Abstract
Purpose of Review
Parental occupational exposures might be associated with neurodevelopmental disorders (NDDs) in offspring. We aimed to conduct a systematic review and meta-analysis to summarize and synthesize the current literature and to estimate the pooled magnitude of the underlying association(s) between parental occupational exposures and subsequent risk of NDDs.
Recent Findings
In the meta-analysis of 20 included studies, significant associations were found between parental occupational exposure to pesticides or solvents and the risk of attention deficit hyperactivity disorder in offspring. Prenatal occupational exposure to pesticides was significantly associated with motor development or cognition disorders in children. Furthermore, some evidence showed that metals might have a role in the development of autism spectrum disorders.
Summary
Further studies need to identify the level of parental occupational exposures that can be significantly associated with NDDs. Moreover, utilizing standardized outcome and exposure scales is recommended to incorporate paternal, maternal, and parental as well as both prenatal and postnatal exposure in future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodevelopmental disorders (NDDs) are several central nervous system conditions including attention deficit hyperactivity disorder (ADHD) and autism spectrum disorders (ASD) [1••, 2]. They impose a significant global burden as well as high community and individual costs [1••, 2]. Importantly, consequent irreversible structural and functional brain damage in those with NDDs [3, 4] might lead to emotional and behavioral problems as well as impairments in social, academic, and occupational functions [3, 5].
A complex interaction between environmental and genetic factors may be responsible for the etiology of NDDs [6,7,8,9]. A study using the Swedish national registry data found that the environmental factors were responsible for 17–50% of autism cases; thus, it reinforced the central role of environmental factors in the NDDs etiology [10, 11].
Evidence also suggests that the occupational exposures including lacquer [12•], pesticides [13,14,15,16], lipophilic chemicals [17], organic solvents [18, 19], heavy metals [20], lead, methyl mercury [21], and N2O [22] can play a role in the onset of NDDs. While there has been an increasing concern regarding the potential health risks of parental occupational exposures because of their potential neurotoxic effect [12•, 23], there are no consistent findings regarding the potential role of occupational exposure. In a case–control study of 537 children with ASD and 414 typically developing children, exposure to solvents in mothers of children with autism was significantly higher than mothers of healthy children (OR: 1.5; 95% CI: 1.01, 2.23) [24]. In a mother–child cohort study of 3005 participants in France, children of solvents-exposed mothers had more susceptibility to ADHD [25]. However, other studies did not demonstrate any association between occupational exposure and child-NDDs [12•, 26, 27]. In a cohort study, no association was found between dialkyl phosphate and psychomotor-development index or behavioral and emotional problems in children [26]. The conclusions drawn by case–control studies about the role of parental occupational exposure in autism onset are not conclusive [12•, 27].
Inconclusive findings exist about the strength of possible underlying association between parental occupational exposure and child NDDs. Inconsistencies across studies may be partly explained by insufficient sample size and choice of confounders.
The aim of this systematic review and meta-analysis study is to summarize and synthesize the current literature to quantify the pooled magnitude and direction of the underlying association between parental occupational exposures and subsequent risk of NDDs in offspring.
Methods
This systematic review and meta-analysis were conducted following the best practice guidelines recommended by the Meta-analyses Of Observational Studies in Epidemiology (MOOSE) [28, 29]. It is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines [30].
Search Strategy
We developed a comprehensive search syntax using MeSH and free text terms for PubMed, and adapted text terms as appropriate for the other searched databases, i.e., Scopus and Web of Science (Table S1). We searched all databases (not registries, websites, or organizations) from inception to January 2021, with an update search conducted in September 2021. No study design or language restrictions were imposed in the search strategy; however, finally we excluded the non-English studies. The search strategy consisted of 3 blocks of terms for (i) occupational exposure, (ii) NDDs, and (iii) terms related to prenatal or childhood life periods. Terms for NDDs were taken from the 4th and 5th editions of the Diagnostic and Statistical Manual for Mental Disorders (DSM-IV and DSM-V) [31] with additional terminology from the International Classifications of Diseases 10th edition (ICD-10) [32]. We applied several combinations of the following keywords for the search strategy: (Neurodevelopmental Disorders OR Anxiety, Separation OR Attention Deficit and Disruptive Behavior Disorders OR Attention Deficit Disorder with Hyperactivity OR Conduct Disorder OR Child Behavior Disorders OR Child Development Disorders, Pervasive OR Autism Spectrum Disorder OR Communication Disorders OR Childhood-Onset Fluency Disorder OR Social Communication Disorder OR Speech Sound Disorder OR Developmental Disabilities OR Intellectual Disability OR Learning Disabilities OR Dyscalculia OR Dyslexia OR Specific Learning Disorder OR Motor Skills Disorders OR Mutism OR Reactive Attachment Disorder OR Schizophrenia, Childhood OR Stereotypic Movement Disorder OR Tic Disorders OR Tourette syndrome) AND (Occupational Exposure OR Maximum Allowable Concentration OR Threshold Limit Values) AND (Prenatal Exposure Delayed Effects OR Infant OR Infant, Newborn OR Child OR Child, Preschool OR Adolescent). Additional studies were identified through forwards and backward citation searching (using Scopus) for papers that may have been missed by the electronic searches. Publications that were not found online were obtained by e-mailing the authors.
Eligibility Criteria
Observational studies, i.e., cohort, case–control, and cross-sectional studies were included if they investigated the association of parental occupational exposure with child NDDs. Excluded studies were those that did not report original results, i.e., reviews, meta-analyses, case reports, commentaries, letters, and editorials. Furthermore, we excluded studies dealing with combined parental occupational and domestic, residential, or environmental exposures without separate reporting of parental occupational data. Studies were included if the children studied were between 0 and 18 years of age. Only studies published in 1994 through September 30, 2021, were included in the analysis. This aligns with the publication of DSM-IV, which introduced changes in terminology used to describe neurodevelopmental outcomes.
