Abstract
Objectives
Older adults are particularly vulnerable to unhealthy sleep. This study examines the relationship between sleep duration and chronic diseases among older Native Hawaiians or Other Pacific Islanders (NHOPIs) and identifies variations with older Asians.
Methods
In this cross-sectional study, data were analyzed using the 2016 Behavioral Risk Factor Surveillance System. The total sample of adults 50 years and older included 1277 NHOPIs and 4655 Asians. Weighted, survey logistic regressions were employed to analyze the relationship between sleep duration (i.e., short, healthy, and long) and seven chronic diseases: coronary heart disease, stroke, heart attack/myocardial infarction, pre-diabetes, diabetes, chronic obstructive pulmonary disease, and depressive disorder. Sleep duration was categorized into short sleep (SS; ≤ 6 h), healthy sleep (7–8 h), and long sleep (LS; ≥ 9 h), with healthy sleep as the reference group.
Results
Among NHOPIs, SS and LS were significantly related to stroke (OR 3.19, 95% CI: 1.35–7.53 for SS and OR 9.52, 95% CI: 2.99–30.34 for LS) and SS was associated with pre-diabetes (OR 2.22 CI: 1.07–4.59), after adjusting for all covariates. In contrast, Asians with SS and LS reported higher odds of depression (OR 2.40, 95% CI: 1.20–4.79 and OR 5.03, 95% CI: 1.57–16.13, respectively).
Conclusions
Findings suggest older NHOPIs with SS or LS experience worse health. NHOPIs and Asians varied on the relationship between sleep and chronic disease, underscoring the need to disaggregate Asian/NHOPI data to understand health disparities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep is a public health concern, due to a growing body of research linking sleep duration to poorer health outcomes [1,2,3,4,5,6,7]. Specifically, short sleep (SS) duration, defined as less than 7 h of sleep, and long sleep (LS) duration, defined as 9 or more hours of sleep, have been associated with cardiovascular disease, diabetes, depression, respiratory conditions, and early mortality, when compared to healthy adult sleepers (i.e., generally adults who receive 7–8 h of sleep during a 24-h period) [1,2,3,4,5,6, 8, 9]. Moreover, excessive sleepers have a greater risk of early mortality compared to short and healthy sleepers [1, 3]. Based on these reports, sleep has been found to encompass a U-shaped pattern with SS and LS duration at higher risk of morbidity and premature death [1,2,3, 5, 10].
Older adults are particularly vulnerable to unhealthy sleep behaviors. Biological changes in the circadian rhythm, melatonin levels, and adenosine concentrations result in less deep, non-rapid eye movement (nREM) sleep and an altered, unsatisfying sleep pattern [7, 9, 11, 12]. In addition, sleep is increasingly fragmented in older adults, at times due to medications, diseases and conditions (e.g., restless leg syndrome), bladder issues, or prolonged periods of grief [7, 9, 13]. These, combined with a rise in a suboptimal sleep hygiene environment, including the widespread use of artificial lighting, caffeine, and computer/smartphone or television screens, contribute to sleep deprivation [5, 7, 14].
Fewer studies have focused on physiological and environmental reasons for longer sleep patterns [1, 3]. Some research has attributed oversleeping to certain conditions, such as depression or stroke, and less physical activity, which are often seen in older populations [1, 3, 15, 16]. Protracted melatonin levels with delayed morning declines, longer periods of decreased body temperature, and greater concentrations of adenosine may also contribute to excessive sleep behaviors [7, 14, 15].
Among older adults, Native Hawaiian or Other Pacific Islander (NHOPI) elders endure a higher burden of chronic diseases (diabetes, heart disease, and cancer) and experience shorter life expectancy when evaluated against other racial/ethnic groups [17,18,19]. NHOPIs who are 65 years and older represent a small percentage of the overall NHOPI population, at 5.6% compared to 13% of the total US population [17]. In a recent epidemiological review, rates of cancer and early mortality were greater among NHOPIs than Asians and non-Hispanic Whites [17]. Furthermore, older NHOPIs live in poverty at a higher proportion than the general older adult population (12.6% vs 9.3%) and notably fewer own their home (16.5% vs 26.3%) [17]. These multidimensional factors have been found to contribute to health disparities [1, 6, 17,18,19], and more recently to abnormal sleep patterns [1, 6, 9, 19]. In fact, the Centers for Disease Control and Prevention (CDC) issued a report listing adult NHOPIs as the second highest racial/ethnic group, after non-Hispanic multi-racial, that sleep fewer than 7 h per night regularly [20].
