Abstract
Aim
The association between visual impairment and mild cognitive impairment (MCI) has not been investigated to date. Thus, we assessed this association among older adults from six low- and middle-income countries (LMICs) (China, India, Ghana, Mexico, Russia, and South Africa) using nationally representative datasets.
Methods
Cross-sectional, community-based data from the WHO Study on global AGEing and adult health (SAGE) were analyzed. Visual acuity was measured using the tumbling ElogMAR chart, and vision impairment (at distance and near) was defined as visual acuity worse than 6/18 (0.48 logMAR) in the better-seeing eye. The definition of MCI was based on the National Institute on Aging-Alzheimer’s Association criteria. Multivariable logistic regression was conducted.
Results
Data on 32,715 individuals aged ≥ 50 years [mean (SD) age 62.1 (15.6) years; 51.2% females] were analyzed. Compared to those without far or near vision impairment, those with near vision impairment but not far vision impairment (OR = 1.33; 95% CI = 1.16–1.52), and those with both far and near vision impairment (OR = 1.70; 95% CI = 1.27–2.29) had significantly higher odds for MCI. Only having far vision impairment was not significantly associated with MCI.
Conclusions
Visual impairment is associated with increased odds for MCI among older adults in LMICs with the exception of far vision impairment only. Future longitudinal and intervention studies should examine causality and whether improvements in visual acuity, or early intervention, can reduce risk for MCI and ultimately, dementia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The World Health Organization (WHO) defines dementia as a syndrome—usually of a chronic or progressive nature—in which there is deterioration in cognitive function (i.e., the ability to process thought) beyond what might be expected from normal aging [1]. Dementia is one of the major causes of disability and dependency in the older population and also has significant social and economic impacts [2]. However, there are currently no pharmacological interventions to prevent or cure dementia. Therefore, it is essential to understand the correlates of its preclinical stage to plan strategies to prevent or delay the onset of dementia.
An identified precursor to dementia is mild cognitive impairment (MCI). It has been estimated that one or two in every 10 people over 65 years may have MCI [3]. Although not all individuals with MCI will go on and develop dementia, MCI is associated with a high conversion rate to dementia. Specifically, 12%, 20%, and 50% at 1, 3, and 5 years, respectively [3, 4].
Previously identified correlates of MCI include factors such as low physical activity, poor diet, alcohol consumption and smoking [5]. However, there are currently no studies on the association between vision impairment and MCI. Vision impairment may increase one’s risk of cognitive impairment via barriers in carrying out daily activities, such as reading or driving, and this may consequently negatively impact cognition, owing to decreased brain stimulation [6]. Several previous studies from high-income countries have found that vision impairment is associated with cognitive decline [6,7,8,9,10,11,12,13,14]. For example, one longitudinal study from Germany measuring vision impairment at baseline found that the onset of severe visual impairment was associated with decline in cognitive function score [9]. However, all these previous studies only focused on cognitive decline in general and not MCI, which carries a particularly high risk of conversion to dementia.
Therefore, the aim of the present study was to investigate the association between objectively measured visual impairment (far and near) and MCI in adults aged ≥ 50 years from six low- and middle-income countries (LMICs). Examining this association in LMICs is of particular importance as people living with dementia is increasing most rapidly in these settings [15], while the prevalence of visual impairment has been estimated to be higher in LMICs than in high-income countries [16].
Methods
The survey
Data from the Study on Global Ageing and Adult Health (SAGE) were analyzed. These data are publically available through http://www.who.int/healthinfo/sage/en/. This survey was undertaken in China, Ghana, India, Mexico, Russia, and South Africa between 2007 and 2010. Based on the World Bank classification at the time of the survey, all countries were LMICs.
Details of the survey methodology have been published elsewhere [17]. Briefly, to obtain nationally representative samples, a multistage clustered sampling design method was used. The sample consisted of adults aged ≥ 18 years with oversampling of those aged ≥ 50 years. Trained interviewers conducted face-to-face interviews using a standard questionnaire. Standard translation procedures were undertaken to ensure comparability between countries. The survey response rates were: China 93%; Ghana 81%; India 68%; Mexico 53%; Russia 83%; and South Africa 75%. Sampling weights were constructed to adjust for the population structure as reported by the United Nations Statistical Division. Ethical approval was obtained from the WHO Ethical Review Committee and local ethics research review boards. Written informed consent was obtained from all participants.
