidism did not decrease the fecal elastase level, which is an indirect indicator of chronic pancreatitis; therefore, chronic hypercalcemia in PHPT may not cause chronic pancreatitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several direct and indirect methods of assessing pancreatic exocrine function are available. Although measuring pancreatic enzyme levels after stimulating pancreatic secretion with secretin and cholecystokinin is the most direct and reliable method of assessing pancreatic exocrine function, the complexity, discomfort and limited availability of this method necessitated the development of indirect measurements of pancreatic function. Indirect pancreatic function tests have lower sensitivity but higher applicability than direct tests. One indirect method is the measurement of fecal elastase levels, which is almost as reliable as direct testing [1, 2]. Elastase-1 is a proteolytic enzyme secreted by pancreatic acinar cells. It is a pancreas-specific carboxypeptidase and catalyzes elastin hydrolysis [3]. Its most important feature is its stability during intestinal transport, which enables measurement of its concentration in stool to assess pancreatic function. There is a significant correlation between fecal elastase concentrations and duodenal elastase, amylase, lipase, trypsin and bicarbonate levels [4, 5]. Measurements of fecal elastase concentrations via monoclonal antibodies are widely used to evaluate pancreatic exocrine function [6].
Primary hyperparathyroidism (PHPT) is a disease characterized by hypercalcemia as a result of excessive secretion of parathormone (PTH) by one or more parathyroid glands. The severities of hyperparathyroidism signs and symptoms are associated with calcium levels, regardless of etiology [7]. Nowadays, hypercalcemia is detected incidentally in the majority of affected patients. The increase in routine measurement of serum calcium levels has increased early diagnosis and estimates of PHPT prevalence. Therefore, approximately half of the patients are classified as having a mild PHPT [8]. When patients are questioned in detail, gastrointestinal symptoms, such as constipation, nausea, vomiting and loss of appetite, are commonly mentioned. Additionally, some patients suffer from urolithiasis, or more rarely, pancreatitis attacks. In a study by Mithöfer et al., hypercalcemia created by calcium infusions in rats was shown to cause morphological changes characteristic of acute pancreatitis in the rat pancreas. Hypercalcemia is believed to cause acute pancreatitis by blocking pancreatic secretory ducts via calcium deposition and by trypsinogen activation in the pancreatic parenchyma [9].
In studies investigating the etiologies of acute and chronic pancreatitis, the frequency of PHPT has been reported to range from 0.1 to 4.0% [10]. In many studies evaluating large numbers of patients with PHPT, the disease has been found increase the risk of pancreatitis [11]. As chronic pancreatitis is a rare complication in patients with PHPT, there are only a limited number of studies in the literature evaluating the effects of chronic hypercalcemia on pancreatic exocrine function. The present study aimed to investigate whether pancreatic exocrine function declines due to chronic hypercalcemia by measuring fecal elastase levels.
Materials and methods
We included patients with hypercalcemia, which was defined as a plasma calcium level above the upper limit of the reference range (10.2 mg/dL), and established diagnoses of PHPT. Patients with high serum calcium and parathormone levels and who does not have low urinary calcium excretion were diagnosed PHPT. None of patients have familial hyperparathyroidism. We excluded all patients with secondary hyperparathyroidism, familial hypocalciuric hypercalcemia, multiple endocrine neoplasia syndromes and other causes of hypercalcemia. We also excluded patients with alcohol abuse, chronic pancreatitis, pancreatic and periampullary carcinoma, previous pancreatic resections and gastrointestinal surgeries, diabetes mellitus, cystic fibrosis, amyloidosis, gluten enteropathy, Zollinger–Ellison syndrome, Crohn’s disease and short bowel syndrome. At the beginning of the study, abdomen ultrasonography was performed and patients with gallstones were also excluded.