Outcome and Main Exposure Classification
Study Main Outcomes
Included studies were classified as those indicating any or a combination of the following NDDs: (1) ADHD or ASD studies (where a diagnosis or a validated scale for measuring these conditions was reported as an outcome measure); (2) motor skill disorder (where motor development was measured); (3) learning disabilities (where cognition/memory was reported as an outcome measure); (4) communication disabilities (where language development was reported as an outcome measure); and (5) intellectual disability (where IQ, global developmental delay, and mental retardation were measured).
Study Main Exposures
In the case of parental occupational exposure, studies were classified as those investigating: (1) pesticides, (2) solvents, (3) metals, (4) anesthetics, (5) radiations, (6) fuels/exhausts, (7) plastic/polymers, (8) automobile/mechanic fluids, (9) asphalt, (10) temperature, (11) teratogens, or (12) asthmagenes/chemicals. Parental occupational exposures were measured in various periods including prior to pregnancy, during pregnancy, after birth until breastfeeding, and after breastfeeding in the case of passive exposure of the child to parental work clothes or residuals on the surface; thus, we had two main time windows as prenatal and postnatal exposure. In the case of postnatal exposure, some previous studies have measured child exposure using urine analysis. These studies indicated the indirect role of parental occupational exposure in child development after birth.
Study Selection
We uploaded the search results to reference management software (Endnote X8.0.2 for Windows). Titles and abstracts were independently screened for relevance by two reviewers (MB and PG). Any disagreement was released by discussion or third reviewer (IA), where necessary. The full texts of potentially relevant papers were retrieved and screened in the same way using the pre-specified inclusion and exclusion criteria. All duplicate papers were double-checked and excluded. “Sibling” papers derived from the same parent study were identified and linked.
Data Extraction
Two independent reviewers (MB and PG) employed a pilot data extraction form to extract relevant data from the included studies on (1) author’s name; (2) year of publication; (3) study design; (4) the location of conducting the survey; (5) study sample size; (6) type of occupational exposure; (7) the parent(s) exposed (mother, father, or both); (8) the measurement method of toxicants exposure; (9) the severity of exposure; (10) the time window(s) of exposure; (11) type of neurodevelopmental disorders/outcomes; (12) neurodevelopmental or neuropsychological tests/questionnaire; (13) the age of the children studied; and (14) the time of follow-up in cohort studies. Any discrepancies were resolved by discussion and involvement of a third reviewer (IA) when necessary. Authors were contacted to provide clarification or additional data if needed.
Quality Assessment
Two independent reviewers (PG and MB) assessed the methodological quality of the included studies through the Joanna Briggs Institute (JBI) critical appraisal checklist [33], a reliable and valid quality index for the appraisal of observational studies. Discrepancies were resolved through discussion and involvement of a third reviewer (IA) where necessary. JBI critical appraisal checklists were structured into fixed sets of questions in three different versions for cohort [34], case–control [35], and cross-sectional [36] studies within 11, 10, and 8 questions, respectively focusing on different aspects of bias in the study design, conduct, and analysis. Bias was assessed as a judgment with yes, no, unclear, or not applicable answers for each question. The decisions about the scoring system and the cut-off for inclusion of a study in the review were made in advance and agreed upon by all participating reviewers before critical appraisal commences [37]. All reviewers agreed that studies with two or more negative responses were assumed as low methodological quality studies that should be excluded from meta-analysis.
Data Synthesis and Analysis
We extracted odds ratio (OR), mean difference (MD), and beta coefficient (β) values from primary studies as the study effect sizes. MD was converted to OR if possible, then meta-analysis was performed on ORs and βs. The potential heterogeneity across studies was assessed using both Cochran’s Q-test and the I2 index. We employed the random effects model for estimating the pooled estimates.
If needed, subgroup analyses based on the exposure time window (prenatal and postnatal) were performed to seek the sources of heterogeneity. In addition, meta-regression was used for assessing the sample size, year of publication, the average age of the child, and percentage of boys in study samples as the possible source of heterogeneity. The sensitivity analyses were done by excluding one or more studies at a time to estimate the robustness of the study results. We employed the Funnel plot along with Egger’s test to evaluate the possible publication bias. All statistical analyses were conducted using software STATA 12.0 (STATA Corp, College Station, TX, USA).
Results
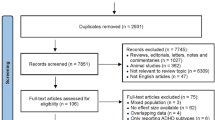
In total, 23 articles were found to be eligible for this systematic review; they consisted of 12 cohort, 8 case–control, and 3 cross-sectional studies [12•, 24,25,26,27, 38,39,40, 41•, 42,43,44,45,46,47,48,49,50,51,52,53,54,55]. Figure 1 demonstrates the whole process of the study selection.
Table 1 provides the main characteristics of the included studies. The studies were published between 1994 and 2019 with 129,105 child-parent pairs of participants. Overall, parental occupational exposure to pesticides and solvents as well as ADHD and ASD were investigated more frequently for the possible underlying association(s). The period of exposure varied from “prior to pregnancy” to “after birth,” as detailed in Table 1. Of 23 included studies, 13 studies reported maternal exposure, one study provided data on paternal exposure, and the other nine studies provided data on parental exposure (maternal/paternal/both). In most studies (N = 18), self-reported information on parental occupational exposure was obtained. The information on the parental occupational exposure have been assessed using different approaches in the previous studies. While studies [12•, 24, 27, 38, 43, 45, 46, 55] applied a combination of self-report information with quantitative estimation by industrial hygienists, or previously developed occupational classification (i.e., asthma-specific job-exposure matrix (JEM) [56••, 57], Standard Occupational Classification (SOC) [58, 59], and North American Industry Classification System (NAICS) [60]), the other studies [25, 39, 41•, 44, 47, 54] typically had the parent(s) self-report exposure using a list of exposures that the study researchers identified in the scientific literature as potentially associated with NDDs. Air monitoring [49], dust analysis [38], blood tests [42, 52, 53], and urine analysis [25, 26, 42, 48, 50] were measured in 1, 1, 3, and 5 studies, respectively. There were two different approaches among included studies further to exposure assessment. Seven studies collected information on multiple agents, while 16 studies focused on a specific exposure. Pesticides and solvents were the exposure of interest in these studies.
We show an overview of the number of studies and the final result of studies in Fig. S1. This figure shows “which potential associations were less considered in previous studies?” Also, susceptible associations, based on the result of our meta-analysis, have been marked differently. The size of the circles shows the number of studies assessing the potential associations. Circles in blue show the significant associations and circles in magenta show the non-significant associations.