Currently, there is a paucity of research examining the relationship between sleep and chronic diseases among older NHOPIs. Therefore, the primary purpose of this study, a first of its kind, was to determine whether older NHOPIs who report inadequate or excessive sleep durations have higher odds of reporting a specific chronic disease. This lack of research on older NHOPIs is partly due to the practice of aggregating NHOPIs and Asians into one racial/ethnic category [21, 22]. As a result, any unique factors that can elucidate health disparities and inequities particular to older NHOPIs are masked by a generally healthier Asian population, hindering the ability of health professionals to improve health outcomes for NHOPIs [21,22,23,24]. Accordingly, variations between older NHOPIs and Asians are noted.
Methods
Participants and Design
Data from the 2016 Behavioral Risk Factor Surveillance System (BRFSS) [25] was employed for this study. The BRFSS uses an automated digit-dialing telephone approach to collect self-reported information on health-related behaviors and risk factors, chronic conditions, service utilization, and demographics on individuals throughout the 50 states plus District of Columbia, Puerto Rico, Guam, and US Virgin Islands. Participants are chosen randomly and must be 18 years and older, noninstitutionalized, and accessible through a landline or nonbusiness cellular/mobile phone. In 2016, the median survey response rate was 47.1% and ranged from 30.7 to 65.0%, resulting in a total sample of 486,303 respondents. Sleep data is collected biennially, during even years only. Further details, including the questionnaire, codebook, and weighting methods, on the BRFSS can be found at https://www.cdc.gov/brfss/annual_data/annual_2016.html; additional information on the response rate can be found in the BRFSS 2016 Summary Data Quality Report [26].
The sample focused on NHOPI adults 50 years and older, narrowing the total sample to 1277 participants. We chose the age threshold of 50 years of age because, as noted above, adults 65 years and older comprise a relatively small segment of the general NHOPI population. Additionally, median age of the NHOPI population was estimated at 10 years lower than the general US population in 2010 [17]. For comparison, Asians 50 years and older were included in the study (N = 4655).
Measures
Sleep duration, the independent variable, was based on the self-reported BRFSS item: “On average, how many hours of sleep do you get in a 24-h period?” with responses being recorded in full hour increments. Sleep duration was then categorized into three groups: short sleep (SS; ≤ 6 h of sleep), healthy sleep (HS; 7–8 h of sleep), and long sleep (LS; ≥ 9 h of sleep). These categories are consistent with the National Sleep Foundation recommended sleep duration for older adults as well as other published studies [1, 5, 27].
Individual chronic diseases, the outcome variables, experienced by participants were determined by a “yes” or “no” response to being told by a “doctor, nurse, or other health professional” that they had a heart attack or myocardial infarction, coronary heart disease, stroke, chronic obstructive pulmonary disease (COPD), or depressive disorder (includes dysthymia and minor to major depression). Regarding diabetes, the BRFSS provides four classifications to the question “(Ever told) you have diabetes”: “(1) yes; (2) yes, but female told only during pregnancy; (3) no; (4) no, pre-diabetes or borderline diabetes.” For purposes of this paper, we dichotomized diabetes and pre-diabetes into yes/no. Participants with diabetes (N = 32) were excluded from the pre-diabetes category, and respondents who only reported gestational diabetes were categorized as not having diabetes or pre-diabetes, since our sample consisted only of older adults.
Based on the previous research in this area, covariates consisted of demographic-related variables (gender, age group, and education) and health behaviors (cigarette smoking, alcohol use, and physical inactivity). We employed the BRFSS calculated 14-level, 5-year age variable, and the four-level educational variable. For health behaviors, items were dichotomized into yes or no. The following items were used to determine current cigarette smoking: “Have you smoked at least 100 cigarettes in your entire life?” and “Do you smoke cigarettes every day, some days, or not at all?” Former smokers were grouped with nonsmokers. We utilized this question from the BRFSS on alcohol use: “During the past 30 days, how many days per week or per month did you have at least one drink of any alcoholic beverage such as beer, wine, a malt beverage or liquor?” Respondents with at least one reported alcoholic drink were recoded into the yes category. Physical inactivity was measured by participants’ yes/no responses to “During the past month, other than your regular job, did you participate in any physical activities or exercises such as running, calisthenics, golf, gardening, or walking for exercise?”.