65 patients with primary hyperparathyroidism (18 men and 47 women, median age 55.4, range 28–83) and 30 healthy subjects (11 men and 19 women, median age 53.2, range 25–75) participated in the study. All patients were questioned regarding symptoms and complications of hyperparathyroidism. The follow-up time after diagnosis of hyperparathyroidism was accepted as the duration of hypercalcemia. The time from diagnosis to operation in patients who underwent curative surgery and the time from diagnosis to the last visit in patients who were being followed without surgery or underwent non-curative surgery were estimated as the duration of hypercalcemia.
Renal function tests, lipid parameters, bone mineral density, and serum calcium, phosphorus, 25 hydroxyvitamin D, parathormone, glucose, and thyroid stimulating hormone (TSH) levels were evaluated. Evaluation of renal function tests BUN, creatinine, sodium and potassium were evaluated. Total cholesterol and triglyceride levels in lipid parameters were assessed. In the bone mineral density measurement, T score and Z score were evaluated. PTH was measured by Abbott Diagnostics assays. Random fecal samples were taken from all participants and stored at −20 °C until elastase measurement. Fecal pancreatic elastase was measured via ELISA (Pancreatic Elastase ELISA; Bioserv Diagnostic GmbH, Rostock Germany), according to the manufacturer’s instructions. Pancreatic morphology was evaluated via abdominal ultrasound in all patients prior to the study.
Comparisons of elastase levels between patients with PHPT and healthy subjects in the control group were made. Correlations between fecal elastase levels and various parameters associated with PHPT were analyzed using Pearson’s correlation coefficient and also Spearmen correlation statistical analysis was performed. The Mann–Whitney U test and a Chi-square test were used for statistical analysis, and p values less than 0.05 were considered statistically significant.
Results
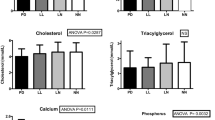
No significant differences were observed between the patients with PHPT and the healthy control group subjects with respect to age, gender, and serum vitamin D levels. The median ages were 55.4 (28.0–83.0) in the PHPT group and 53.2 (25.0–75.0) in the control group. 47 patients were female (female/male ratio 2.6) in the PHPT group, and 19 were female (female/male ratio 1.9) in the control group. The biochemical characteristics of both groups are shown in Table 1. Since the cholesterol and potassium levels were not in the inclusion criteria of the study, the statistical difference was not evaluated.
When patients were questioned in detail regarding symptoms related to hypercalcemia, fatigue was the most frequently mentioned symptom (Table 2). Gastrointestinal symptoms were observed frequently in the PHPT group. Although the patients were not elderly, 55% were hypertensive and receiving treatment. In our study, patients with nephrolithiasis in their anamnestic records or detected nephrolithiasis in ultrasonographic examinations were grouped as patients with nephrolithiasis. 28% patients had nephrolithiasis. None described clinical presentations such as acute pancreatitis attacks.
In our study, the mean fecal elastase level was 335.3 ± 181.4 µg/g in the PHPT group and 317.4 ± 157.3 µg/g in the control group (Fig. 1). There was no significant difference in fecal elastase levels between the two groups (p = 0.5). As a result of statistical analysis, there were no significant differences between serum calcium levels and elastase levels (p = 0.562) (Figure 2). And also patients with calcium levels below 11.5 mg/dL and those with higher levels were compared in the PHPT group. The mean fecal elastase level was 335.9 ± 167.1 µg/g in the patients with calcium level 11.5 mg/dL and higher levels and 335.3 ± 186.4 µg/g in the patients with calcium levels below 11.5 mg/dL. There was no statistically significant difference between the two groups (p = 0.44).