Risk of Bias and Heterogeneity
Tables S2, S3 and S4 show the results of the quality assessment of the primary studies. All studies, except Anderson et al., Grandjean et al., and Till et al., were assessed as high quality using the JBI tools.
Parental Occupational Exposure and NDD Associations
For ADHD, we found 11 studies [25, 26, 38, 39, 42,43,44, 47,48,49, 55] that focused on the association between parental occupational exposure and ADHD in children. The main agent exposures that have been previously investigated for possible association with child NDDs were as follows: pesticides, solvents, anesthetic gases, and metals. We excluded the studies of Anderson et al. [55] and Till et al. [47] for quantitative analysis. The number of studies was not enough for meta-analysis regarding anesthetic gases and metals.
In the meta-analysis for parental occupational exposure to pesticides and ADHD, the random effects model for three studies [26, 38, 42] showed that parental occupational exposure to pesticides significantly increased the risk of ADHD in children (OR = 1.31; 95% CI: 1.14, 1.52; I2 = 0%; P = 0.850) (Fig. 2). The funnel plot was symmetric and the Egger’s test (P = 0.706) did not document any obvious publication bias (Fig. 6).
The results of subgroup analysis based on exposure time window (prenatal and postnatal exposure) showed that prenatal exposure to pesticides increased the risk of ADHD in children (OR = 1.38; 95% CI: 1.16, 1.63; I2 = 0%; P = 0.917). However, the increased risk was not significant for postnatal exposure (OR = 1.18; 95% CI: 0.90, 1.54; I2 = 0%; P = 0.636). In the sensitivity analysis, the pooled OR did not change noticeably after excluding the paternal exposure in the study of Harari et al. (OR = 1.31; 95% CI: 1.13, 1.51; I2 = 0%, P = 0.779). The pooled OR and heterogeneity indices did not change after excluding other studies. The meta-regression showed that sample size and year of publication, the average age of the child, and the percentage of boys in the study were not significantly associated with the heterogeneity between studies.
Results of the meta-analysis using the random effects model on four studies [25, 39, 43, 44] indicated that parental occupational exposure to solvents was associated with a statistically significant increase in the risk of ADHD (OR = 1.65; 95% CI: 1.38, 1.97; I2 = 54.3%, P = 0.067) (Fig. 2). The funnel plot was symmetric and the P-value for Egger’s test was 0.740, indicating no obvious publication bias. While in the sensitivity analysis, the pooled effect size (OR) was not influenced after one-by-one excluding studies, the heterogeneity indices were changed. After excluding the studies of Hoovield et al. (I2 = 65.7%, P = 0.033), Pele et al. (I2 = 64.1%, P = 0.039), and Costet et al. (I2 = 61.8%, P = 0.049), the heterogeneity indices were increased. Based on the meta-regression, none of the variables of sample size, year of publication, the average age of the child, and percentage of boys in the study was significantly associated with the heterogeneity between studies.
The study of Ratzon et al. [49] was the only retrieved article that investigated the association between parental occupational exposure to anesthetic gases and ADHD. This study indicated that children born of women exposed occupationally to waste anesthetic gases during pregnancy might have a higher risk of inattention and/or hyperactivity at school age (β = 0.28; P = 0.030).
There was one study dealing with parental occupational exposure to metals and ADHD. Rudriguez et al. [48] showed that postnatal arsenic exposure might be associated with impaired selective and focused attention (β for impulsivity = 0.6; 95%CI: 0.1, 1.1; β for inattention = 0.5; 95% CI: 0.03, 1.00). Moreover, a significant dose–response relationship was observed between the urine arsenic level and two measures related to inattention and impulsivity.
For ASD, we retrieved seven studies [12•, 24, 27, 40, 41•, 45, 46] dealing with parental occupational exposure and ASD in children. The main agent exposures that have been reported in previous studies were as follows: pesticides, solvents, anesthetic gases, metals, fuels/exhausts, asthmagenes/chemicals, automobile/mechanic fluids, plastic/polymers, radiations, asphalt, pharmaceuticals, temperature, and teratogens. However, the number of retrieved studies was only sufficient for meta-analysis on exposures to solvents, metals, and asthmagenes/chemicals.
Results of the meta-analysis using the random effects model on six studies denoted that parental occupational exposure to solvents was not associated with risk of ASD (OR = 1.22; 95% CI: 0.97, 1.53; I2 = 79.2%, P < 0.001) (Fig. 3). The funnel plot was symmetric and the P-value for Egger’s test was 0.199, indicating no obvious publication bias (Fig. 6). The results of subgroup analysis based on parental exposure (maternal or paternal) showed that maternal (OR: 1.23; 95% CI: 0.85, 1.78; I2 = 72.7%; P = 0.012) and paternal (OR = 1.07; 95% CI: 0.73, 1.57; I2 = 74.9%; P = 0.019) exposure to solvents was not associated with the risk of ASD in children. The results of sensitivity analysis showed that the pooled effect size (OR) and heterogeneity were not influenced after one-by-one excluding studies. Results of meta-regression analysis showed that only year of publication (β (standard error): − 0.10 (0.03); P = 0.022) was significantly associated with the effect of parental occupational exposure to solvents on the risk of ASD (P < 0.050).
We retrieved four studies investigating the underlying association between prenatal occupational exposure to asthmagenes-chemical and ASD. As demonstrated in Fig. 3, prenatal occupational exposure to asthmagenes-chemical was not significantly associated with the risk of ASD in children (OR = 1.06; 95% CI: 0.95, 1.18). The heterogeneity was significant (I2 = 83.5%, P < 0.001), and no evidence of publication bias was detected based on the funnel plot and Egger’s test (P = 0.136) (Fig. 6). The results of subgroup analysis based on parental exposure (maternal or paternal) showed that the increased risk of ASD in children due to maternal exposure to asthmagenes-chemical (OR = 1.38; 95% CI: 0.96, 1.98; I2 = 87.8%; P < 0.001) was not significant. Moreover, paternal exposure to asthmagenes-chemical was not significantly associated with risk of ASD in children (OR = 1.00; 95% CI: 0.97, 1.03; I2 = 0%, P = 0.956). In the sensitivity analysis, the pooled OR changed significantly only after excluding the study of Singer et al. (OR = 1.50; 95% CI: 1.08, 2.09) with non-significant heterogeneity I2 = 45.7%, P = 0.137. The meta-regression showed that sample size and year of publication, the average age of the child, and the percentage of boys were not significantly associated with the heterogeneity between studies.
