Abstract
Purpose
The aim of the study was to evaluate the effects of nutraceuticals containing Equol, Resveratrol, Quecitine and Passiflora (Zemiar®, Avantgarde, Pomezia, Rome, Italy) on quality of life (QoL) and sexual function in perimenopausal women.
Methods
Sixty perimenopausal women having vasomotor symptoms and being in the −1, +1a of the STRAW system (amenorrhea for longer than 60 days and FSH < 20 UI/L) were enrolled. The modified Kupperman Index (KI) was used to evaluate menopause symptoms. The Short Form-36 (SF-36), Female Sexual Function Index (FSFI) and the Female Sexual Distress Scale (FSDS) were used to assess QoL, sexual function and sexual distress, respectively. The study had two follow-ups at 3 and 6 months.
Results
The women reported an improvement in the KI total score from the baseline (35 ± 4) to the 1st (21 ± 3, p < 0.05) and the 2nd (18 ± 2, p < 0.01) follow-ups. At the 1st follow-up, the women reported QoL improvements in some functions (p < 0.05); at the 2nd follow-up, they reported improvements in all categories (p < 0.001). At baseline, the total FSFI score was 23.1 ± 1.2 and the FSDS score was 18.1 ± 1.4, both indicating sexual dysfunction with sexual distress. FSFI and FSDS total scores did not change at the 1st follow-up (p = NS). On the contrary, at the 2nd follow-up, the FSFI score had risen to (27.6 ± 1.5) (p < 0.001) and the FSDS score had dropped to (11.3 ± 1.2) (p < 0.001).
Conclusions
Nutraceuticals can be effective in modulating the perimenopausal symptoms in women. The progressive reduction of the vasomotor symptoms reported by women over the nutraceutical usage could contribute to improve their QoL and sexual life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perimenopausal women are often report as suffering from neurodegenerative and behavioral disorders. Over the past 40 years, a revolution in biological and cultural aspects of menopause have been observed: individual variability of age at natural menopause are partially under genetic control—dates written in female biology—[1] so that the time of menopause has not changed [2]; on the other hand, our culture has changed so evidently the concept of femininity from the psycho-sociological standpoint. In fact, today menopause can no longer be considered the end of femininity, but the beginning of a new phase in which a careful preservation of the health and QoL of women is necessary [3].
The neurovegetative syndrome is quite common in perimenopausal women. In this period, they are commonly associated with hot flushes and women refer daytime fatigue, mood labiality and irritability. Therefore, during perimenopause, psychological and cognitive changes can appear such as depression, mood swings, difficulty concentrating and impaired memory [4]. Hormonal fluctuations during the initial stages of menopause are also responsible, at least in part, for a certain affective instability and emotional distress [5–8]. These hormonal changes are responsible for the symptoms of menopause influencing the woman’s lifestyle, body image and the kind of diet, depending on the social context in which she lives [9].
In the latter stages of perimenopause, vasomotor syndrome appears disturbing the subjective QoL and social status of women; furthermore, the reduced level of estrogen triggers the process that will lead to atrophy of the genitourinary system with implications for the QoL of women [10] such as urinary incontinence [11] and changes in sexual desire [12]. Sexual health often starts to become dysfunctional, usually following the onset of pains during intercourse. Dyspareunia is also associated with an initial drop in sexual desire [13, 14].
Today, perimenopause is defined by STRAW: Stages of reproductive aging workshop [15] (Fig. 1) of the early (−2 stage) and the late (+1 stage) phases; the latter is characterized not only by vasomotor symptoms but also by the characteristics of the cycle, where the interval of amenorrhea has to be ≥60 days, and the level of FHS > 25 UI/L. It ends 12 months after the final menstrual period (FMP). Perimenopause includes menopausal transition having the same characteristics but ends at the FMP. The late menopausal transition may last 1–3 years.
Nutraceuticals have been gaining ever more approval for the treatment of perimenopausal disorders, particularly when using the synergistic effect of different natural substances. The term “nutraceutical” refers to products derived from food sources that are purported to provide extra health benefits, in addition to the basic nutritional value found in the foods [16]. Depending on specific properties, products may claim to prevent chronic diseases, improve health, delay the aging process, increase life expectancy, or support the structure or function of the body [17].