Statistical Analysis
All analyses were conducted using SAS version 9.4 [28] and complex survey sampling weights were applied. Selected demographics were examined. Rao-Scott chi square tests were used to evaluate participant characteristic differences between NHOPI and Asian older adult populations. A series of survey logistic regressions were employed to analyze the relationship between sleep duration (i.e., short, healthy, and long) and each chronic disease, with healthy sleep as the reference group. Step 0 represents the unadjusted model. In step 1, the partially adjusted model included the covariates: gender, age group, and education. For the final, fully adjusted model, the health behavior covariates of cigarette smoking, alcohol use, and physical inactivity were added. For each logistic regression, the DOMAIN feature was used to analyze older NHOPIs and Asians separately. Specific to the depressive disorder category, odds ratios for NHOPIs and Asians were further analyzed to detect significant differences between the two racial groups using the LSMEANS statement with DIFF and SLICEBY options [29]. Multicollinearity was assessed, with tolerance for all variables exceeding standard thresholds. Results were deemed significant at p ≤ 0.05.
Results
Table 1 presents demographic characteristics for both NHOPI and Asian groups. Based on weighted figures, NHOPIs reported less household income, lower educational achievement, and fewer owned their home, compared to Asians. NHOPIs were more likely to be 50–64 years (71.5%) compared to Asians (61.9%); Asians tended to be 65 years or older (38.1%) compared to NHOPIs (28.5%).
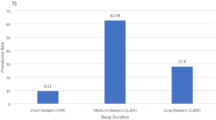
Table 2 compares participant self-reported sleep duration and health characteristics. NHOPIs and Asians were similar on sleep duration, alcohol consumption, and physical inactivity. On the other hand, NHOPIs reported higher rates of current smoking (17.82%) when contrasted with Asians (5.36%) and higher rates of obesity (33.68% vs 11.73%). In addition, NHOPIs reported overall poorer health status with a mean of 3.01 (SE = 0.08; scale with 1 = excellent and 5 = poor) in contrast to Asians with a mean of 2.67 (SE = 0.04).
Table 3 provides significant and non-significant results from the logistic regressions of sleep duration and selected chronic diseases. After adjusting for all covariates in the final model, older NHOPI SSs had 3.19 greater odds of reporting a stroke and 2.22 greater odds of having pre-diabetes. Likewise, LSs were found to have higher odds of reporting a stroke (OR 9.52, 95% CI: 2.99–30.34). Conversely, coronary heart disease, heart attack/myocardial infarction, diabetes, COPD, and depressive disorder were non-significant in the fully adjusted model for both SSs and LSs. Similarly, LS was not significantly associated with pre-diabetes.
For comparison, unlike older NHOPIs, older Asian SSs and LSs had greater odds of having a depressive disorder after accounting for all covariates (OR 2.40, 95% CI: 1.20–4.79 and OR 5.03, 95% CI: 1.57–16.13, respectively). Where older NHOPI and Asian SSs reported significant associations with depression in the partially adjusted model (i.e., adjustment for demographic characteristics only), the odds ratios were not significantly different between the two racial groups. As illustrated in Table 3, no other chronic disease was related to SS or LS among older Asians, which underscores the need to disaggregate data from a larger Asian population to fully understand NHOPI health disparities.
Discussion
The aim of this paper was to investigate the association between extreme sleep patterns and certain chronic conditions among older NHOPIs. The findings from this analysis of the 2016 BRFSS indicate that excessive or inadequate sleep patterns were related to stroke, pre-diabetes, COPD, depressive disorder, and, to a lesser degree, coronary heart disease. After eliminating the effects of certain demographic conditions (i.e., gender, age group, and education) and health behaviors (i.e., cigarette smoking, alcohol use, and physical inactivity), SS and LS were associated with stroke, and a link between SS and pre-diabetes emerged. Comparisons with older Asians were also made. Notably, depressive disorder was the only chronic condition significantly associated with SS and LS among both older NHOPIs and Asians in the unadjusted model. However, once all covariates were accounted for in the final model, SS and LS were not related to depression among older NHOPIs, while SS and LS among older Asians remained linked to a depressive disorder.
Stroke
Consistent with other studies [8, 10, 30, 31], SSs and LSs had significantly higher odds of having a stroke compared to healthy sleepers, suggesting a U-shaped curve. Moreover, our research indicated that LSs had greater odds of reporting a stroke compared with SSs. In separate systematic reviews, SS and LS were associated with increased relative risk for experiencing or dying from a stroke [8, 30]. Additionally, a CDC study, using data from 14 states within the BRFSS, found that SS and LS were significantly related to having a stroke among adults 45 years and older [32].
Biological mechanisms can provide explanations into the consistency of these findings. SS is associated with elevated blood pressure [7, 30, 33] and inflammation [8, 30, 34], increased heart rate [30], and greater cortisol levels [7, 8, 33], all of which are known risk factors for stroke. However, research is only beginning to delve into the relationship between LS and stroke. Similar to SS, one explanation is chronic inflammation due to increased levels of proinflammatory biomarkers (e.g., IL-6, CRP, and fibrinogen) [30, 34]. In addition, sedentary lifestyles, prolonged stress due to racism, or the presence of co-morbidities are potential contributing factors [8, 17]. The underlying causes that link sleep duration specifically with stroke and not the other cardiovascular diseases are beyond the scope of this study. Future research should explore key mechanisms for this relationship found between extreme sleep patterns and strokes among older NHOPIs.
