Abstract
Background
The Short Physical Performance Battery (SPPB) and the De Morton Mobility Index (DEMMI) are two commonly used instruments to assess mobility in older patients.
Aims
To compare the two assessments in acute senior trauma patients with regard to sensitivity to change during an acute care, and prediction of discharge destination.
Methods
Medical records were extracted for consecutive trauma patients aged 70 + receiving acute care rehabilitation in the geriatric ward during 9 months. SPPB and DEMMI were obtained at admission and discharge. Sensitivity was analyzed using paired t tests and Cohen’s d, and discharge destination with logistic regression predicting the probability of returning home.
Results
A total of 69 patients were included in the study [83.7 years (SD 6.3), 78% women, length of stay 10 (IQR 8–10) days]. Overall, SPPB improved from 2.0 (SD 2.5) to 3.8 (SD 2.7; p ≤ 0.001) and DEMMI from 41 (SD 19) to 53 (SD 14; p ≤ 0.001) (Cohen’s d: 0.72 for SPPB, 0.62 for DEMMI). Among patients admitted from home each additional point in SPPB at admission and acquired during acute care rehabilitation increased the odds of returning home by 1.7 times (95% CI 1.1–2.8, p = 0.02) and 1.6 times (95% CI 1.1–2.5, p = 0.02). For DEMMI, every 10 points at admission, but not in change, increased the odds of returning home by 2.5 times (95% CI 1.3–5.0, p = 0.007).
Discussion and conclusion
SPPB and DEMMI are both valid mobility assessments for senior patients in acute care. However, SPPB is a better predictor than DEMMI for discharge destination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Good mobility is critical for maintenance of functional independence at older age [1], while reduced mobility has been associated with higher mortality risk [2], lower quality of life [3], and a higher risk of falls and fall-related injuries [4]. The latter leads to a growing number of senior trauma patients in acute care, who, if bedridden due to their injury, lose function rapidly [5] and may not recover to their former autonomy [6].
One of the primary goals of acute care geriatric rehabilitation is to enable older patients to regain their mobility effectively to maintain their autonomy and return home [7]. Notably, mobility has been identified as a key predictor of length of stay in acute care [8] and of discharge destination among older patients [8, 9]. Moreover, in a prospective study, better mobility at discharge from acute geriatric care rehabilitation, was found to be the strongest predictor for living at home even 3 months after acute care [10]. Therefore, determining a patient’s mobility status is a fundamental component of health assessments in acute care of older patients.
To identify and adequately treat older patients at risk of functional decline, the measurement of mobility is a cornerstone of the comprehensive geriatric assessment. However, although several mobility assessments have been validated in older adults, there is no internationally accepted consensus on one specific gold-standard mobility assessment for older patients [11, 12].
The Short Physical Performance Battery (SPPB) is a widely used mobility test to assess lower extremity function developed to identify the onset of disability in non-disabled older adults [13]. Notably, the SPPB has been shown to be reliable and valid [14, 15]. In an acute geriatric setting, SPPB scores at hospital admission predicted the length of hospital stay with a difference of 4 days comparing patients with better versus poorest SPPB scores [16]. Further, poor SPPB scores at hospital discharge predicted a 5.4-fold increased risk of hospital readmission or death during the 1st year after hospitalization [17]. To our best knowledge, the association between SPPB and immediate discharge destination from acute care has not been determined.
Another mobility assessment tool especially developed for the hospital setting is the De Morton Mobility Index (DEMMI). It was designed for clinicians and researches as an instrument for measuring overall mobility in hospitalized older patients [18]. DEMMI has been validated in sub-acute hospital settings [19] and in the transition from hospital to community [20]. A recent study in hip fracture patients showed a 4.3-times higher chance of returning home after acute care in patients starting with higher DEMMI values (≥ 20) compared to those with lower values (< 20) [21]. To our best knowledge, the association between DEMMI and immediate discharge destination from acute care in a broader patient setting has not been explored to date.
We aimed to compare the SPPB and the DEMMI in a sample of acute care senior trauma patients (1) to assess the tests’ sensitivity to change during an acute care rehabilitation program and (2) to evaluate the tests’ usefulness to predict discharge destination, both based on baseline score and change from baseline to discharge.
Methods
Subjects and study design
We extracted data from the electronic medical records of a sample of consecutive senior patients aged 70 years or older who were admitted to the Department of Geriatrics at the University Hospital Zurich between November 2016 and July 2017 as a consequence of a fall-related injury.