Results of the meta-analysis using the random effects model on four studies indicated that parental occupational exposure to metals significantly increased the risk of ASD in children (OR = 1.14; 95% CI: 1.05, 1.24; I2 = 0%, P = 0.689) (Fig. 3). No evidence of publication bias was detected based on the funnel plot and Egger’s test (P = 0.80) (Fig. 6). The results of subgroup analysis demonstrated that none of maternal (OR = 1.20; 95% CI: 0.96, 1.50; I2 = 0%; P = 0.832) and paternal (OR = 1.03; 95% CI: 0.76, 1.40; I2 = 57.6%; P = 0.125) exposure to metals was associated with the risk of ASD. In the sensitivity analysis, after excluding studies one by one, the pooled OR and heterogeneity indices did not change significantly. Based on the meta-regression, heterogeneity was not significantly associated with the following variables: sample size, year of publication, the average age of the child, and percentage of boys. In two studies, Mccanlies et al. [12•, 24] indicated that parental occupational exposure to pesticides may not be associated with higher rates of ASD in their children (OR = 1.10; 95% CI: 0.20, 6.50) for industrial hygienist (IH)-reported exposures and (OR = 2.00; 95% CI: 0.60, 6.30) for self-reported exposures in one of these studies [12•] and (OR = 1.16; 95% CI: 0.37, 1.65) for moderate IH-reported exposure and (OR = 0.72; 95% CI: 0.20, 2.56) for high IH-reported exposure in the other study [24]). Furthermore, because of the rarity of exposure to pesticides in their study, Windham et al. [46] could not estimate this relevant measure of association.
In the study of Windham et al. [46], the self-reported maternal occupational exposure to fuels/exhausts was significantly higher in the ASD group than in controls (OR: 12.00; 95% CI: 1.40, 104.60) for exhaust IH-reported exposure. Although parental occupational exposure to fuels/exhaust was measured in the study of Mccanlies et al. [12•], sparse data were reported. While Windham et al. [46] and Mccanlies et al. [12•, 24] collected information on parental occupational exposure to automobile/mechanic fluids, the number of reported exposed children was not enough for further analysis.
Mccanlies et al. [12•, 24] collected data on parental occupational exposure to plastic/polymers; however, the number of exposed participants was not enough to be analyzed.
Based on Mccanlies et al. studies [12•, 24], no significant association existed between parental occupational exposure to radiations and ASD (OR = 0.60; 95% CI: 0.20, 1.90 in one of these studies [12•] and OR: 0.79; 95% CI: 0.28, 2.22 in the other one [24]).
The study of Mccanlies et al. demonstrated that parents of children with ASD were more likely to report exposure to asphalt compared to parents of unaffected children (OR = 7.00; 95% CI: 1.50, 32.40 for self-reported exposure) [12•]. However, it was not possible to estimate the relevant OR for the association between parental occupational exposure to asphalt and ASD in the other study of these researchers [24]. Mccanlies et al. [24] found no significant association between parental occupational exposure to pharmaceuticals and ASD. Again, due to sparse data, the OR was not estimable in the other study of these researchers [12•].
Mccanlies et al. [12•] study was the only study that investigated the association between parental occupational exposure to temperature (cold/heat) and ASD in children. However, the number of reported exposed participants was low, and the corresponding OR could not be estimated.
We found only one study dealing with parental occupational exposure to teratogens and ASD in children. The results were not conclusive (OR = 0.66; 95% CI: 0.36, 1.25 for parental exposure, vs. OR = 1.16; 95% CI: 0.47, 2.82 for maternal exposure), but it showed that occupational exposure to teratogens was more likely reported in parents of children with ASD compared to unaffected children [40].
For motor skills disorder, we retrieved 10 studies [26, 38, 42, 44, 47, 49, 50, 52, 53, 55] that investigated the possible association between different types of parental occupational exposure and motor development outcomes. The main agent exposures that were reported in previous studies were as follows: pesticides, solvents, and anesthetic gases. Considering the number of included papers, meta-analysis was only done on pesticide exposure. The studies of Anderson et al. [55] and Grandjean et al. [50] were excluded.
By employing a random effects model for combining the results of five included studies [26, 38, 42, 52, 53], we did not detect a statistically significant association between parental occupational exposure to pesticides and the motor developmental outcomes (β = − 0.13; 95% CI: − 0.64, 0.39; I2 = 67.3%; P = 0.002) (Fig. 4). Considering the significant observed heterogeneity, in the subgroup analysis, we found that with increasing prenatal exposure to pesticides, the performance of motor functions declined significantly (β = − 0.63; 95% CI: − 1.08, − 0.17; I2 = 0%; P = 0.732); there was no significant heterogeneity. However, the decreased performance for the postnatal exposure was not significant (β = − 0.36; 95% CI − 0.33, 1.05; I2 = 73.1%; P = 0.011). An almost symmetrical funnel plot along with Egger’s test (P = 0.090) indicated no obvious publication bias (Fig. 6). Results of the sensitivity analysis showed that after excluding the paternal exposure of the study of Harari et al. (2010) (β = − 0.09; 95% CI: − 0.61, 0.42; I2 = 69.8%; P = 0.002) as well as the other studies, the pooled β did not change significantly. Moreover, the pooled effect size (OR) was not influenced after excluding studies one by one, but the heterogeneity indices were changed. None of the included variables to the meta-regression model, i.e., sample size, year of publication, the average age of the child, and the percentage of boys in the study was significantly associated with the heterogeneity between studies. Laslo-baker et al. [44] and Till et al. [47] investigated the association between parental occupational exposure to solvents and motor development in children. While a lower composite score on graphomotor ability was indicated in children of exposed parents, the fine motor ability was not significantly different between exposed and unexposed groups in the Till et al. survey [47]. Moreover, in the Laslo-baker et al. study [44], the results of the pegboard test were significantly different between parentally exposed and unexposed children. In our review, the study of Ratzon et al. [49] was the only paper that reported the association between parental occupational exposure to anesthetic gases and motor skills disorder. The mean score of gross motor ability was significantly lower in the children born of mothers exposed to waste anesthetic gases when compared to the unexposed group.
