Abstract
Previous studies have reported incidence and mortality declines for colorectal cancer (CRC). We evaluated recent temporal trends of colorectal cancer in the United States for the last 4 decades. Using the Surveillance, Epidemiology, and End Results (SEER) database, we identified primary CRCs diagnosed between 1973 and 2015. Temporal changes were evaluated by 6-year time periods. Age-adjusted incidence rates and annual percentage change (APC) for CRC were calculated by site and gender. Age-standardized relative survival rates were also evaluated. We identified 878,632 CRC patients, 51% of whom were men. For both genders, the proportions of new diagnoses of right-sided colon cancer (RCC) remained relatively stable, with the APC of − 0.8 and − 0.6 for the male and the female, respectively. There was a relative increase in RCC for the younger aged group (< 49 years). In contrast, the proportions of left-sided colon cancer (LCC) and rectosigmoid-cancer (RSC) decreased significantly over time. For those aged 0–49, the age adjusted incidence rates showed a small increase (in both genders), whereas age-adjusted incidence rates declined for those aged 50–64 and > 65 (in both genders). Our study showed near significance in the decline of CRC mortality rates in this population, except the 1-year age-standardized survival of LCC and RSC, and the 5-year age-standardized RCC in females. There was a significant increase in RCC for the younger aged group (< 49 years). In contrast, the proportions of LCC and RSC decreased significantly over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common cancer in men and the second most common in women worldwide [1]. Left-sided CRC (LCC) and right-sided CRC (RCC) exhibit different clinical and biological characteristics and are considered as two different entities [2, 3]. Several epidemiological studies have reported that CRC exhibits a proximal or right-sided shift distribution shift [4, 5].
Since 2001, colonoscopy has been the accepted method of screening individuals at an average risk. The risk appears to have accelerated rapidly from a prevalence of 20% in 2000 to 48% in 2008, among Americans 50 years and older [6, 7]. It is recognized that colonoscopy with polypectomy can reduce colon cancer rates by between 76 and 90% [8]. A follow-up study from the National Polyp Study has shown a decrease in mortality rates associated with colonoscopy and polypectomy [9]. Investigation of whether a change in anatomical distribution shift has occurred is important, because this information will guide the current referral practices of effective cancer prevention strategies where colorectal malignancy is suspected. The past decade has witnessed important advances in CRC screening and it is important to acknowledge that in average-risk individuals, screening reduces CRC incidence and mortality [9, 10].
Differences between genders are apparent in the distribution of CRC, with right-sided tumors being more common in women and left-sided and rectal tumors more common in men [11]. Additionally, as CRC predominantly occurs in older people, the incidence and mortality of the disease are expected to increase in this group [11]. Furthermore, although an upward trend was reported in the late 1990s, very little research has been conducted in the first decade of the twenty-first century. Katanoda et al. [12] did include CRC in their most recent report on cancer incidence trends among the Japanese but did not report trends by anatomical sub-site.
Here, we assessed trends in incidence rates of sub-site specific CRC between 1973 and 2015 using a large data resource that incorporated 18 population-based cancer registries. In this study, we evaluated temporal trends over a 40-year period with incident CRC after considering confounding factors of site, race, and gender, in a US population-based study.
Methods
The study was conducted based on a publicly available data set and, therefore, did not require institutional review board approval. This study included 890,192 patients who were initially diagnosed as having incident CRC during 1973–2015 with 18 cancer centers reporting to the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute, USA. We collected information for each incident cancer, including patient demographics, the primary CRC site, pathological classification according to the International Classification of Diseases for Oncology [13], tumor stage at diagnosis, limited treatment information, and survival data. Only cancers with histological diagnoses of adenocarcinoma and mucinous (signet ring) carcinoma were included. Tumor sites were classified as follows: right-sided colon cancer (RCC, cecum, ascending colon, hepatic flexure, and transverse colon); left-sided colon cancer (LCC, splenic flexure, descending colon, and sigmoid colon), and rectosigmoid colon cancer (RSC, rectosigmoid junction and rectum). We used the SEER summary stage for the analysis, which has been verified across multiple tumor types, and is very consistent with survival [14]. The SEER stage included localized (malignant but confined to primary organ), regional (invasion beyond primary organ but no distant metastasis), or distant (with distant metastasis) and unknown status (when data were unavailable or stage was defined > 4 months after initial diagnosis). Using the direct adjustment method [15], annual percentage change (APC) and age-adjusted incidence rates by site, race, and gender for the 6-year time periods were computed using population estimates derived from the SEER Program. These were then adjusted to the 1970 US population. The proportions of new diagnoses were calculated by site, and age group (< 50, 50–64, > 65) at diagnosis for 6-year time periods. Calculations were performed using SEER*Stat version 2.0 software, which is the statistical package created for the analysis of the SEER database, and SAS version 8.2 (SAS Institute Inc, Cary, NC). P < 0.05 was considered statistically significant and P values were two sided.
