Abstract
Background
While sensor-based daily physical activity (DPA) gait assessment has been demonstrated to be an effective measure of physical frailty and fall-risk, the repeatability of DPA gait parameters between different days of measurement is not clear.
Aims
To evaluate test–retest reliability (repeatability) of DPA gait performance parameters, representing the quality of walking, and quantitative gait measures (e.g. number of steps) between two separate days of assessment among older adults.
Methods
DPA was acquired for 48-h from older adults (age ≥ 65 years) using a tri-axial accelerometer. Continuous walking bouts (≥ 60 s) were identified from acceleration data and used to extract gait performance parameters, including time- and frequency-domain gait parameters, representing walking speed, variability, and irregularity. To assess repeatability, intraclass correlation coefficient (ICC) was calculated using two-way mixed effects F-test models for day-1 vs. day-2 as the independent random effect. Repeatability tests were performed for all participants and also within frailty groups (non-frail and pre-frail/frail identified using Fried phenotype).
Results
Data was analyzed from 63 older adults (29 non-frail and 34 pre-frail/frail). Most of the time- and frequency-domain gait performance parameters showed good to excellent repeatability (ICC ≥ 0.70), while quantitative parameters, including number of steps and walking duration showed poor repeatability (ICC < 0.30). Among majority of the gait performance parameters, we observed higher repeatability among the pre-frail/frail group (ICC > 0.78) compared to non-frail individuals (0.39 < ICC < 0.55).
Conclusion
Gait performance parameters, showed higher repeatability compared to quantitative measures. Higher repeatability among pre-frail/frail individuals may be attributed to a reduced functional capacity for performing more intense and variable physical tasks.
Trial registration
The clinical trial was retrospectively registered on June 18th, 2013 with ClinicalTrials.gov, identifier NCT01880229.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global population of older adults (≥ 60 years) was 600 million in 2000 and is expected to reach two billion by 2050 [1]. Daily physical activity (DPA) gait analysis, in a natural unsupervised environment outside the lab, has become increasingly popular for the assessment of frailty and fall-risk [2,3,4,5] and has been employed for a variety of purposes, including aiding diagnostics, predicting outcomes, and serving as research endpoints. In addition to quantitative gait measures such as total number of steps and walking duration, DPA gait performance measures, representing the quality of walking, may provide additional information about age-related motor function deficits. Moreover, the amount of daily activities, like the number of steps, can be influenced by several factors other than neuromuscular functionality, such as individual (daily schedule and exercise routines) and environmental (weather, transportation, and living conditions) factors. It can be hypothesized that, gait performance parameters extracted from DPA, may not substantially be affected by these factors. Nevertheless, it is important to explore this hypothesis by assessing the repeatability of DPA gait parameters between different days of measurements.
In the clinic, repeatability of time-domain gait parameters is assessed for several purposes. Previous studies have reported excellent repeatability of time-domain gait parameters like stride-time, stride-length, step-length, and step-width in different scenarios of multiple walking sessions on the same day and walking sessions recorded on two different days [6, 7]. Earlier studies have also examined the repeatability of supervised gait parameters extracted from the three axes of a tri-axial accelerometer, and have reported that the vertical acceleration provides the best repeatability in time-domain gait parameters, such as step-time, step-length, cadence and velocity [8]. While these studies provide insights regarding the reliability of sensor-based gait measures, to the best of our knowledge, no study has examined the repeatability of gait parameters in an unsupervised environment.
In this study, we evaluated the repeatability of DPA gait performance parameters and quantitative measures between two separate days among older adults. The gait performance parameters extracted here are temporal gait parameters (step- and stride-time representing walking speed), time-domain gait variability, frequency-domain gait variability, and gait irregularity. Furthermore, gait variability measures were defined to assess the stride-to-stride fluctuation in walking cycles, since it has been associated with high risk of falling and cognitive impairments in elders [9,10,11]. Also, gait studies conducted in the clinic have shown that neurological and neuromuscular diseases, as well as frailty can influence the predictability of walking cycles, which can be described by gait irregularity measures [12,13,14,15]. In addition to all the above gait performance measures, gait quantitative parameters including total number of steps and total walking duration in 2 days were also studied here for comparison.
