Abstract
Purpose of review
To summarize the recent evidence on the various wheezing patterns in early life and provide a case-based review with insights into clinical application of individualized therapy in preschool children with recurrent wheezing.
Recent findings
Preschool wheezing is often characterized predominantly by the risk domain with exacerbations and relatively limited impairment. In children with intermittent disease and a positive Modified Asthma Predictive Index, intermittent therapy with inhaled corticosteroids (ICSs) should be considered as an initial therapy to prevent exacerbations. Early administration of azithromycin at the onset of lower respiratory tract infections (LRTIs) reduces the risks of progression to severe illnesses in children who have a history of recurrent severe LRTIs, and more information is needed regarding the risks of developing drug-resistant organisms. In preschool children with mild persistent asthma, allergic sensitization to aeroallergens and absolute eosinophil count can help identify children most likely to have a good response to daily ICS.
Summary
Recent clinical trials in preschool children with severe episodic wheezing and persistent asthma have made a significant impact on the approach for the care of these children, particularly with evidence directing individualized approaches based on specific clinical features and biomarkers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheezing is a common respiratory symptom in infants and preschool children. Nearly half of children experience at least one episode of wheezing in the first 6 years of life [1]. Recurrent wheezing and asthma in preschool children represent heterogeneous conditions with diverse clinical and biological phenotypes [2•]. Preschool asthma is associated with significant morbidity, with these children experiencing the highest rates of asthma-related health care utilization including outpatient department visits, emergency department visits, and hospitalizations [3, 4].
Diagnosis of recurrent wheezing and asthma in preschool children
Wheezing is a nonspecific symptom shared by multiple respiratory tract conditions, including lower respiratory tract infections (LRTIs) and asthma. Most wheezing episodes in young children are triggered by viral infections, making it challenging to differentiate between isolated viral-triggered wheezing and virus-trigged asthma exacerbations in clinical practice. In the Tucson Children’s Respiratory Study (TCRS), approximately one-third of children had wheezing with acute LRIs in the first year of life [5]. This birth cohort study also identified three distinct wheezing patterns during childhood, including transient infant wheezers, nonatopic wheezers, and atopic wheezers [1]. Transient wheezers were children who wheezed during the first 3 years of life and did not wheeze after the age of 3 years, with the majority of children who wheezed during the first year of life falling into this category. Nonatopic wheezers were children who continued to wheeze beyond the third year of life, with LRI with respiratory syncytial virus (RSV) significantly increasing the risk persistent wheeze at age of 6 years. Atopic wheezers were characterized by sensitization to common aeroallergens, especially Alternaria, at the age of 6 years and could be divided into early atopic wheezers and late atopic wheezers (symptoms started after 3 years of age) [6].
To date, there remains no single marker or set of features that reliably and prospectively differentiates between children who will exhibit the transient wheeze pattern and those who continue to wheeze and have asthma in later life. There are several tools available that help in assigning subsequent asthma risk, but which are neither intended nor validated in directly assigning an asthma diagnosis. One example is the Asthma Predictive Index (API), a composite of several clinical factors identified from TCRS (Table 1). The negative predictive value for active asthma at age 6 years was 92% for the stringent index while the positive predictive value was only approximately 50% (sensitivity 27% and specificity 96%) [8]. The API has been modified by adding an evidence for allergic sensitization to an inhalant allergen as a major criterion, and allergic sensitization to a food (milk, egg, or peanut) replaced physician-diagnosed allergic rhinitis as a minor criterion [9]. The utility of modified API (mAPI) in predicting asthma in school-aged children was validated in a post hoc analysis in a high-risk birth cohort of children with a family history of allergy and/or asthma. The positive mAPI was superior to the original API for future asthma prediction, with the positive likelihood ratio of the mAPI for asthma diagnosis at ages 6, 8, and 11 years ranging from 4.9 to 55. The predictive ability increased if the prediction year and diagnosis year were closer, whereas a negative mAPI did not provide a clinically meaningful predictor of a decrease in future asthma probability [10].
The Pediatric Asthma Risk Score (PARS), developed from the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS) birth cohort, was constructed to predict asthma development in young children. The PARS scoring system consists of parental asthma, eczema before age 3 years, wheezing apart from colds, wheezing before age 3 years, African-American race, and skin prick testing positive to ≥ 2 aeroallergens and/or food allergens (Fig. 1). PARS requires the calculation of a score, and there is a web application available for users. The performance in predicting asthma at age 7 years of PARS is superior to the original API, with a sensitivity of 68% and specificity of 77%. However, there is no direct comparison to the predictive utility to the mAPI [11]. Other approaches for identifying children at high risk for subsequent asthma have been reviewed elsewhere [12].
Pediatric Asthma Risk Score (PARS) scoring sheet. Reproduced with permission from Biagini Myers et al. [11].