For this analysis, patients were included if they had a prospective assessment of functional mobility for both SPPB and DEMMI and gave general consent to use their data for research. Patients who had missing scores or were not enrolled in the acute care rehabilitation program were excluded. The main reason for missing scores was poor health status. The project was approved by the local ethics committee (No. 2017-01643).
Acute care geriatric rehabilitation
Acute care geriatric rehabilitation is an interprofessional treatment developed to minimize the loss of mobility and function among older adults during their hospital stay. Our patients were initially hospitalized in the trauma surgery department for treatment of their fall-related injury, and then transferred to the acute care geriatric department. Upon admission to the geriatric department a standardized geriatric assessment was performed to plan individualized acute care and rehabilitation treatments. Acute care rehabilitation includes ten physiotherapy and occupational therapy sessions per 7 days, activating treatment by nursing staff and interprofessional team meetings to define individualized goals and to assess progress. Depending on the patient’s acute care needs and rehabilitation progress, the program may take between 7 and 21 days. The geriatric mobility assessment including both the SPPB and DEMMI was repeated prior to patients’ discharge. The decision on a patient’s discharge location is usually made 5–7 days before discharge, and therefore also before the geriatric mobility assessment at discharge. Entry values of SPPB, but not DEMMI are available for the interprofessional team making the decision.
Measurements
SPPB and DEMMI assessments were performed upon admission to the geriatric ward and prior to discharge. The SPPB was applied by an assessment nurse and the DEMMI was administered by a physiotherapist.
Short Physical Performance Battery (SPPB)
The SPPB consists of three components: (1) a balance test, (2) a timed 4-m walk, and (3) a timed chair rise test. For the balance test, patients had to stand with their feet in a side-by-side, semi-tandem, and tandem position for 10 s without the use of assistance. Gait speed was measured over a 4-m distance. Walking aids were allowed for patients, who needed them. We retained the faster of two walks. Repeated chair rise test measures the time needed to stand up five times from a regular chair. All the subtests were scored from 0 to 4 points based on community cut points established by Guralnik et al. leading to a final SPPB score ranging from 0 to 12 with higher scores representing better lower extremity function [13, 14]. The SPPB is reported to take an average of 12 min [14].
De Morton Mobility Index (DEMMI)
The DEMMI assesses mobility through 15 mobility exercises, administered from easiest to hardest. It starts with bed mobility (bridge, roll onto side, lying to sitting), progresses to chair mobility (sit unsupported, sit to stand, sit to stand without using arms), to static balance (stand unsupported, stand feet together, toe stand, tandem stand), to walking mobility (walking distance, walking independence), and finally to dynamic balance without gait aid (pick up pen from floor, walking backwards, jump). With these 15 exercises, a patient can achieve up to 19 raw points, which are then converted to an interval score ranging from 0 to 100 with higher levels indicating a better status. The DEMMI is reported to take an average of 8.8 min [18].
Further data from clinical information system
Patients’ demographic and clinical characteristics were obtained from electronic medical records. Patients’ characteristics included age, gender, body mass index, Barthel Index at admission and discharge, type of trauma that led to hospitalization, living conditions before entry (community dwelling or coming from a nursing home), discharge destination (return home, admitted to a rehabilitation centre, admitted to an acute and transitional care unit). Further, we extracted quality of life (first question of the SF-36 questionnaire), number of frailty criteria defined by Fried et al. [22], cognitive status by Folstein Mini Mental State Examination (MMSE) [23], and the risk of malnutrition by the full version of Mini Nutritional Assessment (MNA) [24].
Statistical analysis
Descriptive statistics were used to summarize patients’ characteristics and are presented as means ± standard deviations (SD), unless stated otherwise. To describe the two mobility tests, we determined: (1) the proportion of patients with SPPB scores of 0 (floor) and 12 (ceiling), respectively, with DEMMI scores of 0 (floor) and 100 (ceiling) was calculated for admission and discharge. A proportion of ≥ 15% was considered as a notable floor or ceiling effect. (2) Responsiveness to change was determined using paired t tests, standardized effect sizes (Cohen’s d) and standardized response means (SRM) [25]. (3) A minimal clinically important difference (MCID) was determined using a distribution-based approach using 0.5 SD of admission scores as an estimate for the MCID [26]. Additionally, an anchor-based approach was used to determine a MCID where a clinically important change was considered to have occurred in patients who rated their overall health at discharge at least 1 point better on the 5-point Likert scale than at admission.