Zemiar® (Avantgarde, Pomezia, Rome, Italy) contains soy fermented with Equol, having estrogen, autonomic and cardioprotective action [18]; Resveratrol, with strong antioxidant activity [19], and Quecitine that synergistically contribute to the upward trend in weight [20]; Passiflora whose main activity is to modulate mood disorders and anxiety through GABA-benzodiazepine activity [21, 22].
The aim of this prospective study was to evaluate the efficacy of Zemiar on QoL and sexual function in perimenopausal women over 6 months of usage.
Materials and methods
This prospective observational study was performed at the Service for Menopause, Research Group for Sexology, Department of General Surgery and Medical Surgical Specialties, School of Medicine, University of Catania, Italy. The study protocol was approved by the Institutional Review Board of the Department and conformed to the ethical guidelines of the 1975 Helsinki Declaration. Informed written consent was obtained from each woman before entering the study, and they did not receive any monetary payment. The time of enrollment was from July 2014 to May 2015.
Subjects and setting
Women attending the Service for Menopause for counseling because of vasomotor symptoms, having an interval of amenorrhea more than ≥60 days, and level of FSH > 25 UI/L, being in the late menopausal transition (−1) or in early perimenopause (+1a) as defined by STRAW:Stages of Reproductive Aging Workshop were invited to participate in the study. Consequently, FSH was measured for each woman at enrollment by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (Elecsys Systems 2010, Roche, Monza, Italy).
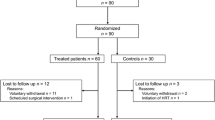
The sample consisted of 72 perimenopausal women aged 46–53 years (mean age 52.4 ± 3.1), with body mass index ≤28, sexually active having at least one sexual activity during the month before counseling. Women with a history of sexual dysfunction, or having a partner affected by sexual dysfunction, or having used hormonal steroid treatment for less than 3 months or who had received phytoestrogens within 1 month before the start of the study, were excluded. Furthermore, women with endometrial thickness equal to or greater than 4 mm measured by transvaginal ultrasound before study initiation and/or abnormal uterine bleeding, hormone-dependent malignancies, or affected by diabetes, or on antihypertensives or on cholesterol lowering medication, or affected by chronic medical illness, were also excluded from the study.
Instruments
The modified Kupperman Index (KI) is widely used to evaluate menopause symptoms and consists of 13 items [23], including hot flushes/night sweats, paresthesia, dizziness, arthralgia/myalgia, headache, palpitations and formication (categorized as somatic symptoms); insomnia/sleep disturbance, depression, irritability and fatigue (categorized as psychological symptoms); and urinary infection and sexual complaints (categorized as urogenital symptoms). The scores are 0 = none, 1 = mild, 2 = moderate and 3 = severe. The total KI score ranges from 0 to 63, and this score is calculated as the sum of all of the scores for each item. Score ranges of 0–6 (none), 7–15 (mild), 16–30 (Moderate) and >30 (severe) were used to rate the degree of severity.
The Short Form-36 (SF-36) questionnaire was used to assess QoL [24]. The questionnaire contains 36 questions grouping four categories of somatic aspects [physical activity (10 items), physical role (4 items), bodily pain (2 items), general health (6 items)], and four mental aspects [vitality (4 items), social activity (2 items), emotional role (3 items) and mental health (5 items)]. Women were instructed to place a mark on a 0–100 scale for each item that best corresponded to their feelings, from the lowest to the highest score of a given category of the QoL. Thereafter, the sum of all items of each category was made. Mean values were calculated on the basis of individual items within a given category. Consequently, eight scale scores were obtained, with higher scores indicating better functioning.
A sexual history interview was adopted to investigate the quality of sexual life of women and their partners. To define FSD, the revised definition and classification of the international consensus development conference on FSD was used [25]. Sexual behavior was assessed using the self-administered Female Sexual Function Index (FSFI) validated in the Italian gynecological population [26]. The FSFI consists of six domains, which include desire (two items), arousal (four items), lubrication (four items), orgasm (three items), satisfaction (three items) and pain (three items), answered on a five point Likert scale, ranging from 0 (no sexual activity) or 1 (never/very low) to 5 (always/very high). A score is calculated for each of the six domains, and the total score is obtained from summing all the items. The total score range is 2–36. A cut-off of ≤26.55 is usually accepted for diagnosis of sexual dysfunction in women within a wide age range. Moreover, for diagnosis of sexual dysfunction, an essential element is that the condition causes significant personal distress for the woman. Therefore, the Female Sexual Distress Scale (FSDS) was used [27]. The FSDS consists of 12 items. The maximum score is 48. An FSDS score of ≥15 corresponds to clinically significant distress. We considered women with an FSFI score of less than 26.55 to be affected by sexual dysfunction if they also had an FSDS score of 15 or greater.