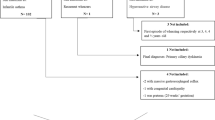
The substantial heterogeneity in early-life recurrent wheezing and asthma likely explains the inconsistent responses reported in therapeutic trials in this age group. Given our improved understanding of these wheezing phenotypes, supplemented by evidence from several clinical trials, it is now possible to begin to make clinical therapeutic decisions guided by disease phenotypes, in an effort to optimize clinical outcomes by selecting the most effective therapy for a given patient first. For phenotype-based management, one potential construct to consider divides preschool children into two groups: (1) persistent disease characterized by the presence of significant impairment and risks of exacerbation and (2) intermittent disease with exacerbations during acute LRTIs and limited to no day-to-day asthma symptom burden [13]. Here, we review the current treatment options of recurrent wheezing with LRTIs and persistent asthma in preschool-aged children, based on these 2 clinical phenotypes.
Phenotype-based approach for asthma and intermittent wheezing in the context of lower respiratory tract illnesses in preschool children. ICS, inhaled corticosteroids; LTRA, leukotriene receptor antagonist; AEC, absolute eosinophil count (cells/mcl); mAPI, Modified Asthma Predictive Index; LRTI, lower respiratory tract illness. The asterisk symbol indicates if the child failed to respond to intermittent azithromycin therapy, high-dose intermittent ICS can be considered.
Case 1
A 3-year-old girl presents with a history of frequent cough, wheezing, and shortness of breath for 2 years. Her symptoms are triggered by viral respiratory tract infections and weather change. She has made two emergency room visits over the past year and was prescribed albuterol and oral corticosteroids (OCS) on both visits. Outside of these periods of exacerbation, the mother notes wheezing and cough 2 days/week on average and nocturnal awakenings due to cough or wheezing requiring albuterol 2 nights/month. Albuterol provides consistent improvement in these symptoms. Her family history was significant for asthma in her father. Her physical exam was unremarkable on the visit. Her chest radiographs were normal and sweat chloride test was negative.
Questions: What is the next step of management? Which would be preferable—starting a daily ICS, a daily LRTA, or continuing with the use of albuterol as needed? Are there additional tests that may guide the treatment for this patient?
Literature review
Diagnosis of persistent asthma in preschool children
The diagnosis of asthma for preschool-aged children is based on symptom patterns, presence of risk factors, careful consideration of differential diagnoses, and therapeutic responses [14]. There are several disorders that present with wheezing in young children, and these include airway malacia, vascular ring, respiratory tract infections, bronchopulmonary dysplasia, cystic fibrosis, foreign body aspiration, aspiration syndromes, and pulmonary edema. A thorough history and physical examination are highly important, not only for the diagnosis and identification of the severity of asthma, but also for exclusion of other wheezing-related disorders. Chest radiographs should be considered in the first step of evaluation in children with recurrent wheezing to evaluate for vessel anomalies (right-sided aortic arch for certain types of vascular rings), radio-opaque foreign bodies, infiltrates, and masses. Reconsideration of the differential diagnosis is imperative, especially when patients exhibit inadequate response to asthma-related interventions.
Current asthma guidelines for preschool-aged children
Based on the stepwise approach for long-term management of asthma by Global Initiative for Asthma (GINA) 2019, daily low-dose ICS is the preferred initial treatment in children 5 years and younger if the symptom pattern suggests a diagnosis of asthma and respiratory symptoms are uncontrolled, while leukotriene receptor antagonists (LTRA) or intermittent ICSs are alternative therapies [15]. For the assessment of asthma control in young children, the guideline recommends to take 2 domains of asthma into consideration: (1) symptom control and (2) future risk for poor asthma outcomes. For the symptom control domain, asthma is considered well controlled if, over the past 4 weeks, the child had daytime asthma symptoms less than once a week without activity limitation, had no nighttime awakening or nighttime cough due to asthma, and reliever medication was needed no more than once a week. The risk domain includes risk factors for asthma exacerbation (one or more severe exacerbations in the past year was included in this element), fixed airflow limitation, and medication side effects [15]. Here, we review the recent literature on the treatment options for persistent asthma in preschool children.