Pre-Diabetes/Diabetes
Interestingly, the absence of a significant relationship between unhealthy sleep behaviors and diabetes found in our study is partly inconsistent with the previous research [19, 32, 35,36,37,38]. Sleep data frequently show a U-shaped relationship with diabetes, with both SS and LS linked to diabetes [32, 35,36,37,38]. However, a study of NHOPI adults found that only SS (defined as 5–6 h of sleep per night) was significantly related to diabetes after adjusting for covariates, though extremely short sleep (< 5 h/night) was not meaningfully associated with diabetes [19].
Less research has been conducted on extreme sleep patterns and pre-diabetes. Based on our results, LSs had lower odds of pre-diabetes when accounting for gender, age group, and education, but not in the unadjusted model or after additional adjustments for health behaviors. On the other hand, SSs had two times greater odds of pre-diabetes, after taking into consideration alcohol consumption, cigarette smoking status, and physical inactivity in the final model. Engenda et al. [39] found a relationship between SS duration (≤ 5 h) and clinically diagnosed pre-diabetes but not undiagnosed pre-diabetes. The authors speculate the discrepancy may be due to underlying co-morbidities, prompting the clinical diagnosis, or particular biological mechanisms inherent in the progression of diabetes [39]. Studies suggest two biological mechanisms: endocrine function and glucose metabolism are more adversely affected in SS than LS, producing separate health outcomes [15, 35, 40, 41]. For that reason, older adults with habitual SS patterns may be prone to pre-diabetes earlier than longer sleepers [40, 41].
Older NHOPIs have a higher proportion of diabetes compared to Euro-Americans [17, 18, 42] so there may be other contributing factors not accounted for in this study, such as sleep quality, historical trauma, and energy intake, that would provide a better explanation [7, 18, 23, 37, 42]. Additionally, the reclassification of SS into two categories: 6 h and ≤ 5 h, comparable to some studies referenced above, may have generated different results [35, 39]. Future research should investigate any association between these factors and sleep behaviors among older NHOPIs.
Chronic Obstructive Pulmonary Disease (COPD)
SS had a modest effect on COPD [43] and was significant in step 1 (i.e., adjusted for gender, age group, and education), which is consistent with other, somewhat limited, research [44,45,46]. In a recent study, SS was linked to COPD compared to healthier sleep patterns (i.e., 7–8 h per night) among Chinese older adults 60–79 years old living in the Jilin Province of China [46]. In other research, shorter sleep duration and less REM sleep were associated with patients over 40 years of age with mild-to-moderate COPD when compared to matched controls without COPD [47].
In our study, however, the relationship became non-significant after adjusting for unhealthy behaviors, probably due to the confounding effects of smoking on the predictor variable. Nicotine acts as a stimulant that promotes alertness, light sleep, and frequent sleep disruption, exacerbating sleep-related problems already inherent in aging populations [9, 19, 48]. Furthermore, nocturnal nicotine withdrawal appears to contribute to sleep fragmentation [48].
It is important to note that factors associated with COPD, for example chronic coughing or dyspnea, have also been found to result in disrupted, shorter sleep patterns, producing a constant detrimental loop between SS and COPD that worsens the symptoms of COPD [45, 49]. Therefore, future studies should employ longitudinal data of older NHOPIs to determine whether SS predicts onset of COPD or SS is an outcome of COPD.
Depression
Research findings on sleep duration and depression are mixed [7, 15, 16, 50,51,52,53,54]. In our study of older NHOPIs, SSs and LSs had greater odds of reporting a depressive disorder in the unadjusted model but were not associated after adjusting for all covariates, and the magnitude of the relationship decreased. In contrast, a combined cross-sectional and prospective study of older adults 65 years or older residing in the northern Manhattan area of New York City found SS was significantly related to depression, defined by a score of 16 or more on the Center for Epidemiological Studies — Depression scale (CES-D) or antidepressant use [51]. Moreover, non-depressed SSs were more apt to be depressed after 5 years [51]. The link between SS, but not LS, and depression is supported by a larger study using data from the English Longitudinal Study of Ageing [50]. However, older SSs and LSs (58–100 years of age) in the Rotterdam Study reported significantly higher odds of having a depressive disorder, determined by their CES-D score and psychiatric interview, compared to the reference group [16]. SSs scored higher on the CES-D than LS, which the authors speculate is indicative of major depression [16]. Conversely, a meta-analysis found no connection between unhealthy sleep durations and depression among studies with a mean age of 60 years or older [54].
