Abstract
Background
It was reported that inhaled corticosteroids (ICS) treatment may affect local immunity and microbial community of the airway. However, whether ICS treatment increases the risk of influenza in patients with asthma remains unclear. This meta-analysis aimed to compare the risk of influenza between ICS and non-ICS treatment in patients with asthma.
Methods
PubMed, Embase, Cochrane Library and Clinical Trials.gov were searched from inception until November 2019. Randomized controlled trials (RCTs) were included that compared ICS treatment with non-ICS treatment on the risk of influenza in patients with asthma. Meta-analyses were conducted by the Peto approach and Mantel–Haenszel approach with corresponding 95% CIs.
Results
Nine trials involving 6486 patients were included in this meta-analysis. The risk of influenza was not different between ICS treatment and the control groups (Peto OR: 1.01, 95% CI 0.74–1.37, P = 0.95). The results of subgroup analyses based on durations (long-term and short-term treatment), doses (high-, medium- and low-dose treatment) and types (fluticasone and budesonide treatment) of ICS were consistent with the above pooled results. Moreover, subgroup analysis based on patients’ age also revealed that use of ICS did not increase the risk of influenza. Results of the two meta-analysis approaches were similar.
Conclusions
Use of ICS does not increase the risk of influenza in patients with asthma. This study adds to safety evidence of ICS as a regular controller treatment for patients with asthma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asthma is a common, chronic respiratory disease affecting 1–18% of the population in different countries. Inhaled corticosteroids (ICS) constitute the cornerstone of asthma treatment [1, 2]. Although ICS treatment is generally considered safe and well tolerated in patients, regular use of ICS may affect local immunity and lead to respiratory infections of the patients [3]. Recently, ICS-related respiratory infections have been widely concerned. Development of pneumonia [4, 5], tuberculosis [6], and upper respiratory tract infection [7] due to daily use of ICS have been observed in patients with chronic obstructive pulmonary disease (COPD). Fewer studies assessed the association between ICS treatment and the risks of various respiratory infections in patients with asthma. McKeever et al. reported increased risks of pneumonia and lower respiratory tract infections in patients with asthma using ICS [8]. A meta-analysis of 4 observational studies involving 44,016 participants revealed a significantly increased risk of pneumonia in patients with asthma [9]. In addition, a significantly increased risk of upper respiratory tract infection has been observed in patients with asthma [10]. However, to our knowledge, no study has systematically assessed the possible link between use of ICS and the risk of influenza in patients with asthma.
Influenza, one of the most common respiratory infectious, is a highly contagious disease caused by influenza viruses which can be severe, and result in hospitalization, even death [11, 12]. Influenza causes an estimated 5 million severe cases and 500 thousand deaths each year worldwide [13]. Patients with asthma are more susceptible to severe influenza due to chronic airway inflammation and type 2 immune responses [14, 15]. Moreover, influenza may lead to severe asthma attacks [16]. Clarifying the possible link between ICS treatment and risk of influenza is helpful to guide the medication of asthmatic patients. Therefore, we conducted this meta-analysis of all available randomized controlled trials (RCTs) to assess the association between the effects of various doses and types of ICS on the risk of influenza in patients with asthma.
Methods
The study protocol was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [17].
Search strategy
Two reviewers (HC, ZX) independently searched PubMed, Embase, Cochrane Library and Clinical Trials.gov from inception until November 2019. To maximize the search for relevant references, we also conducted a manual search using the reference lists of key articles. The search strategy was as follows: asthma AND (“inhaled corticosteroids” OR “ICS” OR “fluticasone” OR “flunisolide” OR “budesonide” OR “beclomethasone” OR “mometasone” OR “ciclesonide” OR “triamcinolone”) and clinical trial design. Disagreements between two reviewers were resolved by discussion, and consultation with a third investigator if necessary (LH). The search was limited to English language publications in human subjects.