Daily ICS and montelukast are both effective in improving asthma control in preschool children with persistent disease
Daily ICS is a well-established, effective therapy in improving asthma control in preschool-aged children. The ICSs that have been studied for daily use in preschool children are fluticasone propionate metered-dose inhaler (MDI) [16, 17], budesonide by nebulization [18, 19], and ciclesonide MDI [20]. The Prevention of Early Asthma in Kids (PEAK) trial demonstrated daily low-dose ICS (fluticasone propionate, 88 mcg, twice daily) for 2 years in 238 children aged 2–3 years with a positive mAPI resulted in a higher proportion of episode-free days, a lower rate of exacerbations (50/100 children-years in fluticasone group versus 89/100 children-years in placebo group), and a lower rate of supplementary use of controller medication, compared with placebo [16]. However, the beneficial effects ceased after the ICS was discontinued. In post hoc analysis, certain subgroups of children demonstrated greater responses to ICS, and these subgroups included Caucasians, boys, those with a history of ED visit or hospitalization for asthma within the past year, and aeroallergen sensitization, thereby demonstrating substantial heterogeneity in ICS response even within the mAPI-positive study population. A meta-analysis reviewing the efficacy of ICS in infants and preschoolers with recurrent wheezing asthma found children who received ICS had significantly fewer asthma exacerbations requiring OCS than those receiving placebo, with relative risk of 0.59 (95% CI 0.52–0.67, P = 0.001), and the treatment of 7 patients with ICS therapy prevented 1 patient from experiencing an asthma exacerbation requiring OCS compared with placebo [21].
Daily montelukast has been shown to improve respiratory symptoms and reduce asthma exacerbations in preschool children [22,23,24]. Montelukast is also effective in improving symptom scores as an add-on therapy for asthmatic children aged 4–11 years whose symptoms are not well controlled on ICS [25].
Limited head-to-head comparisons between daily ICS and LTRA therapy in preschool children have been reported, with an open-label comparison trial [26] and a recent meta-analysis [27•], both concluding that daily ICS is more effective than LTRA.
The majority of preschool children with persistent asthma have a differential response to controller therapies
Given the clinical challenges facing physicians to select the most appropriate controller medications for a given child with persistent asthma, combined with the heterogeneity of asthma with its various clinical phenotypes, it would be helpful to be able to identify certain subgroups among preschool children with asthma who derive greater benefit from a given therapy. The Individualized Therapy for Asthma in Toddlers (INFANT) study was a multicenter, randomized, double-blinded, double-dummy clinical trial of children aged 12–59 months old with persistent asthma. The definition of persistent asthma included daytime asthma symptoms more than 2 days per week, nighttime awakening from asthma at least once over the previous 4 weeks, or 4 or more wheezing episodes, each lasting 24 h or more, in the preceding 12 months. Approximately 75% of participants had a differential response to either daily ICS (fluticasone propionate, 88 μg twice daily), as needed ICS (fluticasone 88 μg given whenever albuterol was needed), and daily montelukast, based on composite outcomes incorporating both risks and impairment domains of asthma control [28•]. Daily low-dose ICS was found to be the therapy most likely to exhibit a preferential response, as reflected by asthma control days and exacerbations, and the likelihood of experiencing the best response increased in the presence of sensitization to aeroallergens and/or blood eosinophil counts of 300/μl or greater. No factor predicted a preferred response to LTRA or as needed ICS, although substantial numbers of children experienced their best responses when receiving one of these two treatments.
A recent latent class analysis, combining the findings of 5 major clinical trials of recurrent preschool wheezing, showed that the daily ICS treatment improved exacerbation rates only in children characterized by sensitization with indoor pet exposure, multiple sensitizations, and eczema, but not in the group with minimal sensitization or the group that had sensitization with tobacco smoke exposure [29].
Discussion on the approach to case 1
Our index case has a history of recurrent respiratory symptoms and response to bronchodilators. After the exclusion of other wheezing-related diseases based on clinical history, exam findings and normal radiographs, and sweat chloride testing, she was diagnosed with mild persistent asthma. Since she had only intermittent nasal symptoms concerning for viral respiratory tract infection, determination of allergic sensitization and/or peripheral blood eosinophilia could help with determining if she would likely achieve better responses with daily ICS over montelukast based on the evidence described above.
For the implementation of results from the INFANT study in clinical practice, determination of aeroallergen sensitization, either by skin prick testing or by in vitro allergen-specific IgE testing, should be considered in preschool children with mild persistent asthma. If allergy testing is negative, complete blood count with differentials should be obtained to evaluate for absolute eosinophil count. If the child has positive skin testing to aeroallergens and/or absolute eosinophil count ≥ 300/μl, he/she will most likely respond best to daily ICS with higher asthma control days and fewer exacerbations compared with daily LTRA or as needed ICS with albuterol. If the child fails initial therapy with daily ICS, a trial of an alternative therapy such as daily LTRA or intermittent ICS should be considered before escalating to next step of treatment since in the INFANT study, there were substantial numbers of children who responded best to either daily LTRA or the as-needed ICS regimen. However, if the child has negative allergy testing and absolute eosinophil count < 300/μl, providers can choose either daily ICS, daily LTRA, or as-needed ICS with each dose albuterol (Fig. 2).