Visual impairment
Visual acuity was measured using the tumbling ElogMAR chart for distance and near acuity separately for each eye. For distance vision, the participant was asked to be seated in a chair positioned so that the respondent’s head is 4 m from the eye chart. A string was used to measure 40 cm as the test distance for near visual acuity. The interviewer was instructed to check that the vision charts are well lit and to make sure that the surface does not reflect glare. Furthermore, the respondent was instructed to use glasses or contact lenses if they usually wear them. We defined vision impairment (at distance and near) according to the World Health Organization definition for moderate vision impairment, which refers to visual acuity worse than 6/18 (0.48 logMAR) in the better-seeing eye [18]. We created different categories of visual impairment based on different combinations of far and near vision impairment. Specifically, the following four categories were created: (a) no far or near vision impairment; (b) only far vision impairment and no near vision impairment; (c) only near vision impairment and no far vision impairment; and (d) both far and near vision impairments.
Mild cognitive impairment (MCI) (Outcome)
MCI was ascertained based on the recommendations of the National Institute on Aging-Alzheimer’s Association [19]. We applied the identical algorithms used in previous SAGE publications to identify MCI [4, 20]. Briefly, individuals fulfilling all of the following conditions were considered to have MCI:
-
(a) Concern about a change in cognition: individuals who replied ‘bad’ or ‘very bad’ to the question “How would you best describe your memory at present?” and/or those who answered ‘worse’ to the question “Compared to 12 months ago, would you say your memory is now better, the same or worse than it was then?” were considered to have this condition.
-
(b) Objective evidence of impairment in one or more cognitive domains: was based on a < − 1 SD cut-off after adjustment for level of education and age. Cognitive function was assessed through the following performance tests: word list immediate and delayed verbal recall from the Consortium to Establish a Registry for Alzheimer’s disease [21], which assessed learning and episodic memory; digit span forward and backwards from the Wechsler Adult Intelligence Scale [22], that evaluated attention and working memory; and the animal naming task [21], which assessed verbal fluency.
-
(c) Preservation of independence in functional abilities: was assessed by questions on self-reported difficulties with basic activities of daily living (ADL) in the past 30 days [23]. Specific questions were: “How much difficulty did you have in getting dressed?” and “How much difficulty did you have with eating (including cutting up your food)?” The answer options were none, mild, moderate, severe, and extreme (cannot do). Those who answered either none, mild, or moderate to both of these questions were considered to have preservation of independence in functional activities. All other individuals were deleted from the analysis (935 individuals aged ≥ 50 years).
-
(d) No dementia: individuals with a level of cognitive impairment severe enough to preclude the possibility to undertake the survey were not included in the current study.
Control variables
The control variables used were selected based on past literature and included sex, age, years of education, wealth quintiles based on income, physical activity, smoking (never, current smoking, past smoking), obesity (body mass index ≥ 30 kg/m2 based on measured weight and height), diabetes, stroke, hypertension, and depression [24, 25]. Levels of physical activity were assessed with the Global Physical Activity Questionnaire and were classified as low, moderate, and high based on conventional cut-offs [26]. Diabetes and stroke were based solely on lifetime self-reported diagnosis. Hypertension was defined as having at least one of: systolic blood pressure ≥ 140 mmHg; diastolic blood pressure ≥ 90 mmHg; or self-reported diagnosis. Questions based on the World Mental Health Survey version of the Composite International Diagnostic Interview [27] were used for the endorsement of DSM-IV depression [28].
Statistical analysis
The statistical analysis was performed with Stata 14.1 (Stata Corp LP, College station, Texas). The analysis was restricted to those aged ≥ 50 years as MCI is an age-related condition. We included the middle-aged in this analysis as assessment of cognitive function and its risk factors at earlier ages is important for prevention of dementia given that cognitive dysfunction can manifest up to 10 years prior to the actual dementia diagnosis [29], and current evidence shows that intervening in mid-life is important [30,31,32,33]. We conducted multivariable logistic regression analysis to assess the association between visual impairment (exposure) and MCI (outcome). The variable on visual impairment was a four-category variable with different combinations of far and near visual impairment. The regression analysis was adjusted for sex, age, education, wealth, physical activity, smoking, obesity, diabetes, stroke, hypertension, depression, and country. Analyses stratified by age groups (50–64 and ≥ 65 years) were also conducted. Adjustment for country was done by including dummy variables for each country in the model as in previous SAGE publications [4, 34].