Eligibility criteria
Eligible studies were identified through the PICOS criteria (participants, interventions, comparators, outcomes and study design) [17]. The inclusion criteria included: (1) patients with asthma of any severity; (2) RCTs; (3) the interventions included ICS, including ICS alone or as an ingredient, with non-ICS treatment as a control (including placebo or other inhaled drugs of corticosteroid free); (4) RCTs providing data on influenza. The exclusion criteria included: (I) non-RCTs, such as observational studies, case series and reviews; (II) patients with COPD or bronchiectasis or ambiguous diagnosis; (III) ICS were used in both the treatment group and the control group; (IV) non-English articles.
Data collection process and risk of bias assessment
Two reviewers (HC, JY) independently and in duplicate extracted relevant data from the included trials. The risk of bias of the included RCTs was assessed using the Cochrane Collaboration risk of bias tool [18]. The included RCTs were assessed according to the following features: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment;(5) selective reporting; (6) incomplete outcome data; (7)other bias. Each item was assessed as low, unclear, or high risk of bias. Disagreements between the two reviewers were resolved by discussion, and a third investigator (KW) was consulted if necessary. The corresponding authors were contacted when relevant data were not available.
Statistical analysis
A meta-analysis was conducted to evaluate whether exposure to ICS was associated with the risk of influenza. The statistical analyses were performed using the Review Manager software (version 5.3.3, Cochrane Collaboration). As the Peto OR approach provides the best confidence interval (CI) coverage when events are rare [19], we calculate the pooled Peto OR with 95% CI for the comparison of ICS treatment vs non-ICS treatment. To account for the potential imbalance of sample size of the included trials and interpret the results more intuitively, we also computed the pooled risk ratio (RR) for the comparison using the Mantel–Haenszel approach [20]. Moreover, we conducted multiply subgroup analyses to minimize the influences of clinical heterogeneity. A two-tailed P value of less than 0.05 was set for statistical significance. Statistical heterogeneity was assessed using the I2 test, with I2 ≥ 50% being considered substantial [21]. A random-effect model would be selected when a substantial statistical heterogeneity was found. The GRADE profiler (version 3.6, GRADE working group) was used to assess the quality of the evidence provided by the results [22].
Results
Study selection and study characteristics
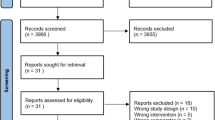
Figure 1 shows the study selection process. Nine published RCTs met the eligibility criteria and were finally included in the meta-analysis [23,24,25,26,27,28,29,30,31]. The 9 included trials enrolled 6486 subjects, of whom 4824 received ICS treatment and 1662 received non-ICS treatment. Of the nine RCTs, eight were multicenter, double-blind, randomized trials. The included trials were published from 2008 to 2019, with population sizes raging from 242 to 2258 subjects. Duration of the included trials ranged from 1.5–12 months, with four trials longer than 6 months, and 5 shorter than 6 months. Of the nine RCTs, two involved the use of high-dose ICS (defined as > 500 ug/day of fluticasone propionate or equivalent), two involved medium-dose ICS (defined as > 250–500 ug/day of fluticasone propionate or equivalent) six involved low-dose ICS (defined as 100–250 ug/day of fluticasone propionate or equivalent) [1]. Of the nine RCTs, six RCTs evaluated fluticasone treatment, three RCTs evaluated budesonide treatment and one RCT evaluated mometasone treatment. The characteristics of the included studies are presented in Table 1.
Risk of bias and quality of evidence
All RCTs were assessed using the Cochrane Collaboration risk of bias assessment tool. The results of risk of bias are presented in Fig. 2a, b. Eight RCTs had a low risk of bias. One RCT had a high risk for performance bias and an unclear risk for detection bias, and one RCT had an unclear risk for allocation concealment. Three RCTs had an unclear risk due to other bias, mainly because of the potential funding bias. Quality of the evidence provided by the results assessed by GRADE is presented in Table 2.
Use of ICS and risk of influenza
For nine included RCTs, the crude risk of influenza was 4.1% (197 of 4824 patients) in the patients receiving ICS treatment, 3.7% (62 of 1662 patients) in the patients receiving non-ICS treatment, and 4% in all patients (259 of 6486 patients). No significant association was found between use of ICS and risk of influenza by the Peto approach (Peto OR: 1.01, 95% CI 0.74–1.37, P = 0.95) (Fig. 3). The result was rated as high-quality evidence by GRADE assessment (Table 2). Result of the Mantel–Haenszel approach also revealed no significant difference in the risk of influenza between the ICS treatment group and non-ICS treatment group (RR: 1.01, 95% CI 0.76–1.35, P = 0.95) (Table 3). There was no obvious heterogeneity among the studies (I2 = 0%).