We hypothesized that unsupervised DPA gait performance parameters, irrespective of the frailty status, would be more repeatable between days than the sensor-based quantitative walking parameters like number of steps and duration of walking. In addition, we explored how frailty, as a common syndrome among older adults, can affect the DPA repeatability. Frailty is primarily indicated by reduction or impairment of physical function [4, 16], and gait performance parameters extracted from DPA are known to provide a more accurate frailty assessment when compared to quantitative parameters [2]. We hypothesized that with frailty gait performance parameters become more repeatable, due to a decline in physiological reserve and incapability of the neuromuscular system to provide a wide range of motor function execution [17,18,19].
Methods
This is an observational cross-sectional study, performed at the Arizona Center on Aging, Tucson, AZ. Study participants were recruited from primary, secondary, and tertiary health-care settings within our academic network and also from community providers and aging service organizations. DPA was recorded from eligible volunteers for two consecutive days (48 h), and the walking data was processed to study the repeatability of gait performance and gait quantitative parameters.
Eligibility for the study included older adults aged 65 years or older, without severe mobility disorder, and the ability to walk at least 10 meters with or without an assistive device. The exclusion criteria for the participant recruitment included dementia identified by a Mini-Mental State Examination (MMSE) [20] score of < 23 or terminal illness. All the recruited participants signed a written consent according to the principles expressed in the Declaration of Helsinki [21], approved by the Institutional Review Board of the University of Arizona.
Demographic and clinical measures
The MMSE, Mobility-Tiredness Scale [22], Center for Epidemiologic Studies Depression Scale (CES-D) [23], Falls Efficacy Scale-International (FES-I) [24], and Barthel Activity of Daily Living (ADL) Scale [25] were part of the interviewer administered questionnaires. Self-reported history of falls and use of assistive device were included as additional clinical measures.
Frailty was assessed using the five criteria proposed by Fried et al. [16]: self-reported weight loss, exhaustion and low energy expenditure, weakness measure by the grip strength, and slowness measure by the walking test. A score of one point was assigned for each of the five criteria recorded, totaling a score in the range of 0–5. Based on this score, frailty was categorized as non-frail (score 0), pre-frail (score 1–2) and frail (score 3–5).
Sensor-based DPA assessment
DPA was quantified for 48 h using a tri-axial accelerometer sensor (PAMSys, BioSensics Cambridge, MA, USA) attached to a tee-shirt, inserted in a device pocket located at the sternum. PAMSys is a small (5.1 cm × 3 cm × 1.6 cm) and light (24 g) recording system containing inertial sensors [26]. Vertical acceleration data was used for analyses here. A previously validated algorithm was employed to identify the walking bouts (PAMWare, BioSensics Cambridge, MA, USA). Briefly, walking bouts were defined by a minimum of three successive steps [26], where steps were estimated by the detection of an acceleration peak beyond a predefined threshold after filtering (Butterworth filter cut-off frequency of 2.5 Hz) [27]. Gait quantitative outcomes including walking bout times (duration of each walking episodes) and number of steps per walking bout were derived for all the walking bouts longer than three steps. Furthermore, gait performance parameters were extracted by defining continuous walking bouts. Continuous walking bouts, longer than 60 s were used to extract gait performance parameters [2, 28, 29]. Gait performance parameters including time- and frequency-domain gait variability and gait irregularity were extracted from continuous walking bouts that lasted 60 s or longer with no pause longer than 1.7 s between gait cycles [3]. Based on the stride-time duration average and standard deviation previously observed in frail participants, we conservatively selected an allowable pause of 1.7 s between gait cycles. Gait variability and irregularity were assessed for each of the continuous walking bouts [2] as defined below.