Furthermore, each woman received a diary to record daily sexual events as well as adverse events during treatment. The diary was in months divided into days; woman had to report their daily nutraceutical usage, sexual activity (SA) and adverse events (AEs) in the boxes for each day specifying the type of AE in a specific space.
After the baseline evaluation, each enrolled woman was prescribed one tablet daily to take orally, for 6 months. All assessments were made at baseline and at the 3rd (the 1st follow-up) and 6th (the 2nd follow-up) months.
Statistical analysis
Paired Student’s t test was used to compare the values obtained at baseline with those of the follow-ups from the KI and the SF-36 domains. For comparisons of the values obtained from the FSFI items between baseline and the follow-ups, the nonparametric Wilcoxon rank-sum test with z values was used. Pearson’s correlation coefficient was used to compare the change in sexual function with the KI. Scores are presented as mean ± SD. The result was statistically significant when p < 0.05. Statistical analysis was carried out using the Primer of Biostatistics statistical computer package (Glantz SA, New York: McGraw-Hill, Inc. 1997).
Results
At enrollment, besides being in amenorrhea for longer than 60 days, women had an FSH level of 42 ± 12 UI/L. Five (8.3 %) women were lost at the 1st follow-up and 7 (11.6 %) at the 2nd follow-up. Consequently, 60 women aged 46–52 (mean age 51 ± 6), being −1, +1a of the STRAW perimenopausal system, completed the study.
Figure 2 shows the changes in the KI. At baseline, women had severe vasomotor symptoms (KI = 35 ± 4). During Zemiar intake, the women reported improvement in the KI total score; at the 1st and the 2nd follow-ups, it was moderate (21 ± 3, p < 0.05) and mild (18 ± 2, p < 0.01), respectively.
Figure 3 shows the changes in QoL of women on nutraceuticals. Except for the vitality and mental health categories, at the 1st follow-up, women reported QoL improvements (p < 0.05) with respect to baseline values. On the contrary, at the 2nd follow-up, they referred improvements in all categories (p < 0.001). The progressive reduction in the KI score was correlated with the QoL improvement in women, at the 1st (r = −0.51, p < 0.05) and the 2nd (r = −0.64, p < 0.001) follow-ups.
Table 1 shows the statistical comparisons, by Wilcoxon rank-sum test, of the FSFI scores for each sexual item observed in both follow-ups with respect to the baseline values. At baseline, the total FSFI score was 23.1 ± 1.2 and the FSDS score was 18.1 ± 1.4, both indicating sexual dysfunction with sexual distress. At the 1st follow-up, neither each FSFI item nor the total FSFI and FSDS scores changed (p = NS). However, at the 2nd follow-up, the FSFI total score improved (27.6 ± 1.5) and the FSDS score reduced (11.3 ± 1.2) and were statistically significant with respect to the baseline values (p < 0.001).
Finally, there was a statistically significant correlation between improvement in the KI and FSFI total score at the 2nd follow-up (r = −0.76, p < 0.001).
Each woman recorded in her diary that she had had the same, and only one, sexual partner throughout the study period, a good quality of relationship and no difficulties in sexual performance of the partner. No partner was suffering from sexual dysfunction during the study. The frequency of sexual activity improved from 1.1 to 2.3 at the 2nd follow-up (p < 0.01).