ICS treatment of different durations and risk of influenza
Of the eligible RCTs, four trials assessed the long-term use of ICS, and five trials assessed the short-term ICS treatment. According to the Peto approach, long-term use of ICS was not associated with the risk of influenza compared to the control group (Peto OR: 1.05, 95% CI 0.75–1.48, P = 0.76) (Fig. 4, Table 3). The pooled result based on the RCTs related to short-term ICS treatment was also consistent with the above (Peto OR: 0.85, 95% CI 0.42–1.69, P = 0.63) (Fig. 4, Table 3). The above results were rated as high-quality evidence by GRADE assessment (Table 2). The results of Mantel–Haenszel approach were also consistent with the results calculated by Peto OR approach (Table 3). There was no obvious heterogeneity among the studies (all I2 < 50%).
ICS treatment of different doses and risk of influenza
Of the eligible trials, two trials assessed the use of high-dose ICS, two trials assessed the medium-dose ICS treatment, and six trials assessed the low-dose ICS treatment, respectively. No significant dose–effect relationship was found. According to the Peto approach, high-dose ICS treatment was not associated with the risk of influenza compared to the control group (Peto OR: 0.68, 95% CI 0.16–2.86, P = 0.6) (Fig. 5, Table 3). The pooled results based on the RCTs related to medium-dose ICS (Peto OR: 1.14, 95% CI 0.41–3.18, P = 0.8) treatment and low-dose ICS treatment (Peto OR: 1.03, 95% CI 0.75–1.42, P = 0.86) were also consistent with the above (Fig. 5, Table 3). The above results were rated as moderate, moderate- and high-quality evidence by GRADE assessment (Table 2). The results of Mantel–Haenszel approach were also consistent with the results calculated by Peto OR approach (Table 3). There was no obvious heterogeneity among the studies (all I2 < 50%).
ICS of different types and risk of influenza
Of the eligible RCTs, six trials assessed fluticasone treatment, and three trials assessed budesonide treatment. According to the Peto approach, fluticasone treatment was not associated with an increased risk of influenza compared to the control group (Peto OR: 0.84, 95% CI 0.56–1.25, P = 0.38) (Fig. 6, Table 3). Budesonide treatment was also not associated with a significant effect on the risk of influenza compared to the control group (Peto OR: 1.3, 95% CI 0.82–2.08, P = 0.27) (Fig. 6, Table 3). The above results were rated as high- and moderate-quality evidence by GRADE assessment (Table 2). The results of Mantel–Haenszel approach were also consistent with the results calculated by Peto OR approach (Table 3). There was no obvious heterogeneity among the studies (all I2 < 50%).
ICS treatment and risk of influenza in different age subgroups
Of the eligible RCTs, eight trials assessed ICS treatment in adults and adolescents (12 years and older, ranging from 12 to 81 years), and one trial assessed ICS treatment in children (6–11 years). According to the Peto approach, ICS treatment did not increase the risk of influenza in adults and adolescents (Peto OR: 1.01, 95% CI 0.74–1.38, P = 0.95) (Fig. 7, Table 3), or did it increase the risk of influenza in children (Peto OR: 1, 95% CI 0.25–4.06, P = 1) (Fig. 7, Table 3). The above results were rated as high- and moderate-quality evidence by GRADE assessment (Table 2). The results of Mantel–Haenszel approach were also consistent with the results calculated by Peto OR approach (Table 3). There was no obvious heterogeneity among the studies (I2 < 50%).