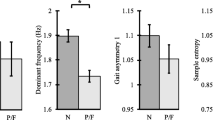
Gait variability Two methods were used to assess gait variability: (1) step- and stride-time variability using time-domain [2]; and (2) power spectral density (PSD) using frequency-domain analyses [2, 30, 31]. For each continuous walking bout, step- or stride-time variability was calculated as the coefficient of variation of the series of step- or stride-times. For the frequency-domain analysis, PSD of the acceleration data was calculated using Welch’s averaged modified periodogram method [32] to represent the frequency components of the acceleration signal [33]. A window size of 512 samples and a fast Fourier transform (FFT) length of two-times the next higher power of the window size was used [32]. A 50% overlap was considered between the windows and 0.5–3.0 Hz locomotion band was analyzed [32]. The PSD components extracted from the raw acceleration signal, included: PSD slope (PSD width to the peak) and dominant walking frequency. A larger slope (shorter and wider PSD peak) was used to identify a higher variability in walking.
Gait irregularity We used the Sample Entropy (SampEn) approach to determine gait-irregularity [34,35,36]. Mutual information method [37] was used to calculate the time-delay of the signal, and the average time-delay of all the continuous walks for each participant was used to calculate the SampEn [2]. We computed the sample entropy of the gait signal using embedding dimension m = 3, and tolerance r = 0.2 times the standard deviation of the signal [34,35,36].
Statistical analysis
Statistical analyses were carried out using JMP (Version 11; SAS Institute Inc., Cary, NC, USA) and MATLAB (Version R2019b; MathWorks Inc., Natick, MA, USA); statistical significance was concluded when p < 0.05. Separate analysis of variance (ANOVA) models were performed to compare sociodemographic parameters between the frailty groups. The DPA gait performance measures were grouped between day-1 and day-2 (i.e., the first and the second 24 h). Repeatability (test–retest reliability) of the parameters between the 2 days was analyzed using Intraclass Correlation Coefficient (ICC), calculated using a two-way mixed effects model with an absolute agreement definition [38]. Repeatability was calculated with a null hypothesis of ICC < 0.6 within a 95% confidence interval (CI). To interpret the results, ICC above 0.75 was considered excellent repeatability, values between 0.6 and 0.74 was considered good, 0.4–0.59 was fair, and 0–0.39 was poor repeatability [39]. Bland–Altman plots [40] were used to visualize between-day repeatability of gait performance and quantitative measures, as well as the between-day repeatability between frailty groups (non-frail vs. pre-frail/frail). Calculated values of agreement and reliability from measurement error studies are estimates of parameters, and as such we report estimates with CIs to indicate the uncertainty with which they have been estimated. All outcomes were normalized using logarithmic transformation before Bland–Altman analyses.
Results
Demographic and clinical measures
We collected DPA measurements from 63 participants: 29 non-frail, 29 pre-frail, and 5 frail according to the Fried frailty criteria [16]. Owing to the small sample size of the frail population, we combined pre-frail and frail participants for all analyses. Demographic and clinical characteristics of the participants are presented in Table 1. Pre-frail/frail participants were significantly older than non-frail participants, used more assistive devices, and had higher BMI (p < 0.05). Compared to non-frail participants, pre-frail/frail participants had significantly higher levels of perceived tiredness and fear of falling (p < 0.05).
Between-day repeatability of gait parameters
For all the participants, the gait performance parameters including PSD slope and dominant frequency showed an excellent repeatability with ICC > 0.75 and significant pICC values (pICC< 0.01), while the quantitative measures showed a poor repeatability with ICC < 0.30 and non-significant pICC values (pICC> 0.90, Table 2). The average ICC for gait performance parameters and quantitative measures were 0.72 ± 0.12 and 0.25 ± 0.03, respectively (Table 2).
Bland–Altman visualizations of stride-time, PSD-slope, and dominant frequency showed that mean differences of gait performance parameters were close to zero (mean ± SD: 0.00 ± 0.01) with majority of the between-day difference in participants within the upper (mean + 1.96 × SD) and lower (mean–1.96 × SD) limits of agreements (LOA) (Fig. 1), demonstrating high between-day repeatability. On the other hand, Bland–Altman visualization of the total number of steps showed that the mean differences of quantitative measures were not close to zero (mean ± SD: 0.54 ± 0.03) (Fig. 1), revealing poor between-day repeatability. Supplementary Table S1 shows mean of between-day differences and the 95% (upper and lower) LOA of all the parameters listed in Table 2.