Overall survival (OS)
For overall survival (OS), 1-year, 3-year, and 5-year age standardized relative survival rates [15] were determined by comparing the patient groups diagnosed from 1973 to 2015.
Results
Study population
878,632 CRC individuals were identified in our analysis during 1973–2015 and the mean age was 70 years, with 51% being men. The ratio of the three sites was as follows: LCC 30.0%; RCC 39.8; RSC 30.2%. When classified by age, 8.5% were aged < 50, 27.2% were middle-aged (50–64), and 64.3% were classified as older people (> 65).
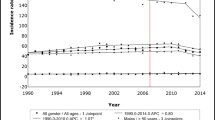
Incidence rate trends for CRC incidence rate is shown in Fig. 1. The overall ASRs for CRC per 100,000 population were 551.5. Over the ensuing period, these rates increased remarkably, with an APC of 1% (95% CI 1–1.6, P < 0.001) until 1985. The rates then decreased significantly until 2010, even though small upward between 1995 and 1997.
RCC incidence rates
Trend analysis according to age groups (for RCC, LCC, and RSC) is shown in Fig. 1a–d. From the beginning of the study period, the ASRs of RCC stable from 1973 to 1999, with APCs of 1.6% (95% CI 1.2–2.2, P < 0.001, from 1973 to 1983). − 0.4% (95% CI − 0.7 to − 0.2, P < 0.001, from 1983 to 1996), and 1.7% (95% CI − 1.8 to 5.3, P < 0.001, from 1996 to 1999) (Fig. 1a; Table 1b). Although the APCs in RCC were increasing over these three periods, they remained negative, meaning that RCC constantly decreased since 1999 (APC = − 1.9 for 1999–2008 and APC = − 4.7 for 2008–2015). Similar trends were observed for both genders (Tables 2, 3).
The trend was not always seen across all three age groups. The overall declines were primarily driven by large decreases in the incidence of disease in the oldest age groups, by − 0.8 per year (95% CI − 1.1 to − 0.5). For the younger age group, there was an overall increase in the incidence rate (APC = 0.5), especially after 2010 (APC = 6.8). For the middle-age group, the overall incidence remained roughly stable (Fig. 1b).
The trend was similar in both men and women, by − 0.8% per year (95% CI 2.0–3.2) in men (Table 2) and by − 0.6% per year (CI − 0.8 to − 0.4) in women (Table 3). There was a tremendous increase in females after 2013 (APC = 24.8, 95% CI − 1.9–58.7).
LCC incidence rates
Decreased ASRs were observed in LCC, decreasing sharply from 1985 to 2013 with an APC of –2% (95% CI –1.3 to –2.7, P < 0.001). The APCs of 1.6% (95% CI 1.2–2, P < 0.001, 1973–1985) and APC of 0.7 (− 5.8 to 7.5) were noted for this time period (Table 1). Similar trends were observed by sex, except that the incidence rate in female patients decreased from 1995 with no increase between 2013 and 2015 (Tables 2, 3).
Incidence rates for younger aged LCC cases decreased significantly during the 1973–1993 period by approximately 1.2% and 2.3% per year, in men and women, respectively. They then moderately increased for between 1996 and 2015. Similarly, a rising incidence between 1973 and 1986 was evident in the eldest age group, with a sustained reduction from 1986. In contrast, there was a reduction during the 1973–1981 period by approximately 2.5% and 0.6% per year, in men and women, respectively. There was then a moderate decrease from 1981 to 2015 for the middle-aged LCC group.
RSC incidence rates
The incidence for RSC fluctuated, with a decrease in 1985–1995 (APC = − 2.1), then increases from 1995–1998 (APC = 2.2), and 1998 (APC = − 2.2). Increases were seen both in women (APC = − 1.3) and men (APC = − 1.4) (Fig. 1a; Tables 1, 2, 3).