Among older Asians, SS and LS were significantly associated with depression. These findings are consistent with an epidemiological study of older adults in Korea, except in our sample excessive sleepers had higher odds of reporting a depressive disorder than older adults with SS duration [55]. Data from the China Health and Retirement Longitudinal Study (CHARLES 2011–2013) showed that SS was related to “incident” depression (defined as reporting depression only during wave two) among older adults 65 years and older, and to a lesser degree among adults 45–64 years of age [56]. Although, “persistent” depression (individuals with depression at both wave one and wave two) was significant only with adults 45–64 who reported less than 6 h of sleep per night [56].
Unlike Asians, our NHOPI analysis suggests sleep may not have a strong association with depressive disorders compared to other unhealthy behaviors, despite high prevalence rates of depressive disorders [57, 58] and, separately, SS duration [19, 26] within this population. Research on adult Asians and NHOPIs (mean age of 51.5 years) found depressive symptoms and current smoking were significantly related among NHOPIs but not Asians [57]. Studies have also observed the significance of physical activity in improving sleep and depression among older adults [59, 60].
Furthermore, the cause or severity of the depressive disorder were not measured within the BRFSS. NHOPIs experience chronic and acute stressors that foster depressive symptoms, for example the marginalization and historical trauma of colonization that resulted in loss of land and basic rights across generations [17, 57, 61, 62]. Additionally, older NHOPIs suffer from the lowest incomes compared to most Asian groups, as well as other racial/ethnic groups [17, 23, 42]. These distinctive psychosocial stressors, along with social norms, dictate coping methods, culminating into a complex interaction between sleep duration and depression beyond the scope of this paper [61].
Strengths and Limitations
Strengths of our study include an investigation of extreme sleep patterns within an understudied population, utilization of a nationwide sample, and examination of a population that is often aggregated yet demonstrates unique health disparities. Concurrently, the study adds to the literature by addressing a crucial gap in our knowledge about older NHOPIs and providing further evidence for the need to disaggregate NHOPIs and Asians.
Despite these strengths, the study has limitations. First, cross-sectional research does not provide insights into causation. Therefore, it cannot be determined whether extreme sleep patterns trigger chronic disease or vice versa, so results should be interpreted with caution. Second, the BRFSS relies on self-report measures for sleep duration, which can be overstated by equating time in bed to time asleep. In addition, self-reported data on chronic disease was unsubstantiated by medical records or other methods by the individual states or the CDC. Third, low representation of LS can affect model outcomes by overestimating or underestimating results [63]. Fourth, the study did not analyze quality of sleep or take into consideration sleep disorders, such as sleep apnea, which can influence sleep duration.
It is important to also note that the inclusion of several covariates employed in the final model to distinguish the direct effect of sleep duration on chronic disease may explain the various non-significant results reported in this study. For those non-significant findings, certain health behaviors (e.g., sedentary lifestyle or tobacco use) may play a larger role than sleep patterns for NHOPIs.
Conclusions
In conclusion, SS and LS were related to stroke, exhibiting a u-shaped curve, among older NHOPIs. SS was also significantly associated with pre-diabetes but not diabetes, and had a moderate effect on COPD when accounting for gender, age group, and education. Notably, there were variations between older NHOPIs and older Asians, which underscores the critical need to disaggregate Asian/Pacific Islander data.
Sleep is an emerging public health concern and unhealthy sleep patterns should be considered a risk factor consistent with smoking, alcohol consumption, and physical inactivity [1, 5, 6, 9, 36]. In support of this concept, our study suggests that older NHOPIs with chronic SS or LS duration experience worse health compared to their HS counterparts. However, further research is needed to substantiate a clear link between sleep behaviors and chronic disease among older, diverse populations, particularly the use of longitudinal studies or the inclusion of objective measures (e.g., polysomnography). Even so, public health professionals and clinicians should consider initiatives to identify and address harmful sleep patterns.
Data Availability
The data that support the findings of this study are publicly available in the Behavioral Risk Factor Surveillance System, Centers for Disease Control and Prevention at https://www.cdc.gov/brfss/annual_data/annual_2016.html.
References
Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18(2):148–58. https://doi.org/10.1111/j.1365-2869.2008.00732.x.
Lubetkin EI, Jia H. Burden of disease due to sleep duration and sleep problems in the elderly. Sleep Health. 2018;4(2):182–7. https://doi.org/10.1016/j.sleh.2017.11.007.
Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2864873/pdf/aasm.33.5.585.pdf.