Between-day repeatability between frailty groups
Overall, the gait performance parameters showed higher repeatability among pre-frail/frail (average ICC: 0.79 ± 0.19) compared to non-frail participants (average ICC: 0.38 ± 0.24, Table 3). However, the quantitative measures showed poor repeatability in both non-frail (average ICC: 0.27 ± 0.03) and pre-frail/frail (average ICC: 0.14 ± 0.01) participants (Table 3).
Discussion
The usefulness of gait performance parameters for the assessment of frailty, as well as fall and neuromuscular diseases (e.g., Parkinson’s disease and low back pain) in a home-based environment has been previously established [2, 3, 41, 42]. However, to the best of our knowledge, this is the first study assessing the repeatability of gait performance parameters between multiple days of DPA recording, especially among frailty groups. As hypothesized, we observed that several sensor-based gait performance parameters show excellent repeatability between the 2 days of measurement.
Advantages of gait performance parameters
Compared to quantitative gait measures, gait performance parameters are more advantageous as they reveal information pertaining the functional performance [2, 3, 28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Previous studies have shown that, gait performance parameters extracted from continuous walks were able to significantly predict frailty with a sensitivity and specificity of 76.8% and 80% [2], while on the same dataset quantitative parameters could not significantly discriminate between frailty groups when adjusted with age [3]. Gait performance measures play a crucial role not only in characterizing gait performance in frailty groups [2, 3], but also to assess fall-risk [29] and motor performance in general [41]. Weiss et al. established that 3-day accelerometer-based gait performance parameters provided a more accurate fall-risk identification compared to performance-based tests such as dynamic gait index, the Berg balance scale, the timed up and go test, and the four square step test [29]. Furthermore, Toosizadeh et al. demonstrated strong discriminatory power in detecting impaired motor function in Parkinson’s disease using in-home DPA measures, especially those related to walking [41].
Repeatability of gait performance parameters
Test–retest reliability or repeatability of gait tests are commonly assessed within clinical settings. In agreement with our findings, results from other supervised studies showed excellent repeatability in gait performance parameters including stride-time, stride-length, step-time, and step-width in between-session (average ICC: 0.92 ± 0.03), within-day (average ICC: 0.97 ± 0.01) and between-day (average ICC: 0.93 ± 0.03) recordings [6, 7]. Overall, higher ICC scores are expected to be achieved in in-clinic supervised studies compared to unsupervised DPA-based studies, as in-clinic studies facilitate a more controlled study environment, whereas DPA-based studies are not controlled. Specifically among older adult participants, Hamacher et al. examined the between-day test–retest reliability of temporal gait variability and observed that repeatability increases in gait variability between days after one familiarization trial has been conducted [43]. Another relevant study performed by Byun et al. used a tri-axial accelerometer for in-clinic acceleration recording, and established excellent repeatability (average ICC: 0.86 ± 0.04) in gait performance parameters including step-time, step-time variability and step asymmetry extracted from the vertical acceleration compared to anteroposterior and mediolateral accelerations [8]. Of note, vertical acceleration in tri-axial accelerometer data contains higher amount of information pertaining to steps and strides associated with walking compared to other directions, and therefore, vertical acceleration has been used for feature extraction and analysis in our study. To the best of our knowledge, currently there are no studies exploring the repeatability of gait performance in a home/community-based environment. We obtained excellent between-day repeatability values in gait performance parameters comparable to other studies with average ICC: 0.82 ± 0.00 (temporal gait parameters), 0.76 ± 0.00 (frequency-domain gait variability) and 0.77 ± 0.10 (gait irregularity).