Based on a fecal elastase cut-off value of 200 µg/g for the diagnosis of pancreatic exocrine dysfunction, 19 patients (29.2%) in the PHPT group and 6 control subjects (20.0%) were diagnosed as having pancreatic exocrine dysfunction. Patients with PHPT were divided into the following groups according to fecal elastase levels: Group I (fecal elastase level <200 ug/g) and Group II (fecal elastase level ≥200 ug/g). Clinical and laboratory features related to hyperparathyroidism were compared between the two groups (Table 3). Fecal elastase levels did not decrease with age. When patients with low fecal elastase levels were compared to those with high fecal elastase levels, no statistically significant differences were observed with respect to serum calcium levels, serum parathormone levels, 24-h urinary calcium excretion and duration of hypercalcemia.
Elastase levels in patients with and without gastrointestinal symptoms were compared, and statistically significant differences were not detected between the patients with or without gastrointestinal symptoms. In our study, 28 (43.0%) patients with hyperparathyroidectomy underwent parathyroidectomy operation. Patients with and without parathyroidectomy were grouped. Elastase levels were compared between those who had operation and those who did not. Mean elastase levels were 351.8 ± 185.8 µg/g in patients who did not have parathyroidectomy and 314 ± 176.7 µg/g in patients who had parathyroidectomy operation. There were no statistically significant differences between the two groups.
The relationship between fecal elastase levels and biochemical parameters related to PHPT was evaluated. Based on correlation analysis, no significant correlations between fecal elastase levels and serum calcium levels, serum parathormone levels, serum vitamin D levels, 24-h urinary calcium excretion and duration of hypercalcemia were observed (Table 4). Correlation analysis of fecal elastase levels and patient bone mineral density showed that Z scores of the total lumbar spine were positively correlated with fecal elastase levels, but Z scores of the femoral neck were not (Fig. 3). Also, it is showed that T scores of total lumbar spine were not positively correlated with fecal elastase, while T scores of femoral neck were positively correlated with fecal elastase (Table 4). The mean femur neck Z scores was −0.02 ± 1.17 and the mean total lumber Z scores was −0.37 ± 1.25 in the Group I. The mean femur neck Z scores was −0.38 ± 1.27 and the mean total lumber Z scores was −1.24 ± 1.08 in the Group II. There were no significant differences between the two groups. None of our patients had a fracture in anamnestic records.
Discussion
Patients with PHPT are usually admitted due to symptoms that are not associated with hypercalcemia. Hyperparathyroidism is diagnosed after detecting elevated serum calcium levels via laboratory examinations. However, when patients are questioned in detail after diagnosis, the majority report having had symptoms of hypercalcemia for long periods of time. Normalization of serum calcium levels after parathyroidectomy results in symptom resolution.
Although the patients included in our study were admitted due to other reasons, the majority described symptoms suggestive of hypercalcemia when questioned in detail, as reported in the literature. Nevertheless, given that hypertension was diagnosed in 55% of patients and that urolithiasis was diagnosed in 28% of patients with PHPT, serum calcium levels should be measured in patients who are followed for hypertension and urolithiasis. Diagnoses of hypercalcemia via tests performed for other reasons, as well as diagnoses of hyperparathyroidism via population screenings, indicate that the majority of patients live for long periods of time without diagnoses. In large-scale studies, patients with PHPT were found to have increased risks of acute and chronic pancreatitis [10,11,12,13]. In many studies, the risk of pancreatitis was reported to decrease after normalization of serum calcium levels post-operatively [14]. In animal studies, it was shown that development of pancreatitis was associated with hypercalcemia [9]. The risk of developing pancreatitis increases with both the severity and the duration of hypercalcemia.
The mean serum calcium level was 11.1 ± 0.93, and the mean duration of hypercalcemia was 11.3 ± 10.7 months in our patient group. Symptoms specific for chronic pancreatitis were not noted upon review of systems. Pancreatic exocrine function was assessed by measuring fecal elastase activity. Significant differences in fecal elastase activity were not observed between the PHPT and control groups.
In the literature, fecal elastase levels lower than 160–200 µg/g have been reported to have sufficient sensitivity for the diagnosis pancreatic exocrine dysfunction [4, 15]. When patients were divided into two groups based on this range, there were no significant differences in serum calcium levels between the two groups. Upon correlation analysis, no significant correlations were detected between fecal elastase levels and serum calcium levels, serum parathormone levels, serum vitamin D levels, 24-h urinary calcium excretion and duration of hypercalcemia.