All variables were included in the models as categorical variables with the exception of age and years of education (continuous variables). The sample weighting and the complex study design were taken into account in the analyses. Results from the regression analyses are presented as odds ratios (ORs) with 95% confidence intervals (CIs). The level of statistical significance was set at p < 0.05.
Results
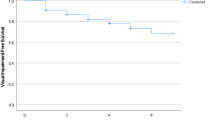
The final analytical sample consisted of 32,715 individuals aged ≥ 50 years with preservation in functional abilities. The sample characteristics are provided in Table 1. The mean (SD) age of the sample was 62.1 (15.6) years and 51.2% were females. The prevalence of MCI was 15.3%, while the prevalence of the four visual impairment categories were: (a) no far or near vision impairment 55.7%; (b) only far vision impairment and no near vision impairment 5.4%; (c) only near vision impairment and no far vision impairment 29.3%; and (d) both far and near vision impairment 9.7%. The prevalence of MCI was lowest in those without far or near vision impairment (13.4%) and highest among those with both far and near vision impairment (22.3%) (Fig. 1). The association between different combinations of far and near vision impairment and MCI estimated by multivariable logistic regression is shown in Table 2. In the overall sample, compared to those without far or near vision impairment, those with near vision impairment but not far vision impairment (OR = 1.33; 95% CI = 1.16–1.52), and those with both far and near vision impairment (OR = 1.70; 95% CI = 1.27–2.29) had significantly higher odds for MCI. Only having far vision impairment was not significantly associated with MCI. Similar results were found for analyses stratified by age groups (i.e., 50–64 and ≥ 65 years).
Discussion
Summary of main findings
In this large sample of older adults from six LMICs, it was found that those with near vision impairment, and those with far and near vision impairment had high odds of MCI; whereas, those with far vision impairment only did not. Findings from the present study support previous literature that has shown an association between vision impairment and cognitive decline [6,7,8,9,10,11,12,13,14] and add to the literature by reporting for the first time that an association exists between vision impairment and MCI in older adults residing in LMICs, after controlling for important covariates.
Potential pathways
Two potential pathways likely explain the present findings. First, vision impairment may reduce the number and type of daily activities that one can engage in (e.g., exercise, driving motor vehicles, socializing) [35], consequently resulting in reduced brain stimulation and thus MCI [6]. Indeed, older cognitively impaired adults have improved cognitive function after cataract surgery, probably reflecting better vision and enhanced brain activity as sensory stimulation increased [24]. However, it is important to note that the present study did not find an association between far vision impairment only and MCI. This is in line with a previous longitudinal study conducted among Mexican American older adults [24], and may be reflecting the fact that near vision is more important for intellectually stimulating activities, but more studies are necessary to assess whether this finding can be corroborated. Second, it is also possible that vision impairment and MCI are not causally related but that their association is explained by a shared common pathway, such as inflammation [6].
Despite the fact that far vision alone was not significantly associated with MCI, those with far and near vision impairment simultaneously had the highest odds for MCI. Although the reason for this is unclear, it is possible that those who have far and near vision impairment are more likely to have non-refractory visual impairment (e.g., cataracts, diabetic retinopathy, glaucoma), and thus, more severe visual impairment, which can severely impair their ability to engage in intellectually stimulating activities.
Implications
If visual impairment is confirmed to be a risk factor for MCI/dementia in future longitudinal studies, one measure that may aid in the prevention of MCI is to ensure that there is an adequate provision of correctly prescribed spectacles. Unfortunately, this is a public health challenge in LMICs since there is a paucity of human resources for refraction and optical services, lack of access to refraction services in rural areas, and the cost of spectacles. Low-cost approaches to provide affordable glasses with the correct prescription in developing countries are critical. Possible approaches to overcome this may include increasing the use of focometers and self-adjustable glasses, among other modalities [36].
Strengths, limitations, and directions for future research
Clear strengths of the present study include the objective measure of vision and a large nationally representative sample of older adults from six LMICs. It is important to interpret findings from the present study in light of its limitations. First the study is cross-sectional in design and, thus, the direction of the association is not known. For example, it is possible that those with MCI are less likely to access health care to correct refractive errors or treat cataracts. Future research of a longitudinal design is required. Second, our study is not able to clinically diagnose dementia. Thus, some people with mild dementia may had been included in our study. However, the present prevalence of MCI was within previously reported figures [37].