Spira AP. Sleep and health in older adulthood: recent advances and the path forward. J Gerontol A Biol Sci Med Sci. 2018;73(3):357–9. https://doi.org/10.1093/gerona/glx263.
Matricciani L, Bin YS, Lallukka T, Kronholm E, Dumuid D, Paquet C, Olds T. Past, present, and future: trends in sleep duration and implications for public health. Sleep Health. 2017;3(5):317–23. https://doi.org/10.1016/j.sleh.2017.07.006.
Laposky AD, Van Cauter E, Diez-Roux AV. Reducing health disparities: the role of sleep deficiency and sleep disorders. Sleep Med. 2016;18:3–6. https://doi.org/10.1016/j.sleep.2015.01.007.
Walker MP. Why we sleep: unlocking the power of sleep and dreams. 1st ed. New York, NY: Scribner, an imprint of Simon & Schuster, Inc.; 2017.
Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–92.
Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12(1):31–8. https://doi.org/10.1016/j.jsmc.2016.10.008.
Liu Y, Wheaton AG, Croft JB, Xu F, Cunningham TJ, Greenlund KJ. Relationship between sleep duration and self-reported health-related quality of life among US adults with or without major chronic diseases, 2014. Sleep Health: J Natl Sleep Found. 2018;4(3):265–72. https://doi.org/10.1016/j.sleh.2018.02.002.
Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1–11. https://doi.org/10.1016/j.jsmc.2017.09.001.
Rodriguez JC, Dzierzewski JM, Alessi CA. Sleep problems in the elderly. Med Clin North Am. 2015;99(2):431–9. https://doi.org/10.1016/j.mcna.2014.11.013.
Schubert CR, Cruickshanks KJ, Dalton DS, Klein BE, Klein R, Nondahl DM. Prevalence of sleep problems and quality of life in an older population. Sleep. 2002;25(8):889–93.
Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA. A longer biological night in long sleepers than in short sleepers. J Clin Endocrinol Metab. 2003;88(1):26–30.
Grandner MA, Drummond SPA. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev. 2007;11(5):341–60. https://doi.org/10.1016/j.smrv.2007.03.010.
van den Berg JF, Luijendijk HJ, Tulen JHM, Hofman A, Neven AK, Tiemeier HW. Sleep in depression and anxiety disorders: a population-based study of elderly persons. J Clin Psychiatry. 2009;70(8):1105–13. https://doi.org/10.4088/JCP.08m04448.
Braun KL, Kim BJ, Ka’opua LS, Mokuau N, Browne CV. Native Hawaiian and Pacific Islander elders: what gerontologists should know. Gerontologist. 2015;55(6):912–9. https://doi.org/10.1093/geront/gnu072.PubMedPMID:2015-56988-001.
Browne CV, Mokuau N, Braun KL. Adversity and resiliency in the lives of Native Hawaiian elders. Soc Work. 2009;54(3):253–61. https://doi.org/10.1093/sw/54.3.253.
Matthews EE, Li C, Long CR, Narcisse M-R, Martin BC, McElfish PA. Sleep deficiency among Native Hawaiian/Pacific Islander, Black, and White Americans and the association with cardiometabolic diseases: analysis of the National Health Interview Survey Data. Sleep Health: J Natl Sleep Found. 2018;4(3):273–83. https://doi.org/10.1016/j.sleh.2018.01.004.
Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of healthy sleep duration among adults — United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(6):137–41
Moy KL, Sallis JF, David KJ. Health indicators of Native Hawaiian and Pacific Islanders in the United States. J Community Health. 2010;35(1):81–92. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2816258/pdf/10900_2009_Article_9194.pdf.
Ro MJ, Yee AK. Out of the shadows: Asian Americans, Native Hawaiians, and Pacific Islanders. Am J Public Health. 2010;100(5):776–8. https://doi.org/10.2105/AJPH.2010.192229 (PubMed PMID: 50615146).
Ka‘opua LS, Braun KL, Browne CV, Mokuau N, Park C-B. Why are Native Hawaiians underrepresented in Hawai‘i's older adult population? Exploring social and behavioral factors of longevity. Journal of Aging Research. 2011;2011:1–8 https://doi.org/10.4061/2011/701232
Srinivasan S, Guillermo T. Toward improved health: disaggregating Asian American and Native Hawaiian/Pacific Islander data. American Journal of Public Health. 2000;90(11):1731–4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1446402/pdf/11076241.pdf.
Centers for Disease Control and Prevention. Behavioral risk factor surveillance system survey data [Data file] 2016. Available from: https://www.cdc.gov/brfss/annual_data/annual_2016.html. Accessed 19 February 2019.