For learning disability, we found six studies [18, 26, 38, 42, 49, 55] dealing with parental occupational exposure and cognition/memory in children. The main agent exposures that have been reported in previous studies were pesticides and solvents. Considering the limited number of included studies, the meta-analysis was only done for pesticide exposure. Because of low methodological quality reasons, the Anderson et al. [55] study was excluded from the meta-analysis.
Pooling the results of three studies [26, 38, 42] on the association between parental occupational exposure to pesticides and memory functions revealed no statistically significant association with increased exposure to pesticides and the performance of memory functions (β = − 0.16; 95% CI: − 0.45, 0.13); I2 = 80.6%; P < 0.001) (Fig. 5). However, the results of subgroup analysis based on prenatal and postnatal exposure showed that increased prenatal exposure to pesticides can lead to significantly limited memory functions (β = − 0.45; 95% CI: − 0.88, − 0.01); I2 = 85.2%; P = 0.001), with significant heterogeneity. The association for postnatal exposure was not significant (β = 0.10; 95% CI: − 0.34, 0.54; I2 = 80.6%; P < 0.001) and had significant heterogeneity. The funnel plot was nearly symmetrical. The P-value for Egger’s test was 0.716, indicating no obvious publication bias (Fig. 6). Results of sensitivity analysis showed that the pooled effect size (β) changed significantly after excluding the Eskenazi study (postnatal) (β = − 0.26; 95% CI: − 0.48, − 0.05); I2 = 63.8%; P = 0.026). However, the pooled effect size (β) and heterogeneity did not change significantly after excluding other studies one by one. Based on the meta-regression, none of the variables of sample size, year of publication, the average age of the child, and percentage of boys in the study was significantly associated with the heterogeneity between studies.
The study of Laslo-baker et al. [44] was the only one dealing with parental occupational exposure to solvents and memory/cognition. It indicated that exposed children with in utero exposure to organic solvents had a significantly reduced ability in recalling sentences.
For communication disabilities, four studies [44, 47, 53, 55] focused on the association between parental occupational exposure and language/communication/speech in children. The main agent exposures that have been reported in previous studies were pesticides and solvents. However, the limited number of retrieved papers in each exposure category did not allow us to run a corresponding meta-analysis. The study of Anderson et al. [55] showed an adverse effect of parental occupational exposure to pesticides on language function in girls, but not in boys. Furthermore, Wang et al. [53] did not report a significant association of parental occupational exposure to pesticides with disorders in the language domain. Laslo-baker et al. [44] and Till et al. [47] investigated the association between parental occupational exposure to solvents and language in children. Till et al. [47] revealed a significant association between this exposure and some specific subtle measures of language development. However, Laslo-baker et al. [44] did not find any statistically significant association. For intellectual disability, six studies [42, 44, 49,50,51, 54] investigated the association between parental occupational exposures and child intellectual disability/IQ/mental retardation. Pesticides, solvents, anesthetic gases, metals, and asthmagenes/chemicals were the main agent exposures that have been reported in previous studies. However, the number of studies in each category was limited for conducting a meta-analysis. No differences were seen on the digit span forward test, a subtest for IQ estimation, in the studies of Grandjean et al. [50] and Harari et al. [42]. Laslo-baker et al. [44] and Decoufle et al. [51] found no significant difference between exposed and unexposed groups regarding intellectual disability. A significant inverse correlation existed between the level of occupational exposure of the mothers to anesthetic gases and the score of IQ performance in the study of Ratzon et al. (r: − 0.39; P: 0.008) [49]. Vahasarga et al. [54] did not find an increased risk for intellectual disability among sons of dental nurses, dentists, and assistant nurses. Decoufle et al. [51] indicated that maternal occupational exposure to natural gas, gasoline, or other fuel products was associated with elevated odds of having a child with severe mental retardation (OR = 4.00; 95% CI: 1.10, 19.30).
Discussion
The results of this meta-analysis demonstrated that parental occupational exposures to pesticides and solvents were significantly associated with an increased risk of ADHD in children. Our results showed a significant role for parental occupational exposures to metals on child ASD development. Furthermore, prenatal occupational exposure to pesticides was significantly associated with poor motor development and cognition.
Undergoing rapid and continuous growth periods make children more susceptible to the harmful effects of toxicant exposures [61]. Any disruption in the development of the central nervous system process from prenatal life to early childhood might lead to major and long-lasting structural and functional consequences [62]. The developing brain of the fetus is extremely vulnerable to environmental agent exposures as the placenta does not adequately protect against toxicants. Also, the blood–brain barrier is not formed until 6 months after birth; thus, prenatal exposure can cause the greatest damage to the brain [63]. Heyer et al. reported that many environmental toxicants have distinct sensitive time windows during which exposure may disrupt critical developmental events and increase the risk of developing NDDs. The majority of these time windows occur during prenatal periods rather than postnatal periods [64].
To the best of our knowledge, this is the first systematic review and meta-analysis investigating the pooled association between parental exposure to solvent and risk of ADHD in children. Our findings on the association between parental occupational exposure to pesticides and increased risk of ADHD and motor development disorder are consistent with the González-Alzaga et al. review. They systematically reviewed a wide variety of studies to estimate the effect of organophosphate pesticide exposure on child neurodevelopment and behavior. Overall, they suggested that exposure to organophosphate pesticides during pregnancy may affect the child’s mental and motor development and behavior during early childhood. Although the effects associated with postnatal exposure were less consistent, they showed that it may increase the risk of attention problems and may affect the child’s cognitive and motor function [65].