Gait performance repeatability and frailty
Among older adults, frailty is an increasingly recognized geriatric syndrome, which is associated with decline in function and reserve across multiple physiologic systems, and high vulnerability to adverse health outcomes such as disability, falls, hospitalization, institutionalization, and mortality [4,5,6,7,8,9,10,11,12,13,14,15,16]. Frailty is primarily indicated by reduction or impairment of physical function [4, 16]. Within the current findings, we observed higher repeatability in gait parameters among pre-frail/frail older adults compared to non-frail individuals. One explanation for this observation may be the reduction in physiologic complexity due to frailty [19]. Lipsitz elaborated on the loss of complexity as a pathway to frailty [18], suggesting that loss of complexity in many physiologic mechanisms eventually lead to frailty in old age. Accordingly, since many of the physiologic inputs and the corresponding connectivity are diminished with aging, this would result in a less complex physiological behavior among frail older adults. As a result, low physiologic complexity in the dynamics of a system, especially under basal conditions, leads to a compromised capacity of the system to respond to unexpected stressors [19]. Therefore, attributing to the reduced physiologic complexity and adoption of a conservative strategy to encounter environmental stressors, pre-frail/frail older adults may adapt a lifestyle with routine and repeatable activities [17, 19, 44]. Our findings showing higher gait performance repeatability among pre-frail/frail compared to non-frail older adults are in agreement with the theory of loss of complexity with aging. While promising, further research is required to more systematically measure overall loss of DPA complexity with frailty, using longer duration of natural activity data collection.
Limitations and future direction
There are limitations to consider in the interpretation of current findings. The repeatability in step- and stride-time variabilities were noticeably lower in the non-frail group compared to the pre-frail/frail group. This was because 5 of the 29 non-frail participants had comparatively fewer walking bouts on day-1 than day-2, and also these walking bouts had a high range of gait-variability causing the step- and stride-time variabilities in the non-frail group to be poorly repeatable between the 2 days. However, the repeatability of frequency-domain variability ranged from fair to excellent in both the non-frail and pre-frail/frail groups. We speculate this pattern could be because frequency-domain parameters are more reliable to measure changes in walking compared to temporal approaches. This is in agreement with previous studies, where it was established that frequency-based measures can serve as objective markers of gait variability in real-settings among patients with PD [32]. When the data from these five volunteers was eliminated, the between-day repeatability in the non-frail group improved from poor to fair [stride-variability(%)—day-1: 7.92 ± 2.20, day-2: 8.67 ± 2.31; ICC: 0.44, pICC: 0.86]. In addition, our sample was predominantly women (80.6% female). Reliability depends on the study population, and not just on the measurement errors of the measurement method. Although we did not observe a gender-specific difference in gait performance parameters, there might be a limited generalizability to a population with a more balanced gender composition. Also, using only the accelerometer data, a very poor distinction of stair climbing from level walking have been reported previously [45]. Due to configuration of the motion sensor and the lack of built-in gyroscope system in the device, we were, therefore, not able to distinguish between level walking and stair ascending/descending; the data representing walking in the current study incorporated both level walking and stair climbing together. Finally, in future studies, several other gait performance measures can be extracted and tested for repeatability within DPA assessment, including gait initiation and dynamic stability (Lyapunov exponents).
Summary and conclusions
From our study, it is evident that gait performance parameters including average step- and stride-time and frequency-domain gait variability parameters provided higher test–retest reliability compared to quantitative measures. Furthermore, gait performance parameters showed higher repeatability among pre-frail/frail participants between 2 days compared to non-frail, which may be attributed to a lack of functional capacity among frail individuals for performing more intense and variable physical tasks. As there is an excellent repeatability observed, it may be sufficient to record DPA for a 24-h duration to assess frailty using gait performance parameters in future research.