To test the associations between SPPB and DEMMI at admission and discharge, we used Spearman correlation coefficients and a linear regression model adjusted for gender, age, BMI, number of comorbidities (0–5 vs. 6–10 vs. > 10 diagnoses) and length of hospital stay.
The association between SPPB and DEMMI test scores with discharge destination was calculated using a logistic regression model with discharge destination (returning home vs. not) as the outcome in the subsample of individuals admitted from home. SPPB at admission and the change in SPPB over time were used as explanatory variables. The model was adjusted for gender, age, BMI, number of comorbidities and length of stay. The same model was used for DEMMI at admission and its change over time. For these models, we additionally estimated receiver operating characteristics (ROC).
All reported p values are two tailed with an alpha value of 0.05. All analyses were conducted with SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
During the data collection period, 128 patients were transferred from the Department of Traumatology to the Department of Geriatrics. A total of 69 (54%) patients were included in this study meeting all inclusion criteria. The reasons to be excluded were a missing consent (n = 20), missing entry assessment (n = 8), missing discharge assessment (n = 29), and death during acute care (n = 2). Table 1 shows admission characteristics of the included patients. The mean age was 83.7 years (SD 6.3). The majority of patients were women (78.3%), 88.4% were community dwelling before admission. Most patients suffered a fall-related fracture (62.4%) or cerebral concussion (21.7%). The median time for transfer from the Department of Traumatology to the Department of Geriatrics was 7 days (IQR 5–9 days) and the median length of stay in the Department of Geriatrics was 10 days (IQR 8–12 days).
Upon admission to the Department of Geriatrics, the mean SPPB score was 2.0 (SD 2.5; median 1; IQR 0–3) with 27 (39%) scoring the lowest possible score (0 points). The mean DEMMI score was 41.3 (SD 18.8; median 41; IQR 30–57) and only 1 (1.4%) patient scored the lowest possible score (0 points). Upon discharge, both SPPB and DEMMI were normally distributed with a mean SPPB score of 3.8 (SD 2.7) and a mean DEMMI score of 52.9 (SD 14.2). No floor or ceiling effects were identified for SPPB or DEMMI at discharge.
Responsiveness to change and minimal clinically important difference (MCID)
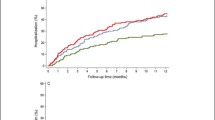
Figure 1 shows the changes of SPPB and DEMMI over time. Both SPPB and DEMMI showed significant improvements over time with an increase in SPPB scores of 1.9 (95% CI 1.3–2.2; p ≤ 0.001) and an increase in DEMMI scores of 11.6 (95% CI 8.8–14.5; p ≤ 0.001). Both instruments revealed a moderate to large effect size (Cohen’s d: SPPB 0.72, DEMMI 0.62; SRM: SPPB 0.92, DEMMI 0.97). Using a distribution-based approach to calculate MCID (0.5 SD of the admission score), a MCID for the SPPB was 1.24 points and DEMMI was 9.38 points. Based on these cut points, 50.1% and 42.0% of patients for SPPB and DEMMI, respectively, reached changes above the MCID. Using an anchor-based approach (any improvement in quality of life), a MCID for SPPB was 2.25 points and DEMMI was 14.65 points.
Change over time of stay in SPPB scores and DEMMI scores. In the box plots of raw values, the boundaries of the box indicate the 25th and 75th percentile and the black line within the box marks indicates the median. Whiskers above and below the box indicate the most extreme value within the × 1.5 interquartile range
Correlation between the two mobility instruments
Spearman correlation showed a significant correlation between SPPB and DEMMI scores upon admission (r = 0.59; p < 0.001) and at discharge (r = 0.72; p < 0.001). This supports convergent validity for both instruments. The association between SPPB and DEMMI scores remained unchanged when adjusting for potential confounder’s age, gender, BMI and number of diagnoses.
Association between SPPB/DEMMI and discharge location
Of the 61 (87%) patients who were admitted from home, 32 (52%) could return home after acute care, 14 (23%) were admitted to a nursing home, 13 (21%) went to an inpatient rehabilitation center, 1 (2%) was admitted to a transitional care setting, and 1 (2%) to a different clinic. Table 2 shows the association of SPPB and DEMMI scores with discharge destination in patients who were admitted from home. Upon admission, SPPB in particular and to a lesser degree DEMMI, predicted a patient’s discharge destination. Upon admission, for each point increase in SPPB, patients had a 1.8-fold higher odds of returning home (OR 1.75, CI 1.11–2.76) and for each 10 points increase in DEMMI, patients had a 2.5-fold higher odds of returning home (OR 2.55, CI 1.30–5.00). In addition, changes in SPPB scores during acute care rehabilitation were predictive of discharge destination (OR 1.63, CI 1.08–2.48), independent of baseline SPPB. Changes in DEMMI scores over time did not predict a patient’s discharge destination (p = 0.57). Both assessment tests had high discriminant ability to predict discharge to home (ROC area under the curve > 0.87 for both tests).