Discussion
To our knowledge, this study is the first meta-analysis to systematically assess the association between the effects of various doses and types of ICS on the risk of influenza in patients with asthma. This meta-analysis of 9 RCTs (including 6486 patients) demonstrated that use of ICS was not associated with an increased risk of influenza compared to the non-ICS treatment. Considering the pooled result may not avoid heterogeneity due to the variables of medication details, subgroup analyses were conducted based on durations, doses and types of ICS. The results of subgroup analyses further verified the above pooled results. To ensure the reliability of the study, we used two different meta-analysis approaches (Peto OR approach and Mantel–Haenszel approach) to calculate the pooled results, and the results were similar with each approach. These results add to safety evidence of ICS as a regular treatment for patients with asthma, which may reduce insufficient use of ICS and help achieve better control of disease in patients with asthma.
A locally high concentration of ICS in the respiratory tract and lung may have immunosuppressive effects and thus lead to respiratory infections. Studies have suggested that corticosteroids could inhibit macrophage functions [32], suppress the activation of T cells in the airways [33, 34], and induce the apoptosis of dendritic cells [35]. Therefore, it is seemingly plausible that ICS treatment may increase the risk of respiratory infections in patients using ICS regularly. There have been growing concerns about the association between ICS treatment and the risk of respiratory infections. Large meta-analyses of RCTs have demonstrated that use of ICS could significantly increase the risk of pneumonia [4, 5], tuberculosis [6] and upper respiratory tract infection [7] in patients with COPD. However, there are fewer studies assessing the possible link between ICS treatment and respiratory infections in patients with asthma. It could be speculated that the possible immunosuppressive effects due to ICS also exists in patients with asthma, which may lead to higher risk of respiratory infections for the asthmatic patients. A cross-sectional study reported that children with asthma taking ICS regularly were almost four times more likely to have oropharyngeal streptococcus pneumonia colonization than those not taking ICS [36]. A meta-analysis of observational studies also revealed a significant increased risk of pneumonia in patients with asthma associated with ICS treatment [9]. Moreover, in 2018, Yang et al. published a meta-analysis including 17 RCTs (15,336 subjects) and reported that ICS treatment significantly increased the risk of upper respiratory tract infection [10]. Studies have reported that patients with asthma are more susceptible to influenza, especially some cases with poor controlled asthma condition may result in severe influenza or even death [12, 14]. However, whether ICS treatment increases the risk of influenza in patients with asthma remains unclear. Since influenza is highly contagious and also one of the most common respiratory infectious, which can be severe, and result in hospitalization, even death, it is important to clarify the possible link between ICS treatment and the risk of influenza. Especially, a recent Cochrane systematic review suggested uncertainty about the effectiveness of influenza vaccination in patients with asthma [37]. Therefore, it is worthy to conduct a meta-analysis of all available RCTs to assess the association between use of ICS and risk of influenza in patients with asthma. However, the results of our meta-analysis are the opposite of the above meta-analyses related to the risk of respiratory infections in patients with COPD or asthma. The pooled results revealed that ICS treatment did not significantly increase the risk of influenza in patients with asthma. In addition, the results of subgroup analyses based on different treatment durations, different doses, different types of ICS and different age subgroups were also consistent with the above pooled results. Our findings could be used for medication reference in the management of asthma.
The results were unexpected. It is not clear why use of ICS increases the risk of some kinds of respiratory infections, such as pneumonia and tuberculosis but not influenza. Possible explanations can be considered. First, influenza is an acute respiratory infection, in which innate immunity of human plays a fundamental role, because type I innate response is essential to limit influenza virus replication and spread [38]. One study by Schleimer et al. suggested that corticosteroids might effectively suppress the adaptive immunity of the airway epithelium but not the innate immunity [35]. Second, use of oral steroids may be a potential confounder, since patients taking placebo are more likely to using oral steroids of higher dose because of a poorer asthma control [39]. Unfortunately, data about the oral steroids used in the included trials can not be obtained. Therefore, further studies are needed to reevaluate the association between ICS treatment and risk of influenza in patients with asthma. Our findings support the findings of a previous meta-analysis of RCTs conducted by Dong et al., which revealed use of ICS was not associated with the risk of influenza compared to the non-ICS treatment in patients with COPD [40]. Moreover, we further verified the association between ICS treatment and risk of influenza through stratified analyses based on durations, doses and types of ICS, which ensured the reliability and precision of the conclusion.