Centers for Disease Control and Prevention. Behavioral risk factor surveillance system 2016 summary data quality report. 2017. Available from: https://www.cdc.gov/brfss/annual_data/2016/pdf/2016-sdqr.pdf. Accessed 10 July 2019.
Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Katz ES, Kheirandish-Gozal L, Neubauer DN, O’Donnell AE, Ohayon M, Peever J, Rawding R, Sachdeva RC, Setters B, Vitiello MV, Ware JC, Adams Hillard PJ. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–3. https://doi.org/10.1016/j.sleh.2014.12.010.
SAS Institute Inc. SAS® proprietary software, version 9.4 for Windows. Cary, NC: SAS Institute Inc.; 2013.
Agnelli R. Examples of logistic modeling with the SURVEYLOGISTIC procedure. Cary, NC: SAS Institute Inc.; 2014.
Li W, Wang D, Cao S, Yin X, Gong Y, Gan Y, Zhou Y, Lu Z. Sleep duration and risk of stroke events and stroke mortality: a systematic review and meta-analysis of prospective cohort studies. Int J Cardiol. 2016;223:870–6. https://doi.org/10.1016/j.ijcard.2016.08.302.
Jike M, Itani O, Watanabe N, Buysse DJ, Kaneita Y. Long sleep duration and health outcomes: a systematic review, meta-analysis and meta-regression. Sleep Med Rev. 2018;39:25–36. https://doi.org/10.1016/j.smrv.2017.06.011.
Liu Y, Wheaton AG, Chapman DP, Croft JB. Sleep duration and chronic diseases among us adults age 45 years and older: evidence from the 2010 Behavioral Risk Factor Surveillance System. Sleep. 2013;36(10):1421–7. https://doi.org/10.5665/sleep.3028.
Kingsbury JH, Buxton OM, Emmons KM, Redline S. Sleep and its relationship to racial and ethnic disparities in cardiovascular disease. Curr Cardiovasc Risk Rep. 2013;7(5):387–94. https://doi.org/10.1007/s12170-013-0330-0.
Grandner MA, Sands-Lincoln MR, Pak VM, Garland SN. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nat Sci Sleep. 2013;5:93–107. https://doi.org/10.2147/NSS.S31063.
Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, Nieto FJ. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Archives of Internal Medicine. 2005;165(8):863–7. https://jamanetwork.com/journals/jamainternalmedicine/articlepdf/486518/ioi40638.pdf.
Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–20. https://doi.org/10.2337/dc09-1124.
Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, Rong Y, Jackson CL, Hu FB, Liu L. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38(3):529–37. https://doi.org/10.2337/dc14-2073.
Zizi F, Pandey A, Murrray-Bachmann R, Vincent M, McFarlane S, Ogedegbe G, Jean-Louis G. Race/ethnicity, sleep duration, and diabetes mellitus: analysis of the National Health Interview Survey. Am J Med. 2012;125(2):162–7. https://doi.org/10.1016/j.amjmed.2011.08.020.
Engeda J, Mezuk B, Ratliff S, Ning Y. Association between duration and quality of sleep and the risk of pre-diabetes: evidence from NHANES. Diabetic Med: A J Bri Diabetic Assoc. 2013;30(6):676–80. https://doi.org/10.1111/dme.12165.
Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet (London, England). 1999;354(9188):1435–9.
Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59(9):2126–33. https://doi.org/10.2337/db09-0699.
Mau MK, Sinclair K, Saito EP, Baumhofer KN, Kaholokula JK. Cardiometabolic health disparities in Native Hawaiians and other Pacific Islanders. Epidemiol Rev. 2009;31(1):113–29. https://doi.org/10.1093/ajerev/mxp004.
Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19(22):3127–31. https://doi.org/10.1002/1097-0258(20001130)19:22%3c3127::AID-SIM784%3e3.0.CO;2-M.
Kutty K. Sleep and chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2004;10(2):104–12. https://doi.org/10.1097/00063198-200403000-00004.
Omachi TA, Blanc PD, Claman DM, Chen H, Yelin EH, Julian L, Katz PP. Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes. Sleep Medicine. 2012;13(5):476–83. https://doi.org/10.1016/j.sleep.2011.12.007.
Wang S, Wu Y, Ungvari GS, Ng CH, Forester BP, Gatchel JR, Chiu HFK, Kou C, Fu Y, Qi Y, Yu Y, Li B, Xiang Y-T. Sleep duration and its association with demographics, lifestyle factors, poor mental health and chronic diseases in older Chinese adults. Psychiatry Res. 2017;257:212–8. https://doi.org/10.1016/j.psychres.2017.07.036.