Case 2
Parents bring a 3-year-old boy for evaluation of his recurrent wheezing. He developed 4 wheezing episodes in the past year, all preceded by rhinorrhea, nasal congestion, and cough for a few days. He made one emergency room visit during these illnesses and was given albuterol with improvement of his cough and wheezing. He received oral prednisolone during 2 of these illnesses with some improvement. He has no respiratory symptoms between these illnesses. His past medical history was significant for eczema. His mother has asthma. The exam was unremarkable. His skin testing to aeroallergens was negative.
Questions: What is the next step of management? Which of the following would be most appropriate—a daily inhaled steroids (ICS) or leukotriene receptor antagonists (LTRA), intermittent therapy with ICS, or continue with albuterol as needed? Are there other potential strategies to consider?
Literature review
Daily low-dose ICS reduces rates of significant exacerbations requiring oral corticosteroids by 40% in preschool children with positive mAPI; however, the daily use for 2 years is associated with evidence of growth suppression
Daily ICS therapy is effective in reducing the risk of asthma exacerbations among young children [27•, 30•]. As discussed in case 1, the PEAK trial provided results favoring the use of daily ICS in preschool children with frequent recurrent wheeze and positive mAPIs [31]. However, there are several concerns and barriers regarding this treatment regimen, including daily medication adherence, parental resistance of daily use of medications for young children with episodic symptoms, and the small effects on growth. Compared with the placebo group, the mean increase in height was 1.1 cm less after 2 years of therapy in the daily ICS group, and the effects on height may be only partially reversed after discontinuation of therapy, especially in children who were younger and of less weight relative to the entire study cohort [16, 32].
Intermittent therapy with high-dose ICS initiated at the onset of LRTIs results in similar benefits as daily low-dose ICS in preschool children with positive mAPI, with lower cumulative exposure to ICS
Due to the issues and barriers surrounding daily ICS use in preschool children noted above, several studies have been conducted to examine the use of intermittent high-dose ICS as an alternative strategy in this phenotype of patients [30•, 33, 34]. A previous study demonstrated the administration of high-dose fluticasone propionate 750 mcg twice daily at the onset of RTI in preschool children aged 1–6 years with moderate-to-severe virus-induced wheezing, and continuing for maximum 10 days reduced the risks of significant exacerbations requiring systemic corticosteroids by half (8% in fluticasone group versus 18% in placebo). However, the preemptive fluticasone group was associated with a smaller gain of both height and weight, compared with placebo [34]. The Maintenance and Intermittent Inhaled Corticosteroids in Wheezing Toddlers (MIST) trial was conducted to compare the frequency of exacerbations requiring oral corticosteroids between daily low-dose (0.5 mg nebulized nightly) versus intermittent high-dose budesonide (1 mg nebulized twice daily for 7 days at the earliest signs of respiratory tract symptoms) in preschool children with positive mAPI who had at least one severe exacerbation requiring systemic corticosteroids, unscheduled doctor visit, or emergency room visit/hospitalization in the prior year and had no evidence of significant day-to-day symptoms during the run-in period. During the 1-year study, both groups had similar rates of exacerbations of LRTIs requiring OCS, with rates per patient-year of 0.97 and 0.99 for daily and intermittent regimens, respectively. However, the lower cumulative dose (a 2/3 lower total dose) to budesonide compared with the daily treatment was the major advantage of using the intermittent regimen [35]. A recent meta-analysis supports the efficacy of intermittent high-dose ICS regimen in preschool children with recurrent wheeze by demonstrating a reduction in exacerbations in the intermittent ICS group, compared with placebo (risk ratio of 0.65, 95% CI, 0.51–0.81) and the treatment of 6 patients with intermittent ICS therapy prevented 1 patient from experiencing an asthma exacerbation requiring OCS compared with placebo [30•]. The doses of ICS found to be effective in the studies varied by the studies and these included budesonide inhalation 1 mg twice daily [33] and 750 mcg of fluticasone propionate via the metered-dose inhaler twice daily as mentioned above [34].
Daily and intermittent therapy with montelukast is not effective in decreasing the frequency of severe RTI or exacerbations requiring OCS among preschool children with severe intermittent wheeze
Montelukast, a leukotriene receptor antagonist, is commonly used for treatment of allergic rhinitis and asthma in children. Although long-term use of montelukast has been shown to reduce the bronchial hyperresponsiveness in preschool children [36] and one of the early studies demonstrated the montelukast for 12 months in young children with a history of intermittent asthma symptoms resulted in a significant but small reduction of exacerbation episodes associated with LRTIs [24], the clinical benefits in preschool children with histories of severe intermittent wheezing episodes are limited. Several studies showed that the regular use of montelukast in children with viral infection–triggered wheeze did not result in improvement of respiratory symptom severity with wheezing episodes or reduction in health care utilization [23, 37,38,39], although one of the studies demonstrated that regular use of montelukast for 8 weeks increased the proportion of symptom-free days within the first week of wheezing illness and a lower wheezing score at the 7th day of illness compared with placebo [23]. Intermittent therapy with montelukast initiated at the onset of respiratory tract illness [33, 40] or during acute exacerbation in the emergency room setting did not reduce the number of LRTI exacerbations requiring OCS [33, 40] or improve pulmonary function [41] in this setting. Recently, a meta-analysis investigated the effectiveness of montelukast on prevention of significant exacerbations requiring OCS in preschool children and did not find significant benefit of using montelukast either by the intermittent or daily regimen [42•].