However, our results were not consistent with the large Toward a Revolution in COPD Health (TORCH) trial, which suggested that long-term use of ICS may increase the likelihood of influenza in patients with COPD. We suggest two possible explanations. First, compared to the COPD patients included in their study (mainly severe or very severe COPD patents), the airway inflammation of almost all asthmatic patients can be better controlled with ICS. Second, another possible explanation might be that the demographic characteristics of patients between the two studies varied widely, especially the patients included in our study were mainly young asthmatic adults whereas in their study were dominantly the aged COPD patients with a greater burden of comorbidities. Similarly, in 2017, Cazeiro et al. published a meta-analysis including a total of 31 randomized trials enrolling 11,615 children with asthma. Findings in their study reported that regular ICS treatment may not increase the respiratory infections in children with asthma [39].
Due to the differences in immune status, pathophysiology and treatment between asthmatic adults and adolescents (12 years and older) and asthmatic children (6–11 years), the Global Initiative for Asthma (GINA) guideline has made recommendations on the use of ICS for them, respectively. Considering that age may be associated with the risk of influenza after ICS use, we conducted a subgroup analysis according to the age subgroups. Our results showed that ICS treatment did not increase the risk of influenza in adults and adolescents or children, which further confirmed the conclusion that ICS did not increase the risk of influenza in patients with asthma.
A major strength of this meta-analysis was that we used a comprehensive search strategy and explicit eligibility criteria including all available RCTs, thus enhance the generalizability. In addition, the rigorously use of the GRADE approach to rate the quality of evidence provided by the results. In addition, an assessment of the results stratified by various medication details ensured the reliability of the study. Moreover, we used two different meta-analysis approaches to calculate the pooled results, which also ensured the reliability of the study. As far as we know, this is the first meta-analysis to assess the association between the effects of various doses and types of ICS on the risk of influenza in patients with asthma.
This study had some limitations mainly owing to the challenges of assessing drug safety in clinical trials [41]. First, the results of the meta-analysis were weakened by the sample size. Only one trial assessed mometasone treatment, which prevented us from performing further subgroup analysis of mometasone treatment. Second, significant clinical heterogeneity weakened the results of this meta-analysis. The baseline characteristics and follow-up periods of the included studies varied, which may limit the generalizability of the present results. However, we conducted multiply subgroup analyses to minimize the influences of clinical heterogeneity. Finally, underreporting of adverse events is common in most clinical trials. And this inherent methodological defect of clinical trials is the main factor limiting the results of all meta-analysis of drug safety. However, as a result of blind outcome assessment, the underestimate of incidence of influenza could not substantially affect the results of the meta-analysis as underreporting may occur equally in both treatment group and the control group.
Conclusion
In conclusion, our results reveal that, use of ICS does not increase the risk of influenza in patients with asthma. These results add to safety evidence of ICS as a regular treatment for patients with asthma.
Code availability
Not applicable.
References
Global Initiative for Asthma (2019) Global strategy for asthma management and prevention. https://ginasthma.org. (Accessed 26 June 2019).