Although different rates have been reported in the literature, the incidence of pancreatitis in patients with primary hyperparathyroidism can reach up to 7% [14]. It has also changed over the years. Bauer et al. evaluated patients with pancreatitis between 1963 and 1992 and reported changes in its incidence during this period; in the first part of the study, the incidence reached 10%; in the final years of the study, the incidence decreased to 1–2% [16]. Bess et al. found that 17 of 1153 patients had pancreatitis in their study. They also showed that only 1.5% of hospitalized patients with PHPT were diagnosed with pancreatitis and that this rate was not different from the incidence of pancreatitis among all-cause hospitalized patients. As a consequence of these studies, it has been argued that the incidence of pancreatitis in patients with PHPT may not be as high as previously thought and that there may not be a cause-and-effect relationship between hypercalcemia and pancreatitis [13]. In patients with PHPT, factors other than hypercalcemia are believed to play a role in the development of pancreatitis. The study by Ward et al. suggested that alcohol use, gallstones and ductal hypertension, ischemia, and viral infections were causes of pancreatitis [17]. In our study, patients with PHPT did not have episodes of pancreatitis. This may be related to the small number of patients included in the study, as well as the presence of mild-to-moderate hypercalcemia, short durations of hypercalcemia, and the absence of additional risk factors.
Pancreatic exocrine function plays an important role in digestion and absorption of nutrients. A wide spectrum of clinical signs can be observed in mild-to-severe exocrine dysfunction in chronic pancreatitis. In cases of severe exocrine dysfunction, steatorrhea, weight loss, abdominal discomfort and bloating all occur secondary to fat malabsorption. Clinical symptoms secondary to deficiencies of fat-soluble vitamins (A, D, E, and K) may also be observed in fat malabsorption. In mild-to-moderate exocrine dysfunction, gastrointestinal symptoms, such as abdominal bloating and cramps, may be observed [18]. In our study, there was no significant difference in vitamin D levels between the patient and control groups. No significant correlation was detected between vitamin D and fecal elastase levels. Some studies have noted decreases in bone mineral density due to deficient vitamin D absorption in patients with chronic pancreatitis [19, 20].
In our study, DXA measurements were not performed in the control group; therefore, comparisons could not be made. However, a significant positive correlation was found between fecal elastase levels and lumbar spine bone mineral density. It was concluded that chronic malabsorption in patients with low fecal elastase levels can lead to decreases in bone mineral density. No significant correlation was observed between fecal elastase levels and bone mineral density of the femoral neck.
One of the major limitations of our study was its evaluation of pancreatic exocrine function with indirect methods. Despite the relatively high sensitivity of the fecal elastase test, studies using direct methods may obtain different results. Studies including more patients and healthy subjects may also obtain different results, and the significance of these results may change. In addition, the inclusion of patients with mild-to-moderate hypercalcemia may be the reason why significant differences were not observed with respect to fecal elastase levels.
In conclusion, chronic hypercalcemia in PHPT does not decrease fecal elastase levels, which are an indirect indicator of chronic pancreatitis; therefore, chronic hypercalcemia in PHPT may not cause chronic pancreatitis. Factors other than hypercalcemia may play a role in decreasing fecal elastase levels. Decreases in bone mineral density (especially that of the lumbar spine) were positively correlated with decreases in fecal elastase levels; therefore, the bone densities of patients with chronic pancreatitis should be monitored.