For the younger group, a substantial increase was observed from 1994 to 1997, with an APC of 8.2 and these rates remained over the remaining period, with an APC of 2% (95% CI 1.6–2.5, P < 0.001) until 2015. The same trend occurred for both men and women within the younger group with an annual increase of 1.6% in men and 1.5% in women.
For the middle-aged group, the proportion of new RSC diagnoses during 1973–1985 was 34% (95% CI 33–34), and decreased to 31% (95% CI 29.0–33) during 1990–2015 (P < 0.0001). The annual incidence continued to decrease in women in the middle-aged group with an annual APC of 0.6%. However, there was a slight increase (1995–1998, APC = 4) in the men, although the overall incidence decreased over the entire period (APC = − 0.6).
The overall incidence in the oldest age group of patients was generally decreasing though there were transient increases in 1973–1979 and 1994–1999. The annual decrease in the old-aged patients was the main contributor for the RSC decrease. Similar trends were observed in both genders.
Incidence rates by age
Distinct patterns of incidence were found when incidence rates were evaluated by age-groups. The overall incidence of colorectal tumors in those < 50 generally increased during 1973 to 2015 at annual rates of 0.9% in men and 0.6% in women, though an interruption occurred from 1973 to 1994 in both sexes (Table 4). For the middle-aged cohort, there was a persistent decrease in the 2000–2012 time period (APC = − 2), and then a germinal increase after 2012 (APC = 2.1). However, in middle-aged females, there is a submit in 2009–2012 with annual rate of 3.4, then a decrease from 2012. For patients in the most senior age group, there has been a large decrease since 1998, despite a sudden increase in 1995–1998.
Cancer-specific survival by sub-site
Overall age-standardized relative survival of patients after CRC diagnosis was found to have significantly improved between 1973 and 2015, for both genders. There was a dramatic increase between 2004 and 2015 compared with 1973–1998, for all sub-sites in both sexes. However, the overall temporal trends between 2004–2010 and 2010–2015 were very similar. No significant differences were observed in the age standardized relative survival, for both men and women (Table 5).
We then compared the survival outcomes between 2004–2015 and 1973–1998. One-year age standardized survival of patients after RCC diagnosis in the period 2004–2015 significantly increased compared with survival after diagnosis in the period 1973–1998, both for males and females. The 1-year survival rate for RCC in males and females was 77% and 81% in 2004–2015 compared to only 66.9% and 67.9% for period 1973–1998, respectively. However, no significant differences were observed in the 1-year age standardized relative survival rates in female patients with RCC and RSC (Table 5). Three-year age standardized survival of patients after diagnosis in the period 2004–2015 significantly increased compared with survival after diagnosis in the period 1973–1998, both for males and females. This was noted for all sub-sites.
Five-year age standardized relative survival of male patients after LCC and RSC diagnosis in both genders across the period 2004–2015 significantly increased compared with survival after diagnosis in the period 1973–1998. The 5-year survival rates for LCC and RSC were 43.4% and 38.8% between 1973 and 1998, respectively. The 5-year survival rates were slightly higher in 2004–2015, with 55% (95% CI 47–57) vs. 55.5% for LCC and RSC, respectively. For the RCC, only in males were there significant differences in the 5-year age standardized relative survival rates (Table 5).
Discussion
Over a 40-year period (1973–2015) the overall tumor location shifted in different age groups. A relative increase in the proportion of RCC was observed, accompanied by a significant decline in the proportion of LCC and RSC. The RCC shift over time was specifically attributed to an increase in the age 0–49 age group. The LCC and RSC declines may result in lower incidence in the older group. The age-standardized survival was approaching significance in the decline of CRC mortality rates in this population.
There were no temporal changes in age-specific rates over time for RCC. The current study does not address the causes of changes in the distribution of CRC over time. However, we may speculate upon the following aspects. First, the RCC shift over time is attributed to a decline in the incidence of LCC, rather than a true increase in the incidence of RCC. The second explanation is the ageing structure-change of the population over time, i.e., an increasingly ageing proportion in recent years, who have a higher RCC rate [11]. Additionally, the RCC shift may be linked to the general use of colonoscopy for screening, previously postulated by Gross and colleagues [6]. However, complete endoscopic examination is more technically arduous for the right-sided colon than the left-sided colon even though non-polypoid lesions are more prevalent in the right-sided colon [16, 17]. These factors may have attenuated the declines in right-sided colon cancer incidence rates because of the efficacy of colonoscopy in the right-sided colon.