Thomson NC, Spears M (2013) Inhaled corticosteroids for asthma: on-demand or continuous use. Expert Rev Respir Med 7:687–699. https://doi.org/10.1586/17476348.2013.836062
Fukushima C, Matsuse H, Saeki S et al (2005) Salivary IgA and oral candidiasis in asthmatic patients treated with inhaled corticosteroid. J Asthma 42:601–604. https://doi.org/10.1080/02770900500216259
Singh S, Amin AV, Loke YK (2009) Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta-analysis. Arch Intern Med 169:219–229. https://doi.org/10.1001/archinternmed.2008.550
Yang M, Du Y, Chen H et al (2019) Inhaled corticosteroids and risk of pneumonia in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Int Immunopharmacol 77:105950. https://doi.org/10.1016/j.intimp.2019.105950
Ni S, Fu Z, Zhao J et al (2014) Inhaled corticosteroids (ICS) and risk of mycobacterium in patients with chronic respiratory diseases: a meta-analysis. J Thorac Dis 6:971–978. https://doi.org/10.3978/j.issn.2072-1439.2014.07.03
Yang M, Chen H, Zhang Y et al (2017) Long-term use of inhaled corticosteroids and risk of upper respiratory tract infection in chronic obstructive pulmonary disease: a meta-analysis. Inhal Toxicol 29:219–226. https://doi.org/10.1080/08958378.2017.1346006
McKeever T, Harrison TW, Hubbard R et al (2013) Inhaled corticosteroids and the risk of pneumonia in people with asthma: a case-control study. Chest 144:1788–1794. https://doi.org/10.1378/chest.13-0871
Bansal V, Mangi MA, Johnson MM et al (2015) Inhaled corticosteroids and incident pneumonia in patients with asthma: systematic review and meta-analysis. Acta Med Acad 44:135–158. https://doi.org/10.5644/ama2006-124.141
Yang M, Zhang Y, Chen H et al (2019) Inhaled corticosteroids and risk of upper respiratory tract infection in patients with asthma: a meta-analysis. Infection 47:377–385. https://doi.org/10.1007/s15010-018-1229-y
Vasileiou E, Sheikh A, Butler C et al (2017) Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 65:1388–1395. https://doi.org/10.1093/cid/cix524
Nair H, Brooks WA, Katz M et al (2011) Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 378:1917–1930. https://doi.org/10.1016/S0140-6736(11)61051-9
Molinari NA, Ortega-Sanchez IR, Messonnier ML et al (2007) The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 25:5086–5096. https://doi.org/10.1016/j.vaccine.2007.03.046
Ritchie AI, Jackson DJ, Edwards MR et al (2016) Airway epithelial orchestration of innate immune function in response to virus infection a focus on asthma. Ann Am Thorac Soc 13:55–63
Gill MA, Bajwa G, George TA et al (2010) Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol 184:5999–6006. https://doi.org/10.4049/jimmunol.0901194
Papadopoulos NG, Christodoulou I, Rohde G et al (2011) Viruses and bacteria in acute asthma exacerbations–a GA2 LEN-DARE systematic review. Allergy 66:458–468. https://doi.org/10.1111/j.1398-9995.2010.02505.x
Shamseer L, Moher D, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 350:g7647. https://doi.org/10.1136/bmj.g7647
Higgins JP, Altman DG, Gøtzsche PC et al (2011) The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Bradburn MJ, Deeks JJ, Berlin JA et al (2007) Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med 26:53–77. https://doi.org/10.1002/sim.2528
Sweeting MJ, Sutton AJ, Lambert PC (2004) What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 23:1351–1375. https://doi.org/10.1002/sim.1761
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Guyatt G, Oxman AD, Sultan S et al (2013) GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol 66:151–157. https://doi.org/10.1016/j.jclinepi.2012.01.006
Chuchalin A, Jacques L, Frith L (2008) Salmeterol/fluticasone propionate via Diskus once daily versus fluticasone propionate twice daily in patients with mild asthma not previously receiving maintenance corticosteroids. Clin Drug Investig 28:169–181. https://doi.org/10.2165/00044011-200828030-00004
Berger WE, Bleecker ER, O'Dowd L et al (2010) Efficacy and safety of budesonide/formoterol pressurized metered-dose inhaler: randomized controlled trial comparing once- and twice-daily dosing in patients with asthma. Allergy Asthma Proc 31:49–59. https://doi.org/10.2500/aap.2010.31.3309