Conclusion
In this large sample of older adults from six LMICs, we showed for the first time that vision impairment increases the odds of having MCI, except for having far vision impairment only. Future longitudinal and intervention studies should examine causality and whether improvements in visual acuity can reduce risk for MCI and ultimately, dementia.
References
World Health Organization (2020) Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia Accessed 25 November 2020
Wimo A, Guerchet M, Ali GC et al (2017) The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 13:1–7. https://doi.org/10.1016/j.jalz.2016.07.150
Alzheimer's Research UK (2019) Mild cognitive impairment. https://www.alzheimersresearchuk.org/dementia-information/types-of-dementia/mild-cognitive-impairment. Accessed 25 November 2020
Koyanagi A, Lara E, Stubbs B et al (2018) Chronic physical conditions, multimorbidity, and mild cognitive impairment in low- and middle-income countries. J Am Geriatr Soc 66:721–727. https://doi.org/10.1111/jgs.15288
Hara Y. Cognitive Vitality (2019) Seven lifestyle interventions evaluated by the WHO for preventing cognitive decline and dementia. August 14, 2019. https://www.alzdiscovery.org/cognitive-vitality/blog/seven-lifestyle-interventions-evaluated-by-who-prevent-cognitive-decline. Accessed 25 November 2020
Zheng DD, Swenor BK, Christ SL et al (2018) Longitudinal associations between visual impairment and cognitive functioning: the salisbury eye evaluation study. JAMA Ophthalmol 136:989–995. https://doi.org/10.1001/jamaophthalmol.2018.2493
Chen SP, Bhattacharya J, Pershing S (2017) Association of vision loss with cognition in older adults. JAMA Ophthalmol 135:963–970. https://doi.org/10.1001/jamaophthalmol.2017.2838
Evans J (2017) Complex relationships between vision and cognition in older people. JAMA Ophthalmol 135:971–972. https://doi.org/10.1001/jamaophthalmol.2017.2843
Hajek A, Brettschneider C, Lühmann D et al (2016) Study on ageing, cognition and dementia in primary care patients group. Effect of visual impairment on physical and cognitive function in old age: findings of a population-based prospective cohort study in Germany. J Am Geriatr Soc 64:2311–2316. https://doi.org/10.1111/jgs.14458
Lim ZW, Chee ML, Soh ZD et al (2020) Association between visual impairment and decline in cognitive function in a multiethnic asian population. JAMA Netw Open 3:e203560. https://doi.org/10.1001/jamanetworkopen.2020.3560
Mandas A, Mereu RM, Catte O et al (2014) Cognitive impairment and age-related vision disorders: their possible relationship and the evaluation of the use of aspirin and statins in a 65 years-and-over sardinian population. Front Aging Neurosci 6:309. https://doi.org/10.3389/fnagi.2014.00309
Marquié M, Castilla-Martí M, Valero S et al (2019) Visual impairment in aging and cognitive decline: experience in a memory clinic. Sci Rep 9:8698. https://doi.org/10.1038/s41598-019-45055-9
Ong SY, Cheung CY, Li X et al (2012) Visual impairment, age-related eye diseases, and cognitive function: the Singapore Malay Eye study. Arch Ophthalmol 130:895–900. https://doi.org/10.1001/archophthalmol.2012.152
Swenor BK, Wang J, Varadaraj V et al (2019) Vision impairment and cognitive outcomes in older adults: the health abc study. J Gerontol A Biol Sci Med Sci 74:1454–1460. https://doi.org/10.1093/gerona/gly244
Ferri CP, Jacob KS (2017) Dementia in low-income and middle-income countries: different realities mandate tailored solutions. PLoS Med 14:e1002271. https://doi.org/10.1371/journal.pmed.1002271
World Health Organization (2020) Blindness and vision impairment. https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment. Accessed 25 November 2020
Kowal P, Chatterji S, Naidoo N, et al. SAGE Collaborators (2012) Data resource profile: the World Health Organization study on global AGEing and adult health (SAGE). Int J Epidemiol 41:1639–1649. https://doi.org/10.1093/ije/dys210
Ehrlich JR, Stagg BC, Andrews C et al (2019) Vision impairment and receipt of eye care among older adults in low- and middle-income countries. JAMA Ophthalmol 137:146–158. https://doi.org/10.1001/jamaophthalmol.2018.5449
Albert MS, DeKosky ST, Dickson D et al (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:270–279. https://doi.org/10.1016/j.jalz.2011.03.008