As we noted, the highest trend for RCC shift was noted to be in the younger age group. In 1998, 90% of Americans aged 65 years and older were insured by the Medicare program, where colonoscopy screening was recommended for individuals at high-risk of CRC; in 2001, beneficiaries at average risk were was expanded for insurance coverage, and this accelerated colonoscopy utilization [18, 19]. This may have been a factor influencing the incidence of RCC in younger people. Additionally, colonoscopy testing requires adequate bowel cleansing, which is necessary for complete visualization of the colonic mucosa. This is more difficult in the right-sided colon [20]. Older patients are not as well prepared for bowel preparation and at substantially higher risk for RCC [21]. However, improved techniques and knowledge of colonoscopy over time with intubation completeness are associated with lower miss rates, and this may have also contributed to improved protection against right-sided tumors in older people. As a consequence, this may result in a relative increase of RCC in younger people [21].
Temporal patterns of screening use are similar by gender, although reported testing is slightly higher in men than for women. For example, in 2008, 50% of men and 47% of women reported recent use of colonoscopy, whereas sigmoidoscopy rates were 3% and 2%, respectively [22]. Our study shows a decrease in RCC incidence rates in 2008–2015 (APC = − 4.7) that coincides with the increase in colonoscopy uptake.
We found that the upward trends in LCC and RSC existed in those aged 0–49 years, both in men and women. We also noted that there was a less drastic change in RSC incidence during the 1990s in those aged 65 years. CRC screening rates were relatively low in those younger than 49 years. Increasing the screening rate especially for those younger than 49 years is not recommended for those persons at average risk [23]. This has clear implications for the choice of CRC screening method. We recommend that for average-risk individuals, even though they may be under 49, they should have an annual FOBT, flexible sigmoidoscopy every 5 years, and colonoscopy every 10 years.
Although the incidence and mortality of CRC are expected to increase by age group [11], we found a decreasing trend in the middle-aged and older aged group, especially the older aged group. One potential explanation for the observed findings may be the lifestyle changes, which include changes in diet (decreased consumption of red/processed meats [24], alcohol [25]), and improved lifestyle (increase in physical activity and decrease in smoking). In addition, the use of medications, such as nonsteroidal anti-inflammatory drugs (NSAID) [26], as well as hormone replacement therapy in women, may have initiated the decrease [26]. Other explanations may be the general use of the colposcopy for whom screening is recommended for persons at average risk [22, 23]. Further studies are needed to resolve these issues.
There are two major limitations of our study. One is the detailed analysis of the stage group since this factor could influence prognosis. However, this is a population based (SEER data account for 11% of the US population), and therefore the results can be generalized to the United States. However, we had analyzed data over a 40-year period, we were able to assess temporal trends.
In conclusion, the proximal migration of CRC over time in all age groups and both genders was not attributed to a true increase in the incidence of right-sided CRC. It is best explained by either a decline in the incidence of LCC and RSC and/or the aging of the population. The increase in disease trend with time is more significant in the younger aged group, in whom the majority of CRCs occurred in the left-sided colon. Our study showed near significance in the decline in CRC mortality rates in this population, except for the 1-year age-standardized of LCC and RSC, and the 5-year age-standardized RCC in females. The potential mechanisms require further study.
Conclusions
These data represent an update of earlier studies with additional data on tumor site and CRC survival. There was a significant increase in RCC disease for the younger-aged group (< 49 years). In contrast, the proportions of LCC and RSC decreased significantly over time, may result from lower incidence in the older group. Our study showed near significance in the decline in CRC mortality rates in this population.