Discussion
This study was the first investigating the QoL and sexual function of symptomatic perimenopausal women taking several nutraceutical substances with synergistic activity such as: Equol, having estrogen activity [18]; Resveratrol, with antioxidant activity [19]; Quecitine, having inhibitory activity on adipogenesis [20]; and Passiflora, having anxiolytic activity [21]. Firstly, the enrollment of perimenopausal women was performed according to the −1 +1a STRAW system. During nutraceuticals usage, women experienced an improvement in perimenopausal symptoms. In fact, the basal 35 ± 4 KI score reduced to 21 ± 3 at the 1st follow-up and to 18 ± 2 at the 2nd follow-up. This progressive reduction of the KI score could have affected the QoL of women, progressively improving all categories over the 6-month study. Interestingly, the quality of sexual life improved at the 2nd follow-up but not at the 1st follow-up. This evidence should lead to some reflections when nutraceuticals are used by premenopausal symptomatic women. In fact, the efficacy latency time may be variable and often some aspects recover more quickly than others, as observed from the results of this study. This evidence shows how counseling should be adopted by clinicians during the prescription of nutraceuticals, underlining the fact that their efficacy depends on the time of their usage; in fact, the information on the benefits of nutraceuticals must stress that their use be continued although their effectiveness tends to be delayed. On the basis of our previous [28] and current investigations, we considered the first 3 months of nutraceutical usage the time during which a series of objective and subjective adjustments could be initiated; moreover, the assessment of the QoL is a crucial parameter to take into account before concluding on the efficacy of a treatment [29]. During the perimenopause, many women can experience several symptoms associated with changes in the menstrual cycle. Hormone changes are associated with neurovegetative symptoms that, at this stage of life, can affect the quality of life and sexual health of women. Symptoms are often characterized by hot flushes, sleep disturbance, and sweats which cause subjective and social inadequacies in women. Mood changes could increase during the menopausal transition with vulnerability to cognitive decline and increased risk of depressive symptoms and depressive disorders [30].
Sexual function also may improve as a result of the decrease in the neurovegetative symptoms. Through the improvements in sexual function and the synchronous reduction of distress shown by our study, women obtain a better sexual activity, not only qualitative but also quantitative. In fact, the monthly frequency of sexual activity increased over the nutraceutical usage.
Nutraceuticals can be effective in modulating the symptoms and are well accepted by the women who usually do not wish to use hormone replacement treatment.
The current study has some limitations. One is the lack of randomization with a control group without treatment or on hormonal therapy on a larger number of women. Moreover, to study the placebo effects that could take place in the first 6 months of nutraceutical usage and to observe the effects on vaginal epithelium of the Equol will be needed. Future investigations are proposed to address this.
References
de Bruin JP, Bovenhuis H, van Noord PA, Pearson PL, van Arendonk JA, te Velde ER, Kuurman WW, Dorland M (2001) The role of genetic factors in age at natural menopause. Hum Reprod 16:2014–2018
He C, Murabito JM (2014) Genome-wide association studies of age at menarche and age at natural menopause. Mol Cell Endocrinol 382:767–779
Deeks AA (2004) Is this menopause? Women in midlife-psychosocial issues. Aust Fam Physician 33:889–893
McKinley NM, Lyon LA (2008) Menopausal attitudes, objectified body consciousness, aging anxiety, and body esteem: European American women’s body experiences in midlife. Body Image 5:375–380
Freeman EW (2010) Associations of depression with the transition to menopause. Menopause 17:823–827
Spinelli MG (2005) Neuroendocrine effects on mood. Rev Endocr Metab Disord 6:109–115
Schipper HM (2015) The impact of gonadal hormones on the expression of human neurological disorders. Neuroendocrinology. doi:10.1159/000440620
Cohen LS, Soares CN, Joffe H (2005) Diagnosis and management of mood disorders during the menopausal transition. Am J Med 118(Suppl 12B):93–97
Pearce G, Thøgersen-Ntoumani C, Duda J (2014) Body image during the menopausal transition: a systematic scoping review. Health Psychol Rev 8:473–489