Koyanagi A, Oh H, Vancampfort D et al (2019) Perceived stress and mild cognitive impairment among 32,715 community-dwelling older adults across six low- and middle-income countries. Gerontology 65:155–163. https://doi.org/10.1159/000492177
Morris JC, Heyman A, Mohs RC et al (1989) The consortium to establish a registry for alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 39:1159–1165. https://doi.org/10.1212/wnl.39.9.1159
The Psychological Corporation (2002) The WAIS III-WMS III Updated Technical Manual. San Antonio, TX
Katz S, Ford AB, Moskowitz RW et al (1963) Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 185:914–919. https://doi.org/10.1001/jama.1963.03060120024016
Reyes-Ortiz CA, Kuo YF, Dinuzzo AR et al (2005) Near vision impairment predicts cognitive decline: data from the hispanic established populations for epidemiologic studies of the elderly. J Am Geriatr Soc 53:681–686. https://doi.org/10.1111/j.1532-5415.2005.53219.x
Lara E, Koyanagi A, Olaya B et al (2016) Mild cognitive impairment in a Spanish representative sample: prevalence and associated factors. Int J Geriatr Psychiatry 31:858–867. https://doi.org/10.1002/gps.4398
Bull FC, Maslin TS, Armstrong T (2009) Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health 6:790–804. https://doi.org/10.1123/jpah.6.6.790
Kessler RC, Ustun TB (2004) The world mental health (WMH) survey initiative version of the World Health Organization (WHO) composite international diagnostic interview (CIDI). Int J Methods Psychiatr Res 13:93–121
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders (4th ed.). American Psychiatric Association, Washington, U.S.
Amieva H, Jacqmin-Gadda H, Orgogozo JM et al (2005) The 9 year cognitive decline before dementia of the Alzheimer type: a prospective population-based study. Brain 128:1093–1101. https://doi.org/10.1093/brain/awh451
Alzheimer’s disease international. World Alzheimer report. 2014.
Kivipelto M, Ngandu T, Laatikainen T et al (2006) Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol 5:735–741. https://doi.org/10.1016/S1474-4422(06)70537-3
Gottesman RF, Albert MS, Alonso A et al (2017) Associations between midlife vascular risk factors and 25 year incident dementia in the atherosclerosis risk in communities (ARIC) Cohort. JAMA Neurol 74:1246–1254. https://doi.org/10.1001/jamaneurol.2017.1658
Johansson L, Guo X, Waern M et al (2010) Midlife psychological stress and risk of dementia: a 35 year longitudinal population study. Brain 133:2217–2224. https://doi.org/10.1093/brain/awq116
Koyanagi A, Garin N, Olaya B et al (2014) Chronic conditions and sleep problems among adults aged 50 years or over in nine countries: a multi-country study. PLoS ONE 9:e114742. https://doi.org/10.1371/journal.pone.0114742
Geda YE, Roberts RO, Knopman DS et al (2010) Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol 67:80–86. https://doi.org/10.1001/archneurol.2009.297
Gudlavalleti VS, Allagh KP, Gudlavalleti AS (2014) Self-adjustable glasses in the developing world. Clin Ophthalmol 8:405–413. https://doi.org/10.2147/OPTH.S46057
Petersen RC (2016) Mild cognitive impairment. Continuum (Minneap Minn) 22:404–418. https://doi.org/10.1212/CON.0000000000000313
Acknowledgements
This paper uses data from WHO’s Study on Global Ageing and Adult Health (SAGE). SAGE is supported by the U.S. National Institute on Aging through Interagency Agreements OGHA 04034785, YA1323–08-CN-0020, Y1-AG-1005–01 and through research grants R01-AG034479 and R21-AG034263.
Funding
Dr. Guillermo F. López-Sánchez. Seneca Foundation—Agency for Science and Technology of the Region of Murcia, Spain (20390/PD/17).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
Ethical approval was obtained from the WHO Ethical Review Committee and local ethics research review boards.
Consent to participate
Written informed consent was obtained from all participants.
Research involving human subjects
The study followed the Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Smith, L., Shin, J.I., Jacob, L. et al. The association between objective vision impairment and mild cognitive impairment among older adults in low- and middle-income countries. Aging Clin Exp Res 33, 2695–2702 (2021). https://doi.org/10.1007/s40520-021-01814-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-021-01814-1