References
Lao VV, Grady WM (2011) Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol 8:686–700
Iacopetta B (2002) Are there two sides to colorectal cancer? Int J Cancer 101:403–408
McCashland TM, Brand R, Lyden E et al (2001) Gender differences in colorectal polyps and tumors. Am J Gastroenterol 96:882–886
Cheng L, Eng C, Nieman LZ et al (2011) Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am J Clin Oncol 34:573–580
Cress RD, Morris C, Ellison GL et al (2006) Secular changes in colorectal cancer incidence by subsite, stage at diagnosis, and race/ethnicity, 1992–2001. Cancer 107:1142–1152
Gross CP, Andersen MS, Krumholz HM et al (2006) Relation between Medicare screening reimbursement and stage at diagnosis for older patients with colon cancer. JAMA 296:2815–2822
Ko CW, Kreuter W, Baldwin LM (2002) Effect of Medicare coverage on use of invasive colorectal cancer screening tests. Arch Intern Med 162:2581–2586
Rex DK, Eid E (2008) Considerations regarding the present and future roles of colonoscopy in colorectal cancer prevention. Clin Gastroenterol Hepatol 6:506–514
Winawer SJ, Zauber AG, Ho MN et al (1993) Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 329:1977–1981
Selby JV, Friedman GD, Quesenberry CJ et al (1992) A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med 326:653–657
Cook AD, Single R, McCahill LE (2005) Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol 12:637–645
Katanoda K, Hori M, Matsuda T et al (2015) An updated report on the trends in cancer incidence and mortality in Japan, 1958–2013. Jpn J Clin Oncol 45:390–401
Ashley J (1990) The international classification of diseases: the structure and content of the tenth revision. Health Trends 22:135–137
Lo NS, Sarr MG (2003) Mucinous cystadenocarcinoma of the appendix. The controversy persists: a review. Hepatogastroenterology 50:432–437
Ogburn EL, Zeger SL (2016) Statistical reasoning and methods in epidemiology to promote individualized health. In celebration of the 100th anniversary of the Johns Hopkins Bloomberg School of Public Health. Am J Epidemiol 183:427–434
Soetikno RM, Kaltenbach T, Rouse RV et al (2008) Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA 299:1027–1035
Matuchansky C (2009) Computed tomographic colonography for detecting advanced neoplasia. JAMA 302:1528–1529
Phillips KA, Liang SY, Ladabaum U et al (2007) Trends in colonoscopy for colorectal cancer screening. Med Care 45:160–167
Schenck AP, Peacock SC, Klabunde CN et al (2009) Trends in colorectal cancer test use in the medicare population, 1998–2005. Am J Prev Med 37:1–7
Rostom A, Jolicoeur E, Dube C et al (2006) A randomized prospective trial comparing different regimens of oral sodium phosphate and polyethylene glycol-based lavage solution in the preparation of patients for colonoscopy. Gastrointest Endosc 64:544–552
Boursi B, Halak A, Umansky M et al (2009) Colonoscopic screening of an average-risk population for colorectal neoplasia. Endoscopy 41:516–521
Siegel RL, Jemal A, Ward EM (2009) Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev 18:1695–1698
Edwards BK, Ward E, Kohler BA et al (2010) Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 116:544–573
Larsson SC, Wolk A (2006) Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer 119:2657–2664
Ferrari P, Jenab M, Norat T et al (2007) Lifetime and baseline alcohol intake and risk of colon and rectal cancers in the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer 121:2065–2072
Chan AT, Giovannucci EL, Meyerhardt JA et al (2005) Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA 294:914–923
Grodstein F, Newcomb PA, Stampfer MJ (1999) Postmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis. Am J Med 106:574–582
Acknowledgements
We would like to thank the native English speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grants 81272641, 81572409, 81472578, 81773051).
Author information
Authors and Affiliations
Contributions
Conception and design: LY, BZ, and L-PX; methodology: LY, S-SL, Z-CX; collection and assembly of data: LY, S-SL, Z-CX, H-JC, W-ZH, Q-KX; data analysis and interpretation: LY, S-SL, CJ, Q-KX; manuscript writing: all authors; final approval of manuscript: all authors. All authors (LY, S-SL, Z-CX, H-JC, W-ZH, Q-KX, CJ, BZ, L-PX) have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors have no conflicts of interest to declare.
Ethics approval and consent to participate
As the data used were from SEER dataset, which is publicly available, ethics approval and consent to participate is not applicable.
Statement of human and animal rights
The research involving humans was carried out according to the principles of the Declaration of Helsinki.
Informed consent
Informed consent was not applicable in this retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, L., Liu, S., Xiong, Z. et al. Changes in colorectal cancer incidence by site and age from 1973 to 2015: a SEER database analysis. Aging Clin Exp Res 33, 1937–1946 (2021). https://doi.org/10.1007/s40520-020-01721-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-020-01721-x