We showed an association between parental occupational exposure to asthmagenes and increased risk of ASD in children. To the best of our knowledge, the underlying assessment approach, i.e., using asthma-JEM, has not been considered in other previous studies in the NDD field. However, there is some evidence that might confirm this result. As traffic-related air pollutions are associated with new-onset asthma assessed at age 7 [66], if we assume traffic air pollution as an asthmagenes, we could refer to some similar findings of previous systematic reviews. Flores-Pajot et al. confirmed that exposure to ambient air pollution might be associated with an increased risk of ASD [67]. Moreover, another systematic review and meta-analysis reported some evidence in favor of the association between air pollutants, especially prenatal exposure to particulate matter, and ASD [68].
We also found an increased risk of ASD in children with a higher level of parental exposure to metals. This finding is consistent with the results of a meta-analysis published in 2019 showed that a higher body burden of exposure to inorganic arsenic may be associated with ASD in children. However, the evidence did not support any consistent relationship between body burden of exposure to lead and ASD [69].
We could only estimate those associations that have been reported in the previous studies. We conducted a review on the previous systematic reviews and meta-analysis on the association between environmental exposures and NDDs. There are still possible associations that have not been explored specifically in previous parental occupational exposure studies. For example, findings of a meta-analysis published in 2013, evaluating the association of arsenic, cadmium, and manganese exposure with neurodevelopment and behavioral disorders in children showed that arsenic and manganese exposures are associated with IQ in children, but there was little information on similar effect of cadmium exposure [70]. Moreover, the meta-analysis of Rodríguez-Barranco et al. provided some evidence supporting the role of manganese in ADHD [71]. Thus, more investigation is needed to explore these susceptible associations between parental occupational exposure to metals and NDDs. On the other hand, Lam et al. found an association of polybrominated diphenyl ethers (PBDEs) with decrements on IQ (3.7-point reduction in IQ per tenfold increase in PBDE exposure) in their meta-analysis (65). PBDEs are used in paints, plastics, foam furniture padding, textiles, televisions, building materials, airplanes, and automobiles [72]. Overall, inconsistent findings and inconclusive data available about some susceptible associations reinforce the need for conducting separate studies in each category.
Although some previous systematic review, meta-analysis, or narrative reviews exist on the association between environmental toxicants exposure, not occupational exposures, and NDDs in children, to the best of our knowledge, this is the first systematic review and meta-analysis that specifically investigated the association between parental occupational exposure and NDDs in children. While we aimed to systematically review all of the potential associations between parental occupational exposures and NDDs, the limited number of previously published studies would not allow for recording some susceptible underlying associations in the meta-analysis. The retrieved studies were different in the term of employed methodology, the recruited study populations, the age range of recruited children (under 18 years), the method of outcome ascertainment and/or the utilized neuropsychological tests, the method of exposure measurement (biomarkers vs. questionnaires), the exposure time window (prenatal vs. postnatal), and the parent (s) exposed (mother, father, or both). The findings of cohort, case–control, and cross-sectional studies were combined if there was low or no evidence of between-study heterogeneity.
Exposure misclassification is a major concern in parental occupational exposure studies. The great majority of currently published studies have not used a common standardized method to collect data on occupational exposures. Despite using some previously developed occupational classifications and some statistical models for quantifying the exposures, the combination of self-reported questionnaires and quantitative estimations still is accompanied by bias and is not the gold standard [73]. Recall bias in self-reported parental occupational exposure may lead to an underestimation or overestimation of the underlying associations [74]. While in most studies, parental occupational exposures were categorized based on occupational groups, i.e., farm/agriculture-related jobs, dentists, etc., these jobs may entail exposure to other agents including biological, physical, and chemical factors. On the other hand, parents who were exposed to occupational exposure may also be exposed to cumulative environmental exposures leading to some degree of residual confounding. Moreover, the lack of data on the potential confounders including the history of smoking, genetic susceptibility, dietary habits, and exposure to other chemicals was another limitation of the included studies. The different intensity levels of exposures, changes in routine work practices or levels of exposure over time, and the underlying job differences in the levels of exposure should also be considered in this issue. The use of unspecific terms including “pesticides,” “solvents,” and “metals” as exposure categories may impose some degree of exposure misclassification. These terms belong to various classes of chemicals with different in vivo effects. However, because of the limited number of retrieved studies, we combined all related agents in one category. The aggregation of all types of these agents would lead to the dilution of the true effects of one or more individual types and can result in additional bias. Information on exposure time windows might provide insight into the underlying outcome mechanism [75]. However, in the present review, almost all included studies investigated prenatal exposure and only a few studies were focusing on postnatal exposures.
Finally, the inconsistent use of the neurodevelopmental outcome/disorder terms, as well as the different utilized questionnaires or neuropsychological tests, might impose outcome misclassification. For example, we consider all ADHD subtypes (i.e., AD/HD or combined type of ADHD) as one disorder, while the terms were not consistent through different studies.
Conclusion
We found a significant association between parental occupational exposure to pesticides or solvents and an increased risk of ADHD in their children. Moreover, parental occupational exposures to metals was significantly associated with ASD. Furthermore, we showed that prenatal occupational exposure to pesticides was significantly associated with disorders in motor development and cognition. Future preventive programs should be implemented in this regard.
Further studies need to identify the level of agents’ exposures that can be significantly associated with NDDs. Moreover, utilizing standardized outcome and exposure scales is recommended to incorporate paternal, maternal, and parental as well as both prenatal and postnatal exposure in future studies.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Bitta M, Kariuki SM, Abubakar A, Newton CRJC. Burden of neurodevelopmental disorders in low and middle-income countries: A systematic review and meta-analysis. Wellcome Open Res. 2017;2:121. https://doi.org/10.12688/wellcomeopenres.13540.3. This review indicated that the burden of NDD in LAMIC is considerable. Perinatal complications were the commonest risk factor for NDD based on this study.
Erskine HE, et al. The global burden of conduct disorder and attention-deficit/hyperactivity disorder in 2010. J Child Psychol Psychiatry. 2014;55(4):328–36.
Edition F. Diagnostic and statistical manual of mental disorders. Am Psychiatric Assoc. 2013;21:591–643.
Tordjman S, et al. Gene× environment interactions in autism spectrum disorders: role of epigenetic mechanisms. Front Psych. 2014;5:53.
Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383(9920):896–910.
Chaste P, Leboyer M. Autism risk factors: genes, environment, and gene-environment interactions. Dialogues Clin Neurosci. 2012;14(3):281.
Pietropaolo S, Crusio WE, Feldon J. Gene-environment interactions in eurodevelopmental disorders. Neural Plast. 2017;2017:9272804. https://doi.org/10.1155/2017/9272804.
Thapar A, et al. Gene–environment interplay in attention-deficit hyperactivity disorder and the importance of a developmental perspective. Br J Psychiatry. 2007;190(1):1–3.
Palladino VS, et al. Genetic risk factors and gene–environment interactions in adult and childhood attention-deficit/hyperactivity disorder. Psychiatr Genet. 2019;29(3):63–78.
Sandin S, et al. The familial risk of autism. JAMA. 2014;311(17):1770–7.
Sandin S, et al. The heritability of autism spectrum disorder. JAMA. 2017;318(12):1182–4.
• McCanlies EC, et al. Parental occupational exposures and autism spectrum disorder. J Autism Dev Disord. 2012;42(11):2323–34. This study had a comprehensive and well-designed occupational exposure assessment (a combination of self-reported and specialist estimation) among included studies.
Roberts EM, et al. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect. 2007;115(10):1482–9.
Shelton JF, et al. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Perspect. 2014;122(10):1103–9.
Saravi SSS, Dehpour AR. Potential role of organochlorine pesticides in the pathogenesis of neurodevelopmental, neurodegenerative, and neurobehavioral disorders: a review. Life Sci. 2016;145:255–64.
Ornoy A, Weinstein-Fudim L, Ergaz Z. Prenatal factors associated with autism spectrum disorder (ASD). Reprod Toxicol. 2015;56:155–69.
Zeliger HI. Exposure to lipophilic chemicals as a cause of neurological impairments, neurodevelopmental disorders and neurodegenerative diseases. Interdiscip Toxicol. 2013;6(3):103–10.
Laslo-Baker D. Child neurodevelopment following in utero exposure to organic solvents. University of Toronto (Canada); 2012.
Bulman C. Defining neurodevelopmental domains in children with prenatal solvent exposure. Paediatrics & Child Health 2012;17(suppl_A):20A-20A. https://doi.org/10.1093/pch/17.suppl_A.20A.
Ijomone OM et al. Environmental influence on neurodevelopmental disorders; potential association of heavy metal exposure and autism. J Trace Elem Med Biol. 2020;62:126638. https://doi.org/10.1016/j.jtemb.2020.126638.
Julvez J, Grandjean P. Neurodevelopmental toxicity risks due to occupational exposure to industrial chemicals during pregnancy. Ind Health. 2009;47(5):459–68.
Fluegge K. Does environmental exposure to the greenhouse gas, N2O, contribute to etiological factors in neurodevelopmental disorders? A mini-review of the evidence. Environ Toxicol Pharmacol. 2016;47:6–18.
Shi L, Chia SE. A review of studies on maternal occupational exposures and birth defects, and the limitations associated with these studies. Occup Med. 2001;51(4):230–44.
McCanlies EC, et al. The CHARGE study: an assessment of parental occupational exposures and autism spectrum disorder. Occup Environ Med. 2019;76(9):644–51.
Pelé F, et al. Occupational solvent exposure during pregnancy and child behaviour at age 2. Occup Environ Med. 2013;70(2):114–9.
Eskenazi B, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115(5):792–8.
Singer AB, et al. Maternal exposure to occupational asthmagens during pregnancy and autism spectrum disorder in the study to explore early development. J Autism Dev Disord. 2016;46(11):3458–68.
Stroup DF, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–12.
Brooke BS, Schwartz TA, Pawlik TM. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021;156(8):787–8. https://doi.org/10.1001/jamasurg.2021.0522.
Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34.
Spitzer RL, Md KK, Williams JB. Diagnostic and statistical manual of mental disorders. In American psychiatric association 1980.
World Health Organization. International statistical classification of diseases and related health problems, 10th revision, Fifth edition, 2016. World Health Organization 2015. https://apps.who.int/iris/handle/10665/246208. Accessed 1 Sep 2021.
Porritt K, Gomersall J, Lockwood C. JBI’s systematic reviews: study selection and critical appraisal. Am J Nurs. 2014;114(6):47–52.
Institute JB. Critical appraisal checklist for cohort studies. 2017. Acedido em http://joannabriggs.org/research/critical-appraisal-tools.html. Accessed 1 Nov 2021.
Institute JB. Checklist for case control studies. Joanna Briggs Institute Critical Appraisal tools. 2016: p. 1–6.
Institute JB. Critical appraisal checklist for analytical cross sectional studies. Adelaide: The Joanna Briggs Institute; 2017.
Moola S et al. Chapter 7: Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; Joanna Briggs Institute Reviewer’s Manual. The Joanna Briggs Institute, 2017. 5. https://doi.org/10.46658/JBIMES-20-08.
Butler-Dawson J, et al. Organophosphorus pesticide exposure and neurobehavioral performance in Latino children living in an orchard community. Neurotoxicology. 2016;53:165–72.
Costet N, et al. Occupational exposure to organic solvents during pregnancy and childhood behavior: findings from the PELAGIE birth cohort (France, 2002–2013). Environ Health. 2018;17(1):1–9.
Dickerson AS, et al. Role of parental occupation in autism spectrum disorder diagnosis and severity. Res Autism Spectr Disord. 2014;8(9):997–1007.
• Grossi E, Migliore L, Muratori F. Pregnancy risk factors related to autism: an Italian case–control study in mothers of children with autism spectrum disorders (ASD), their siblings and of typically developing children. J Dev Orig Health Dis. 2018;9(4):442–9. This study reported the strongest power among reported associations in parental occupational exposure and neurodevelopmental disorders in children. This study has focused on solvent exposure and autism spectrum disorder.
Harari R, et al. Neurobehavioral deficits and increased blood pressure in school-age children prenatally exposed to pesticides. Environ Health Perspect. 2010;118(6):890–6.