Woodcock A, Bateman ED, Busse WW et al (2011) Efficacy in asthma of once-daily treatment with fluticasone furoate: a randomized, placebo-controlled trial. Respir Res 12:132. https://doi.org/10.1186/1465-9921-12-132
Busse WW, Bleecker ER, Bateman ED et al (2012) Fluticasone furoate demonstrates efficacy in patients with asthma symptomatic on medium doses of inhaled corticosteroid therapy: an 8-week, randomised, placebo-controlled trial. Thorax 67:35–41. https://doi.org/10.1136/thoraxjnl-2011-200308
Meltzer EO, Kuna P, Nolte H et al (2012) Mometasone furoate/formoterol reduces asthma deteriorations and improves lung function. Eur Respir J 39:279–289. https://doi.org/10.1183/09031936.00020310
O'Byrne PM, Woodcock A, Bleecker ER et al (2014) Efficacy and safety of once-daily fluticasone furoate 50 mcg in adults with persistent asthma: a 12-week randomized trial. Respir Res 15:88. https://doi.org/10.1186/s12931-014-0088-z
Busse WW, Bateman ED, O'Byrne PM et al (2014) Once-daily fluticasone furoate 50 mcg in mild-to-moderate asthma: a 24-week placebo-controlled randomized trial. Allergy 69:1522–1530. https://doi.org/10.1111/all.12480
Meltzer EO, Pearlman DS, Eckerwall G et al (2015) Efficacy and safety of budesonide administered by pressurized metered-dose inhaler in children with asthma. Ann Allergy Asthma Immunol 115:516–522. https://doi.org/10.1016/j.anai.2015.09.007
Beasley R, Holliday M, Reddel HK et al (2019) Controlled Trial of Budesonide-formoterol as needed for mild asthma. N Engl J Med 380:2020–2030. https://doi.org/10.1056/NEJMoa1901963
Ek A, Larsson K, Siljerud S et al (1999) Fluticasone and budesonide inhibit cytokine release in human lung epithelial cells and alveolar macrophages. Allergy 54:691–699. https://doi.org/10.1034/j.1398-9995.1999.00087.x
Hogg JC, Chu FS, Tan WC et al (2007) Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med 176:454–459. https://doi.org/10.1164/rccm.200612-1772OC
Fauci AS, Dale DC, Balow JE (1976) Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med 84:304–315. https://doi.org/10.7326/0003-4819-84-3-304
Schleimer RP (2004) Glucocorticoids suppress inflammation but spare innate immune responses in airway epithelium. Proc Am Thorac Soc 1:222–230. https://doi.org/10.1513/pats.200402-018MS
Zhang L, Prietsch SO, Mendes AP et al (2013) Inhaled corticosteroids increase the risk of oropharyngeal colonization by Streptococcus pneumoniae in children with asthma. Respirology 18:272–277. https://doi.org/10.1111/j.1440-1843.2012.02280.x
Cates CJ, Jefferson TO, Rowe BH (2013) Vaccines for preventing influenza in people with asthma. Cochrane Database Syst Rev 22:000364
Hale BG, Albrecht RA, García-Sastre A (2010) Innate immune evasion strategies of influenza viruses. Future Microbiol 5:23–41. https://doi.org/10.2217/fmb.09.108
Cazeiro C, Silva C, Mayer S et al (2017) Inhaled corticosteroids and respiratory infections in children with asthma: a meta-analysis. Pediatrics. https://doi.org/10.1542/peds.2016-3271
Dong YH, Chang CH, Wu FL et al (2014) Use of inhaled corticosteroids in patients with COPD and the risk of TB and influenza: a systematic review and meta-analysis of randomized controlled trials. a systematic review and meta-analysis of randomized controlled trials. Chest 145:1286–1297. https://doi.org/10.1378/chest.13-2137
Singh S, Loke YK (2012) Drug safety assessment in clinical trials: methodological challenges and opportunities. Trials 13:138. https://doi.org/10.1186/1745-6215-13-138
Funding
Chengdu Science and Technology Project, Project Number: 2015-HM0100621-SF.
Author information
Authors and Affiliations
Contributions
Study design: HC, KW. Drafting of the manuscript: HC. Literature search: HC, ZX, LH. Risk of bias assessment: HC, KW, JY. Statistical analysis: HC, ZX, KW. Agree with the manuscript’s results and conclusions: HC, ZX, JY, LH, KW.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
For this type of study, informed consent is not required.
Availability of data and material
The data of this study are available from the corresponding author on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, H., Xu, Z., Yang, J. et al. Inhaled corticosteroids and risk of influenza in patients with asthma: a meta-analysis of randomized controlled trials. Aging Clin Exp Res 33, 1771–1782 (2021). https://doi.org/10.1007/s40520-020-01688-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-020-01688-9