The use of azithromycin at the earliest symptoms or signs of RTI reduces the risks of progression to severe LRTI in preschool children with history of recurrent severe LRTI independent of mAPI status
In school-aged children, the presence of certain bacteria including Streptococcus pneumoniae and Moraxella catarrhalis was associated with asthma exacerbations and increased asthma symptoms in the presence of rhinovirus [43], the most common virus-triggered wheezing exacerbation in children [44]. Rhinovirus triggering more severe respiratory tract illnesses and asthma exacerbations could be partly related to overgrowth of bacterial pathogens including S. pneumoniae, Haemophilus influenzae, and M. catarrhalis, and the frequency of these pathogens was more common in younger children [45].
Given the increased recognition of bacterial pathogens in early-life wheezing, recent studies have examined the role of the macrolide antibiotic azithromycin in this clinical context. Azithromycin also has an impact on airway microbiota with reduced abundance in certain bacteria in asthma [46] and has well-described anti-inflammatory effects on neutrophils [47]. The efficacy of early azithromycin in preventing the progression from early respiratory tract symptoms to severe LRTI was investigated in a multicenter, randomized, double-blind, placebo-controlled trial in children aged 12 through 71 months with recurrent severe wheezing in the context of clinically significant LRTIs that required systemic corticosteroids, an unscheduled physician office visit, an urgent or emergency department visit, or hospitalization. Children who were in the treatment group and received azithromycin (12 mg/kg once daily for 5 days) had a significantly lower risk of progressing to severe LRTI than the placebo group, hazard ratio of 0.64 (95% CI, 0.41–0.98), after adjustment for other factors including the presence of mAPI [48•]. The most common side effects were mild gastrointestinal symptoms and none led to study discontinuation. The study did not find differences in rates of resistant bacterial isolates from throat swabs in a subgroup of participants.
A second randomized, double-blinded, placebo-controlled trial in children aged 1–3 years with recurrent asthma-like symptoms from Copenhagen Prospective Studies on Asthma in Childhood (COPSAC) cohort demonstrated the use of azithromycin, in this case given at 10 mg/kg/day for 3 days after participant had asthma-like symptoms lasting at least 3 days, and reduced the duration of respiratory episode after treatment by approximately 4 days, and the effect size increased with early initiation of treatment [49]. It remains uncertain if the effects of azithromycin in preventing the progression to severe LRTI and reduction of duration of respiratory illnesses in these children are mediated via the anti-inflammatory effects or via the modification of the microbiome.
Discussion on the approach to case 2
This patient had recurrent wheezing in the context of viral RTIs and he had positive mAPI based on his history of recurrent wheezing for at least 4 episodes including one episode diagnosed by a physician, and maternal history of asthma. His clinical history was consistent with the severe intermittent wheezing phenotype as he did not have day-to-day respiratory symptoms. In this setting, the primary goal is to prevent subsequent episodes of viral respiratory tract infection from progressing to significant LRTIs requiring OCS. The options based on the above review would include daily ICS, intermittent therapy with ICS at the onset of LRTI, or azithromycin at the onset of RTI. As he has a positive mAPI, intermittent therapy with high-dose budesonide 1 mg twice daily is a preferred initial treatment of choice, as it was shown to be comparable with daily low-dose ICS in preventing the progression of respiratory illnesses to significant LRTIs in preschool children with severe intermittent wheezing episodes in the context of LRTIs and it was associated with the lower cumulative dose of ICS, compared with the daily regimen. Daily low-dose ICS therapy is also a consideration, especially for families who are not able to understand or appropriately implement the episodic high-dose ICS treatment approach.
Currently, there are no trials directly comparing the efficacy of intermittent ICS therapy and azithromycin administration at the earliest signs of LRTI in patients with positive mAPI. Thus, in children with positive mAPI, intermittent therapy with ICS should be considered the initial treatment of choice (Fig. 2). Early azithromycin should be considered in children with negative mAPIs who have severe intermittent wheezing episodes in the context of RTI. Furthermore, a therapeutic trial of azithromycin early in the course of RTI could be considered to prevent progression to severe LRTI and need for OCS in children who previously demonstrated an azithromycin response, in those children with positive mAPIs for whom intermittent high-dose or daily low ICSs were not effective.