Availability of data and material
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Ageing and Life Course, WHO (2004) WHO, 2019. https://www.who.int/ageing/en/. Accessed 11 Sep 2019
Pradeep Kumar D, Toosizadeh N, Mohler J et al (2020) Sensor-based characterization of daily walking: a new paradigm in pre-frailty/frailty assessment. BMC Geriatr 20:164
Schwenk M, Mohler J, Wendel C et al (2015) Wearable sensor-based in-home assessment of gait, balance, and physical activity for discrimination of frailty status: baseline results of the Arizona frailty cohort study. Gerontology 61:258–267
Mohler MJ, Fain MJ, Wertheimer AM et al (2014) The Frailty syndrome: clinical measurements and basic underpinnings in humans and animals. Exp Gerontol 54:6–13
Mohler MJ, Wendel CS, Taylor-Piliae RE et al (2016) Motor performance and physical activity as predictors of prospective falls in community-dwelling older adults by frailty level: application of wearable technology. Gerontology 62:654–664
Al-Amri M, Al Balushi H, Mashabi A (2017) Intra-rater repeatability of gait parameters in healthy adults during self-paced treadmill-based virtual reality walking. Comput Methods Biomech Biomed Eng 20:1669–1677
Choi J-S, Kang D-W, Seo J-W et al (2015) Reliability of the walking speed and gait dynamics variables while walking on a feedback-controlled treadmill. J Biomech 48:1336–1339
Byun S, Han JW, Kim TH et al (2016) Single tri-axial accelerometer-based gait analysis in older adults with normal cognition. PLoS One 11:158956
Hausdorff JM, Edelberg HK, Mitchell SL et al (1997) Increased gait unsteadiness in community-dwelling elderly failers. Arch Phys Med Rehabil 78:278–283
Hausdorff JM, Rios DA, Edelberg HK (2001) Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil 82:1050–1056
Sheridan PL, Solomont J, Kowall N et al (2003) Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer’s disease. J Am Geriatr Soc 51:1633–1637
Lamoth CJ, van Deudekom FJ, van Campen JP et al (2011) Gait stability and variability measures show effects of impaired cognition and dual tasking in frail people. J Neuroeng Rehabil 8:2
Liao F, Wang J, He P (2008) Multi-resolution entropy analysis of gait symmetry in neurological degenerative diseases and amyotrophic lateral sclerosis. Med Eng Phys 30:299–310
IJmker T, Lamoth CJC (2012) Gait and cognition: the relationship between gait stability and variability with executive function in persons with and without dementia. Gait Posture 35:126–130
Riva F, Toebes MJP, Pijnappels M et al (2013) Estimating fall risk with inertial sensors using gait stability measures that do not require step detection. Gait Posture 38:170–174
Fried LP, Tangen CM, Walston J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol Ser A Biol Sci Med Sci 56:M146–M157
Lipsitz LA (2004) Physiological complexity, aging, and the path to frailty. Sci Aging Knowl Environ 2004:pe16
Lipsitz LA (2007) Aging as a process of complexity loss. In: Complex systems science in biomedicine, pp 641–654
Lipsitz LA, Goldberger AL (1992) Loss of ‘complexity’ and aging: potential applications of fractals and Chaos theory to senescence. JAMA 267:1806–1809
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
World Medical Association (2013) World Medical Association Declaration of Helsinki. JAMA 310:2191
Fieo RA, Mortensen EL, Rantanen T et al (2013) Improving a measure of mobility-related fatigue (the Mobility-Tiredness Scale) by establishing item intensity. J Am Geriatr Soc 61:429–433
Orme JG, Reis J, Herz EJ (1986) Factorial and discriminant validity of the center for epidemiological studies depression (CES-D) scale. J Clin Psychol 42:28–33
Yardley L, Beyer N, Hauer K et al (2005) Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 34:614–619
Mahoney FI, Barthel DW (1965) Functional evaluation: the Barthel Index. Md State Med J 14:61–65
Najafi B, Aminian K, Paraschiv-Ionescu A et al (2003) Ambulatory system for human motion analysis using a kinematic sensor: monitoring of daily physical activity in the elderly. IEEE Trans Biomed Eng 50:711–723
Najafi B, Armstrong DG, Mohler J (2013) Novel wearable technology for assessing spontaneous daily physical activity and risk of falling in older adults with diabetes. J Diabetes Sci Technol 7:1147–1160
Weiss A et al (2013) Does the evaluation of gait quality during daily life provide insight into fall risk? A novel approach using 3-day accelerometer recordings. Neurorehabil Neural Repair 27:742–752
Orendurff MS (2008) How humans walk: bout duration, steps per bout, and rest duration. J Rehabil Res Dev 45:1077–1090