Yanikkerem E, Koltan SO, Tamay AG, Dikayak Ş (2012) Relationship between women’s attitude towards menopause and quality of life. Climacteric 15:552–562
Terauchi M, Hirose A, Akiyoshi M, Owa Y, Kato K, Kubota T (2015) Prevalence and predictors of storage lower urinary tract symptoms in perimenopausal and postmenopausal women attending a menopause clinic. Menopause 22:1084–1090
Thornton K, Chervenak J, Neal-Perry G (2015) Menopause and Sexuality. Endocrinol Metab Clin North Am 44:649–661
Dennerstein L, Hayes RD (2005) Confronting the challenges: epidemiological study of female sexual dysfunction and the menopause. J Sex Med 2(Suppl 3):118–132
Nappi RE, Lachowsky M (2009) Menopause and sexuality: prevalence of symptoms and impact on quality of life. Maturitas 63:138–141
Hale GE, Robertson DM, Burger HG (2014) The perimenopausal woman: endocrinology and management. J Steroid Biochem Mol Biol 142:121–131
Nutraceuticals/Functional Foods and Health Claims on Foods: Policy Paper”. Health Canada. June 24, 2013. Retrieved January 30, 2014
Hardy G (2000) Nutraceuticals and functional foods: introduction and meaning. Nutrition 16:688–689
Wu J, Oka J, Ezaki J, Ohtomo T, Ueno T, Uchiyama S, Toda T, Uehara M, Ishimi Y (2007) Possible role of equol status in the effects of isoflavone on bone and fat mass in postmenopausal Japanese women: a double-blind, randomized, controlled trial. Menopause 14:866–874
Mikstacka R, Rimando AM, Ignatowicz E (2010) Antioxidant effect of trans-resveratrol, pterostilbene, quercetin and their combinations in human erythrocytes in vitro. Plant Foods Hum Nutr 65:57–63
Yang JY, Della-Fera MA, Rayalam S, Ambati S, Hartzell DL, Park HJ, Baile CA (2008) Enhanced inhibition of adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin. Life Sci 82:1032–1039
Appel K, Rose T, Fiebich B, Kammler T, Hoffmann C, Weiss G (2011) Modulation of the γ-aminobutyric acid (GABA) system by Passiflora incarnata L. Phytother Res 25:838–843
Ngan A, Conduit R (2011) A double-blind, placebo-controlled investigation of the effects of Passiflora incarnata (passionflower) herbal tea on subjective sleep quality. Phytother Res 25:1153–1159
Cao ZY (2005) Chinese obstetrics and gynecology. People’s Medical Publishing House, Beijing
Ware JE, Kosinski M, Gandek B et al (1998) The factor structure of the SF-36 Health Survey in 10 countries: results from the International Quality of Life Assessment (IQOLA) project. J Clin Epidemiol 51:1159–1165
Basson R, Leiblum S, Brotto L et al (2004) Revised definitions of women’s sexual dysfunction. J Sex Med 1:40–48
Nappi RE, Albani F, Vaccaro P et al (2008) Use of the Italian translation of the Female Sexual Function Index (FSFI) in routine gynecological practice. Gynecol Endocrinol 24:214–219
Derogatis LR, Rosen R, Leiblum S, Burnett A, Heiman J (2002) The Female Sexual Distress Scale (FSDS): initial validation of a standardized scale for assessment of sexually related personal in distress women. J Sex Marital Ther 28:317–330
Caruso S, Iraci SM, Casella E, Ventura B, Fava V, Cianci A (2015) Chronic pelvic pain, quality of life and sexual health of women treated with palmitoylethanolamide and α-lipoic acid. Minerva Ginecol 67:413–419
Caruso S, Iraci M, Cianci S, Casella E, Fava V, Cianci A (2015) Quality of life and sexual function of women affected by endometriosis-associated pelvic pain when treated with dienogest. J Endocrinol Invest 38:1211–1218
Weber MT, Maki PM, McDermott MP (2014) Cognition and mood in perimenopause: a systematic review and meta-analysis. J Steroid Biochem Mol Biol 142:90–98
Acknowledgments
The authors wish to thank The Scientific Bureau of the University of Catania for language support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
Ethical approval
I declare that this study was approved by the Institutional Review Board (IRB) of our Department.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Unfortunately, one of the co-author first name was wrongly published in the original publication and the error has now been corrected.
An erratum to this article is available at http://dx.doi.org/10.1007/s40618-017-0630-1.
An erratum to this article is available at http://dx.doi.org/10.1007/s40618-016-0556-z.
Rights and permissions
About this article
Cite this article
Caruso, S., Cianci, S., Cariola, M. et al. Effects of nutraceuticals on quality of life and sexual function of perimenopausal women. J Endocrinol Invest 40, 27–32 (2017). https://doi.org/10.1007/s40618-016-0500-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-016-0500-2