For the implementation of intermittent/episodic ICS therapy in clinical practice, parents need to be instructed to recognize the early signs of RTI in order to appropriately start the therapy (either high-dose ICS or azithromycin) early during the predefined respiratory tract illness. Parents of toddlers with history of recurrent severe wheezing in the setting of RTI have been shown to be confident in their ability to identify a specific set of signs and symptoms that preceded and signaled the development of severe wheezing during RTI, and the most commonly identified symptoms included cough, breathing problem, or noisy chest [35]. Thus, prior to the implementation of an intermittent therapy regimen, physicians should ask the parents to identify the symptoms and signs of early RTI that typically progress to chest involvement in their children (see Table 2 on the Parental Respiratory Tract Illness Questionnaire from MIST trial) [35], and then a personalized action plan should be developed and reviewed with the parents on the timing of initiation of therapy. As recurrent wheezing in young children is a heterogeneous disease and the course of illness can change over time, it is necessary to monitor the response in these children on the follow-up visits and adjust treatment if needed. Finally, careful attention should be paid to frequency of use of these regimens in order to minimize the risk of adverse outcomes such as the growth effects seen with high-dose fluticasone [34] or the potential development of antimicrobial resistance with azithromycin [48•].
Gaps in the evidence for management of preschool asthma
There are several research questions that should be explored in the future to fill the gaps of knowledge in treatment for preschool wheezing, including the comparison of efficacy in prevention of progression of LRTIs between intermittent therapy with budesonide inhalation and azithromycin in preschool children with recurrent severe LRTIs. There is limited information on the efficacy and safety of adding a long-acting β2 agonist to ICS in preschool children. A recent study that prospectively evaluated the use of inhaled salmeterol/fluticasone propionate combination in 35 preschool children with mild-to-moderate persistent asthma demonstrated the combination approach appears to be safe in this age group, with an improvement in nighttime sleep symptom scores [50]. Additional studies are also needed to identify effective strategies to prevent significant exacerbation episodes in preschool children with persistent disease who developed an acute loss of asthma control while already receiving a daily controller medication.
Conclusions
In conclusion, clinical trials dedicated to investigating preschool children with severe episodic wheezing and persistent asthma have helped move the care of these children towards the era of precision medicine by identifying the factors that maximize the efficacy of interventions to prevent significant exacerbations in these children. While current approaches include careful clinical histories and basic laboratory markers, future strategies including genetics and other biomarkers could potentially improve the decision making in determining individualized therapy.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children’s Respiratory study: 1980 to present. J Allergy Clin Immunol. 2003;111(4):661–75 quiz 76.
• Fitzpatrick AM, Bacharier LB, Guilbert TW, Jackson DJ, Szefler SJ, Beigelman A, et al. Phenotypes of recurrent wheezing in preschool children: identification by latent class analysis and utility in prediction of future exacerbation. J Allergy Clin Immunol Pract. 2019;7(3):915–24 e7. This article showed sensitization and exposure assessments are useful predictors of future exacerbation in children aged 12 to 71 months.
Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(Suppl 3):S131–45.
Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, et al. National surveillance of asthma: United States, 2001–2010. Vital Health Stat 3. 2012;35:1–58.
Taussig LM, Wright AL, Morgan WJ, Harrison HR, Ray CG. The Tucson Children’s Respiratory study. I. Design and implementation of a prospective study of acute and chronic respiratory illness in children. Am J Epidemiol. 1989;129(6):1219–31.
Halonen M, Stern DA, Lohman C, Wright AL, Brown MA, Martinez FD. Two subphenotypes of childhood asthma that differ in maternal and paternal influences on asthma risk. Am J Respir Crit Care Med. 1999;160(2):564–70.
Guilbert TW, Morgan WJ, Zeiger RS, Bacharier LB, Boehmer SJ, Krawiec M, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol. 2004;114(6):1282–7.
Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1403–6.
Guilbert TW. Identifying and managing the infant and toddler at risk for asthma. J Allergy Clin Immunol. 2010;126(2):417–22.
Chang TS, Lemanske RF Jr, Guilbert TW, Gern JE, Coen MH, Evans MD, et al. Evaluation of the Modified Asthma Predictive Index in high-risk preschool children. J Allergy Clin Immunol Pract. 2013;1(2):152–6.
Biagini Myers JM, Schauberger E, He H, Martin LJ, Kroner J, Hill GM, et al. A Pediatric Asthma Risk Score to better predict asthma development in young children. J Allergy Clin Immunol. 2018.
Bacharier LB. The recurrently wheezing preschool child-benign or asthma in the making? Ann Allergy Asthma Immunol. 2015;115(6):463–70.
Beigelman A, Bacharier LB. Management of preschool recurrent wheezing and asthma: a phenotype-based approach. Curr Opin Allergy Clin Immunol. 2017;17(2):131–8.
Bacharier LB, Guilbert TW. Diagnosis and management of early asthma in preschool-aged children. J Allergy Clin Immunol. 2012;130(2):287–96; quiz 97–8.