Valipour A, Lavie P, Lothaller H, Mikulic I, Burghuber OC. Sleep profile and symptoms of sleep disorders in patients with stable mild to moderate chronic obstructive pulmonary disease. Sleep Med. 2011;12(4):367–72. https://doi.org/10.1016/j.sleep.2010.08.017.
Wetter DW, Young TB. The relation between cigarette smoking and sleep disturbance. Preventive Medicine. 1994;23(3):328. https://www.sciencedirect.com/science/article/abs/pii/S0091743584710462?via%3Dihub.
Klink ME, Dodge RR, Quan SF. The relation of sleep complaints to respiratory symptoms in a general population. Chest. 1994;105(1):151–4. https://doi.org/10.1378/chest.105.1.151.
Jackowska M, Poole L. Sleep problems, short sleep and a combination of both increase the risk of depressive symptoms in older people: a 6-year follow-up investigation from the English Longitudinal Study of Ageing. Sleep Med. 2017;37:60–5.
Lippman S, Gardener H, Rundek T, Seixas A, Elkind MSV, Sacco RL, Wright CB, Ramos AR. Short sleep is associated with more depressive symptoms in a multi-ethnic cohort of older adults. Sleep Med. 2017;40:58–62. https://doi.org/10.1016/j.sleep.2017.09.019.
van Mill JG, Vogelzangs N, van Someren EJW, Hoogendijk WJG, Penninx BWJH. Sleep duration, but not insomnia, predicts the 2-year course of depressive and anxiety disorders. Journal of Clinical Psychiatry. 2014;75(2):119–26. http://www.psychiatrist.com/jcp/article/pages/2014/v75n02/v75n0206.aspx.
Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006;29(7):881–9. https://doi.org/10.1093/sleep/29.7.881.
Zhai L, Zhang H, Zhang D. Sleep duration and depression among adults: a meta-analysis of prospective studies. Depress Anxiety. 2015;32(9):664–70. https://doi.org/10.1002/da.22386.
Kim W-H, Kim B-S, Kim S-K, Chang S-M, Lee D-W, Cho M-J, Bae J-N. Sleep duration and associated factors in a community sample of elderly individuals in Korea. Psychogeriatrics. 2015;15(2):87–94. https://doi.org/10.1111/psyg.12072 (PubMed PMID: 25515971).
Yujie L, Yili W, Long Z, Tong W, Yongye S, Dongfeng Z. Longitudinal association of sleep duration with depressive symptoms among middle-aged and older Chinese. Sci Rep. 2017;7(1):1–7. https://doi.org/10.1038/s41598-017-12182-0.
Aczon-Armstrong M, Inouye J, Reyes-Salvail F. Depression and chronic illness: Asian/Pacific Islander adults in Hawaii. Issues Ment Health Nurs. 2013;34(3):169–79. https://doi.org/10.3109/01612840.2012.738356.
Hooker K, Phibbs S, Irvin VL, Mendez-Luck CA, Doan LN, Li T, Turner S, Choun S. Depression among older adults in the United States by disaggregated race and ethnicity. Gerontologist. 2019;59(5):886–91. https://doi.org/10.1093/geront/gny159.
Garfield VV. The relationship between physical activity, sleep duration and depressive symptoms in older adults: the English Longitudinal Study of Ageing (ELSA). Preventive Med Rep. 2016;4:512–6.
Bazargan M. Self-reported sleep disturbance among African-American elderly: the effects of depression, health status, exercise, and social support. Int J Aging Hum Dev. 1996;42(2):143–60.
Kim M. Racial/ethnic disparities in depression and its theoretical perspectives. Psychiatr Q. 2014;85(1):1–8. https://doi.org/10.1007/s11126-013-9265-3.
Lee CH, Sibley CG. Sleep duration and psychological well-being among New Zealanders. Sleep Health. 2019. https://doi.org/10.1016/j.sleh.2019.06.008.
Knutson KL, Turek FW. The U-shaped association between sleep and health: the 2 peaks do not mean the same thing. Sleep. 2006;29(7):878–79. https://doi.org/10.1093/sleep/29.7.878.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study concept. Data analysis was performed by Michelle A. Rainer and Bin Xie. All authors contributed to the interpretation of the results. The initial manuscript draft was written by Michelle A. Rainer and every author provided comments on subsequent versions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study is exempt from Internal Review Board since it solely uses secondary analysis of publicly available, de-identified data.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rainer, M.A., Palmer, P.H. & Xie, B. Sleep Duration and Chronic Disease Among Older Native Hawaiians or Other Pacific Islanders and Asians: Analysis of the Behavioral Risk Factor Surveillance System. J. Racial and Ethnic Health Disparities 10, 2302–2311 (2023). https://doi.org/10.1007/s40615-022-01409-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40615-022-01409-0