Hooiveld M, et al. Adverse reproductive outcomes among male painters with occupational exposure to organic solvents. Occup Environ Med. 2006;63(8):538–44.
Laslo-Baker D, et al. Child neurodevelopmental outcome and maternal occupational exposure to solvents. Arch Pediatr Adolesc Med. 2004;158(10):956–61.
Singer AB, et al. Parental exposures to occupational asthmagens and risk of autism spectrum disorder in a Danish population-based case-control study. Environ Health. 2017;16(1):31.
Windham GC, et al. Use of birth certificates to examine maternal occupational exposures and autism spectrum disorders in offspring. Autism Res. 2013;6(1):57–63.
Till C, Koren G, Rovet JF. Prenatal exposure to organic solvents and child neurobehavioral performance. Neurotoxicol Teratol. 2001;23(3):235–45.
Rodríguez-Barranco M, et al. Postnatal arsenic exposure and attention impairment in school children. Cortex. 2016;74:370–82.
Ratzon NZ, et al. Developmental evaluation of children born to mothers occupationally exposed to waste anesthetic gases. Birth Defects Res A. 2004;70(7):476–82.
Grandjean P, et al. Pesticide exposure and stunting as independent predictors of neurobehavioral deficits in Ecuadorian school children. Pediatrics. 2006;117(3):e546–56.
Decouflé P, et al. Mental retardation in ten-year-old children in relation to their mothers’ employment during pregnancy. Am J Ind Med. 1993;24(5):567–86.
Handal AJ, et al. Occupational exposure to pesticides during pregnancy and neurobehavioral development of infants and toddlers. Epidemiology. 2008;19(6):851–9. https://doi.org/10.1097/EDE.0b013e318187cc5d.
Wang Y, et al. Prenatal and postnatal exposure to organophosphate pesticides and childhood neurodevelopment in Shandong, China. Environ Int. 2017;108:119–26.
Vähäsarja N, et al. Neurological disease or intellectual disability among sons of female Swedish dental personnel. J Perinat Med. 2016;44(4):453–60.
Andersen HR, et al. Occupational pesticide exposure in early pregnancy associated with sex-specific neurobehavioral deficits in the children at school age. Neurotoxicol Teratol. 2015;47:1–9.
•• Kennedy SM, et al. Development of an asthma specific job exposure matrix and its application in the epidemiological study of genetics and environment in asthma (EGEA). Occup Environ Med. 2000;57(9):635–41. This asthma JEM could be a useful tool in general population studies of asthma for estimating 22 exposure groups.
Le Moual N, et al. Update of an occupational asthma-specific job exposure matrix to assess exposure to 30 specific agents. Occup Environ Med. 2018;75(7):507–14.
Emmel A, Cosca T. The 2010 Standard Occupational Classification (SOC): a classification system gets an update. OCCUP Outlook Q. 2010;54(2):13–9.
Elias P, McKnight A, Kinshott G. SOC 2000: redefining skill: revision of the Standard Occupational Classification. 1999.
Murphy JB. Introducing the North American industry classification system. Mon Lab Rev. 1998;121:43.
Hassanien MA, El Shahawy AM. Environmental heavy metals and mental disorders of children in developing countries. In: Environmental heavy metal pollution and effects on child mental development. Springer, 2011. p. 1–25. https://doi.org/10.1007/978-94-007-0253-0_1.
Rodier PM. Developing brain as a target of toxicity. Environ Health Perspect. 1995;103(suppl 6):73–6.
Landrigan PJ. What causes autism? Exploring the environmental contribution. Curr Opin Pediatr. 2010;22(2):219–25.
Heyer DB, Meredith RMJN. Environmental toxicology: sensitive periods of development and neurodevelopmental disorders. Neurotoxicology. 2017;58:23–41.
González-Alzaga B, et al. A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicol Lett. 2014;230(2):104–21.
Carlsten C, et al. Traffic-related air pollution and incident asthma in a high-risk birth cohort. Occup Environ Med. 2011;68(4):291–5.
Flores-Pajot M-C, et al. Childhood autism spectrum disorders and exposure to nitrogen dioxide, and particulate matter air pollution: a review and meta-analysis. Environ Res. 2016;151:763–76.
Lam J, et al. A systematic review and meta-analysis of multiple airborne pollutants and autism spectrum disorder. PLoS One. 2016;11(9):e0161851.
Wang M, et al. Exposure to inorganic arsenic and lead and autism spectrum disorder in children: a systematic review and meta-analysis. Chem Res Toxicol. 2019;32(10):1904–19.
Rodríguez-Barranco M, et al. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ. 2013;454:562–77.
Rodríguez-Barranco M, et al. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ. 2013;454:562–77.
Sjödin A, Patterson DG Jr, Bergman Å. A review on human exposure to brominated flame retardants—particularly polybrominated diphenyl ethers. Environ Int. 2003;29(6):829–39.
Olshan AF, van Wijngaarden E. Paternal occupation and childhood cancer. Adv Exp Med Biol. 2003;518:147–61.
Infante-Rivard C, Jacques L. Empirical study of parental recall bias. Am J Epedimiol. 2000;152(5):480–6.
Dich J, et al. Pesticides and cancer. Cancer Causes Control. 1997;8(3):420–43.
Funding
This project has been supported by Isfahan University of Medical Sciences (Project Number: 299255, Ethics approval code: IR.MUI.MED.REC.1399.1096).
Author information
Authors and Affiliations
Contributions
Conception and study design: RK, IA, MB. Search strategy: MB and PG. Study selection: MB and PG. Data extraction: MB, PG. Data management, synthesis, and analysis: MB, IA, MKH. Data interpretation: RK, IA, MKH. Manuscript drafting: MB and MKH. Manuscript edition: IA, RK. Manuscript revision and review: IA, RK. Accepted the final version of the manuscript: MB, MKH, PG, IA, RK.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Occupational Health
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bemanalizadeh, M., Khoshhali, M., Goli, P. et al. Parental Occupational Exposure and Neurodevelopmental Disorders in Offspring: a Systematic Review and Meta-analysis. Curr Envir Health Rpt 9, 406–422 (2022). https://doi.org/10.1007/s40572-022-00356-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40572-022-00356-6