2019 Available from: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed 13 July 2019.
Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354(19):1985–97.
Schokker S, Kooi EM, de Vries TW, Brand PL, Mulder PG, Duiverman EJ, et al. Inhaled corticosteroids for recurrent respiratory symptoms in preschool children in general practice: randomized controlled trial. Pulm Pharmacol Ther. 2008;21(1):88–97.
Murphy KR, Fitzpatrick S, Cruz-Rivera M, Miller CJ, Parasuraman B. Effects of budesonide inhalation suspension compared with cromolyn sodium nebulizer solution on health status and caregiver quality of life in childhood asthma. Pediatrics. 2003;112(3 Pt 1):e212–9.
Szefler SJ, Baker JW, Uryniak T, Goldman M, Silkoff PE. Comparative study of budesonide inhalation suspension and montelukast in young children with mild persistent asthma. J Allergy Clin Immunol. 2007;120(5):1043–50.
Brand PL, Luz Garcia-Garcia M, Morison A, Vermeulen JH, Weber HC. Ciclesonide in wheezy preschool children with a positive asthma predictive index or atopy. Respir Med. 2011;105(11):1588–95.
Castro-Rodriguez JA, Rodrigo GJ. Efficacy of inhaled corticosteroids in infants and preschoolers with recurrent wheezing and asthma: a systematic review with meta-analysis. Pediatrics. 2009;123(3):e519–25.
Nagao M, Ikeda M, Fukuda N, Habukawa C, Kitamura T, Katsunuma T, et al. Early control treatment with montelukast in preschool children with asthma: a randomized controlled trial. Allergol Int. 2018;67(1):72–8.
Keskin O, Arik Yilmaz E, Motzkus C, Sackesen C, Lilly CM, Kalayci O. The effect of montelukast on early-life wheezing: a randomized, double-blinded placebo-controlled study. Pediatr Allergy Immunol. 2018;29(1):50–7.
Bisgaard H, Zielen S, Garcia-Garcia ML, Johnston SL, Gilles L, Menten J, et al. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am J Respir Crit Care Med. 2005;171(4):315–22.
Wang XP, Yang LD, Zhou JF. Montelukast and budesonide combination for children with chronic cough-variant asthma. Medicine (Baltimore). 2018;97(30):e11557.
Szefler SJ, Carlsson LG, Uryniak T, Baker JW. Budesonide inhalation suspension versus montelukast in children aged 2 to 4 years with mild persistent asthma. J Allergy Clin Immunol Pract. 2013;1(1):58–64.
• Castro-Rodriguez JA, Rodriguez-Martinez CE, Ducharme FM. Daily inhaled corticosteroids or montelukast for preschoolers with asthma or recurrent wheezing: a systematic review. Pediatr Pulmonol. 2018;53(12):1670–7. The article demonstrated daily inhaled corticosteroids is more effective than daily montelukast for improving asthma control and decreasing exacerbations in preschool children.
• Fitzpatrick AM, Jackson DJ, Mauger DT, Boehmer SJ, Phipatanakul W, Sheehan WJ, et al. Individualized therapy for persistent asthma in young children. J Allergy Clin Immunol. 2016;138(6):1608-18 e12. The majority of preschool children with mild persistent asthma had differential responses to either daily ICS, daily LTRA or symptom-driven ICS treatment. Aeroallergen sensitization and blood eosinophil counts are useful to identify children who will most likely have a good response to daily ICS.
Depner M, Fuchs O, Genuneit J, Karvonen AM, Hyvarinen A, Kaulek V, et al. Clinical and epidemiologic phenotypes of childhood asthma. Am J Respir Crit Care Med. 2014;189(2):129–38.
• Kaiser SV, Huynh T, Bacharier LB, Rosenthal JL, Bakel LA, Parkin PC, et al. Preventing exacerbations in preschoolers with recurrent wheeze: a meta-analysis. Pediatrics. 2016;137(6). The article emphasized the efficacy of daily ICS to prevent exacerbations in preschool children with persistent asthma and of intermittent ICS to prevent exacerbations in children with intermittent diseases.
Bacharier LB, Guilbert TW, Zeiger RS, Strunk RC, Morgan WJ, Lemanske RF Jr, et al. Patient characteristics associated with improved outcomes with use of an inhaled corticosteroid in preschool children at risk for asthma. J Allergy Clin Immunol. 2009;123(5):1077–82 82 e1–5.
Guilbert TW, Mauger DT, Allen DB, Zeiger RS, Lemanske RF Jr, Szefler SJ, et al. Growth of preschool children at high risk for asthma 2 years after discontinuation of fluticasone. J Allergy Clin Immunol. 2011;128(5):956–63 e1–7.
Bacharier LB, Phillips BR, Zeiger RS, Szefler SJ, Martinez FD, Lemanske RF Jr, et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J Allergy Clin Immunol. 2008;122(6):1127–35 e8.