References
Naruse S, Ishiguro H, Ko SBH et al (2006) Fecal pancreatic elastase: a reproducible marker for severe exocrine pancreatic insufficiency. J Gastroenterol 41:901–908
Stevens T, Conwell DL. Pancreatic exocrine function tests. http://www.uptodate.com/contents/pancreatic-exocrine-function-testssource=search_result&search=pancreatic+exocrine+function+tests&selectedTitle=1~7. Accessed June 2017
Sziegoleit A (1984) A novel proteinase from human pancreas. Biochem J 219:735–742
Stein J, Jung M, Sziegoleit A et al (1996) Immunoreactive elastase I: clinical evaluation of a new noninvasive test of pancreatic function. Clin Chem 42(2):222–226
Löser C, Mollgaard A, Folsch UR (1996) Faecal elastase 1: a novel, highly sensitive and specific tubeless pancreatic function test. Gut 39:580–586
Dominic R, Franzini C (2002) Fecal Elastase-1 as a test for pancreatic function: a review. Clin Chem Lab Med 40(4):325–332
Shane E, Dinaz I (2006) Hypercalcemia: pathogenesis, clinical manifestations, differential diagnosis and management. In: Favus MJ (ed) Primer on the metabolic bone disease and disorders of mineral metabolism, 6th edn. Lippincott, Williams, and Wilkins, Philadelphia, p 176
Marcocci C, Brandi ML, Scillitani A et al (2015) Italian society of endocrinology consensus statement: definition, evaluation and management of patients with mild primary hyperparathyroidism. J Endocrinol Invest 38:577–593
Mithöfer K, Fernandez-del Castillo C, Frick TW et al (1995) Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat. Gastroenterology 109(1):239–246
Bhadada SK, Updawat HP, Bhansali A et al (2007) Chronic pancreatitis in primary hyperparathyroidism: comparison with alcoholic and idiopathic chronic pancreatitis. J Gastoenterol Hepatol 28:959–964
Jacob JJ, Chacko A, Selvan B et al (2008) Primary hyperparathyroidism and chronic pancreatitis. J Gastroenterol Hepatol 233:164
Niederle B, Roka R, Woloszczuk W (1987) Successful parathyroidectomy in primary hyperparathyroidism: a clinical follow-up study of 212 consecutive patients. Surgery 102(6):903–909
Bess MA, Edis AJ, van Heerden JA (1980) Hyperparathyroidism and pancreatitis. Chance or a causal association? JAMA 243:246–247
Carnaille B, Oudar C, Pattou F (1998) Pancreatitis and primary hyperparathyroidism: forty cases. Aust NZ J Surg 68(2):117–119
Wolkowiak J, Nousia-Arvanitakis S, Cade A et al (2002) Fecal elastase-1 cut of levels in assessment of exocrine pancreatic function in cystic fibrosis. J Cystic Fibrosis 1(4):260–264
Bauer S, Amman R (1994) Chronic pancreatitis and primary hyperparathyroidism. Scweiz Med Wochenschr 124(30):1344–1348
Ward JB, Peterson OH, Jenkins SA et al (1995) Is an elevated concentration of acinar cytosolic free ionized calcium the trigger for acute pancreatitis? Lancet 346:1016–1019
Hart PA, Conwell DL (2015) Diagnosis of exocrine pancreatic insufficiency. Curr Treat Options Gastroenterol 13(3):347–353
Mann ST, Stracke H, Lange U et al (2003) Alterations of bone mineral density and bone metabolism in patients with carious grades of chronic pancreatitis. Metabolism 52(5):579–585
Moran CE, Sosa EG, Martinez SM et al (1997) Bone mineral density in patients with pancreatic insufficiency and steatorrea. Am J Gastroenterol 92:867–871
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical approval
This study was approved by the Institutional Review Board of the Uludag University of the Turkey.
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Our research involved human participants who had obtained informed consent.
Rights and permissions
About this article
Cite this article
Sisman, P., Avci, M., Akkurt, A. et al. The effect of primary hyperparathyroidism on pancreatic exocrine function. J Endocrinol Invest 41, 293–298 (2018). https://doi.org/10.1007/s40618-017-0727-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-017-0727-6