Toosizadeh N et al (2015) Does integrative medicine enhance balance in aging adults? Proof of concept for the benefit of electroacupuncture therapy in Parkinson’s disease. Gerontology 61:3–14
Weiss A, Sharifi S, Plotnik M et al (2011) Toward automated, at-home assessment of mobility among patients with Parkinson disease, using a body-worn accelerometer. Neurorehabil Neural Repair 25:810–818
Lei H, Toosizadeh N, Schwenk M et al (2016) A pilot clinical trial to objectively assess the efficacy of electroacupuncture on gait in patients with Parkinson’s disease using body worn sensors. PLoS One 11:e0155613
Stoica P, Moses RL (2005) Spectral analysis of signals. Pearson, Upper Saddle
Yentes JM, Hunt N, Schmid KK et al (2013) The appropriate use of approximate entropy and sample entropy with short data sets. Ann Biomed Eng 41:349–365
McCamley JD, Denton W, Arnold A et al (2018) On the calculation of sample entropy using continuous and discrete human gait data. Entropy (Basel) 20:764
Shi L, Duan F, Yang Y et al (2019) The effect of treadmill walking on gait and upper trunk through linear and nonlinear analysis methods. Sensors (Basel) 19:2204
Albers DJ, Hripcsak G (2012) Estimation of time-delayed mutual information and bias for irregularly and sparsely sampled time-series. Chaos Solitons Fractals 45:853–860
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Hallgren KA (2012) Computing inter-rater reliability for observational data: an overview and tutorial. Tutor Quant Methods Psychol 8:23–34
Martin Bland J, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 327:307–310
Toosizadeh N, Mohler J, Lei H et al (2015) Motor performance assessment in Parkinson’s disease: association between objective in-clinic, objective in-home, and subjective/semi-objective measures. PLoS One 10:e0124763
Chuan Yen T, Mohler J, Dohm M et al (2018) The effect of pain relief on daily physical activity: in-home objective physical activity assessment in chronic low back pain patients after paravertebral spinal block. Sensors 18:3048
Hamacher D, Hamacher D, Krowicki M et al (2017) Between-day test-retest reliability of gait variability in older individuals improves with a familiarization trial. Aging Clin Exp Res 29:327–329
Chaves PHM, Varadhan R, Lipsitz LA et al (2008) Physiological complexity underlying heart rate dynamics and frailty status in community-dwelling older women. J Am Geriatr Soc 56:1698–1703
Lugade V, Fortune E, Morrow M et al (2014) Validity of using tri-axial accelerometers to measure human movement—part I: posture and movement detection. Med Eng Phys 36:169–176
Acknowledgements
We thank the students of University of Arizona and Arizona Frailty Cohort coordination staff for volunteer recruitment and data collection.
Funding
This study was supported in part by award No. 2R42AG032748 from the National Institute on Aging. Sponsors had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
DPK: data analysis, interpretation of findings, statistical analysis, manuscript writing and critical review of manuscript. CW: data analysis and interpretation, critical review of manuscript and approval of final manuscript. JM: subject recruitment, study management, study design, interpretation of findings, critical review of manuscript and approval of final manuscript. KL: concept, study design, data analysis and interpretation, statistical analysis, manuscript writing and editing, critical review of manuscript and approval of final manuscript. NT: concept, study design, subject recruitment, study management, data analysis and interpretation, statistical analysis, manuscript writing and editing, critical review of manuscript and approval of final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicting interests.
Statement of human and animal rights
This observational cross-sectional study has been performed in accordance with the ehtical standards stated in the Declaration of Helsinki (1964). No animals were involved.
Ethical approval
The study was approved by the University of Arizona and Banner Sun Health Research Institute’s Institutional Review Boards.
Informed consent
Before participation, written informed consent according to the principles expressed in the Declaration of Helsinki was obtained from all subjects.
Code availability
The software and code used for the current study available from the corresponding author on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pradeep Kumar, D., Wendel, C., Mohler, J. et al. Between-day repeatability of sensor-based in-home gait assessment among older adults: assessing the effect of frailty. Aging Clin Exp Res 33, 1529–1537 (2021). https://doi.org/10.1007/s40520-020-01686-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-020-01686-x