Ducharme FM, Lemire C, Noya FJ, Davis GM, Alos N, Leblond H, et al. Preemptive use of high-dose fluticasone for virus-induced wheezing in young children. N Engl J Med. 2009;360(4):339–53.
Zeiger RS, Mauger D, Bacharier LB, Guilbert TW, Martinez FD, Lemanske RF Jr, et al. Daily or intermittent budesonide in preschool children with recurrent wheezing. N Engl J Med. 2011;365(21):1990–2001.
Hakim F, Vilozni D, Adler A, Livnat G, Tal A, Bentur L. The effect of montelukast on bronchial hyperreactivity in preschool children. Chest. 2007;131(1):180–6.
Krawiec M, Strzelak A, Krenke K, Modelska-Wozniak I, Jaworska J, Kulus M. Fluticasone or montelukast in preschool wheeze: a randomized controlled trial. Clin Pediatr (Phila). 2015;54(3):273–81.
Proesmans M, Sauer K, Govaere E, Raes M, De Bilderling G, De Boeck K. Montelukast does not prevent reactive airway disease in young children hospitalized for RSV bronchiolitis. Acta Paediatr. 2009;98(11):1830–4.
Bisgaard H, Flores-Nunez A, Goh A, Azimi P, Halkas A, Malice MP, et al. Study of montelukast for the treatment of respiratory symptoms of post-respiratory syncytial virus bronchiolitis in children. Am J Respir Crit Care Med. 2008;178(8):854–60.
Valovirta E, Boza ML, Robertson CF, Verbruggen N, Smugar SS, Nelsen LM, et al. Intermittent or daily montelukast versus placebo for episodic asthma in children. Ann Allergy Asthma Immunol. 2011;106(6):518–26.
Wang X, Zhou J, Zhao X, Yi X. Montelukast treatment of acute asthma exacerbations in children aged 2 to 5 years: a randomized, double-blind, placebo-controlled trial. Pediatr Emerg Care. 2018;34(3):160–4.
• Hussein HR, Gupta A, Broughton S, Ruiz G, Brathwaite N, Bossley CJ. A meta-analysis of montelukast for recurrent wheeze in preschool children. Eur J Pediatr. 2017;176(7):963–9. The meta-analysis found montelukast was ineffective in reducing oral corticosteroid uses for preschool wheeze.
Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 2014;133(5):1301–7, 7 e1–3.
Lee WM, Lemanske RF Jr, Evans MD, Vang F, Pappas T, Gangnon R, et al. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med. 2012;186(9):886–91.
Bashir H, Grindle K, Vrtis R, Vang F, Kang T, Salazar L, et al. Association of rhinovirus species with common cold and asthma symptoms and bacterial pathogens. J Allergy Clin Immunol. 2018;141(2):822–4 e9.
Slater M, Rivett DW, Williams L, Martin M, Harrison T, Sayers I, et al. The impact of azithromycin therapy on the airway microbiota in asthma. Thorax. 2014;69(7):673–4.
Culic O, Erakovic V, Cepelak I, Barisic K, Brajsa K, Ferencic Z, et al. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur J Pharmacol. 2002;450(3):277–89.
• Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, Fitzpatrick AM, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314(19):2034–44. The article demonstrated the early use of azithromycin during an apparent lower respiratory tract illnesses reduced the likelihood of severe LRTI in young children with histories of recurrent severe RTI, compared to placebo.
Stokholm J, Chawes BL, Vissing NH, Bjarnadottir E, Pedersen TM, Vinding RK, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1–3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4(1):19–26.
Yoshihara S, Fukuda H, Tamura M, Arisaka O, Ikeda M, Fukuda N, et al. Efficacy and safety of salmeterol/fluticasone combination therapy in infants and preschool children with asthma insufficiently controlled by inhaled corticosteroids. Drug Res (Stuttg). 2016;66(7):371–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Leonard Bacharier reports personal fees from Aerocrine, personal fees from GlaxoSmithKline, personal fees from Genentech/Novartis, personal fees from Merck, personal fees from DBV Technologies, personal fees from Teva, personal fees from Boehringer Ingelheim, personal fees from AstraZeneca, personal fees from WebMD/Medscape, personal fees from Sanofi/Regeneron, personal fees from Vectura, and personal fees from Circassia outside the submitted work. Maleewan Kitcharoensakkul declares no conflicts of interest relevant to this manuscript.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Allergic Asthma
Rights and permissions
About this article
Cite this article
Kitcharoensakkul, M., Bacharier, L.B. A Case-Based Review on the Diagnosis and Treatment Options for Recurrent Wheezing and Asthma in Preschool Children. Curr Treat Options Allergy 6, 423–437 (2019). https://doi.org/10.1007/s40521-019-00227-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40521-019-00227-w