Discussion
This study supports the use of both the SPPB and DEMMI as valid measures of mobility in acute senior trauma patients. Both instruments were sensitive to change with regard to early rehabilitation during acute care, and both test results upon entry to acute care predicted discharge destination. However, with regard to change in test performance with early rehabilitation, only change in SPPB but not in DEMMI predicted discharge destination.
At hospital admission, 39% of our patients scored the lowest possible SPPB score. Fisher et al. [14] reported a 9% incidence of SPPB score “0” and Corsonello et al. [27] a 18% incidence of SPPB score “0” among seniors admitted to acute care due to an acute medical event. The higher proportion in our study is likely due to fall-injured patients in our study compared to community-dwelling patients with less comorbidities [14] or by a later time in SPPB assessment [27]. Only a small proportion of patients scored the lowest possible DEMMI score in our study (1.4%), consistent with results of other studies reporting DEMMI scores in acute care [19, 28]. This discrepancy between the proportion of DEMMI and SPPB entry scores can be explained by the different composition of mobility aspects included in these two instruments. The DEMMI covers a number of aspects of early mobilization including mobility in bed and in sitting position, whereas the focus of the SPPB is measuring lower extremity function, which is likely to be impaired after a fall-related trauma. Therefore, the low SPPB scores found in our study represent the immobility of these patients upon admission to acute care. Although the floor effect of the SPPB is a downside as the discrimination of patients at the lower end of the scale is limited, together with other results of this current study, we still consider SPPB as a valid instrument in the functional and prognostic evaluation of a patient.
Both instruments showed significant improvement in mobility during acute care with moderate to large effect sizes. Patients increased their SPPB scores by 1.8 points [from 2.0 (SD 2.5) to 3.8 (SD 2.7)] representing 14.7% of the SPPB scale width, which is in line with another study analyzing SPPB during acute care [16], although the authors of this study reported higher SPPB scores upon admission (SPPB of 6.0). This difference can be explained by the selected patient group entering acute care due to a pulmonary or cardiovascular event. DEMMI scores improved by 12 points [from 41.3 (SD 18.8) to 52.9 (SD 14.1)] representing 11.6% of the DEMMI scale width. These values were slightly lower than those reported in the DEMMI validation study by de Morton et al. [19] where an admission score of 51.5 points (SD 20.9) was shown and higher than in another study of the de Morton group showing admission DEMMI scores of 30.7 points (SD 16.0) and an improvement to 45.7 points (SD 16.5) [29]. These different admission scores indicate that mobility instruments differ among different patient groups depending on their diagnoses. Distribution-based MCID point estimates of 9.4 for the DEMMI and 1.2 for the SPPB represent approximately 10% of their scale widths and are consistent with MCID estimates from previous reports for the DEMMI (10) [18] and the SPPB (0.54–1.34) [30], respectively. Larger MCID values were obtained using an anchor-based method for both DEMMI (2) and SPPB (14.4). This could be explained by the anchor we used for this analysis, which was based on the less number of 20 patients who reported better quality of life at discharge compared to admission.
Several studies have shown the efficacy of geriatric rehabilitation on patients’ mobility [31]. On one hand, geriatric rehabilitation assessments are used to improve patients’ outcomes and on the other hand these assessments also have a predictive value for patients’ outcome [31]. Kool et al. showed the importance of mobility as a predictive factor for living at home after geriatric rehabilitation [10]. Further, the predictive value of lower extremity function has been demonstrated in various studies for different geriatric outcomes including disability [15], nursing home admission [13], and risk of mortality [17].
In this study, we analyzed the predictive value of the DEMMI and SPPB for discharge location. SPPB’s admission scores predicted discharge destination by a 74.5% increased odds of returning home for each point increase in SPPB scores, which shows that this instrument is a valid mobility assessment tool for comprehensive geriatric assessments during acute care. In addition, an improvement in SPPB scores from admission to discharge further predicted the chance of returning home, supporting the fact that improving lower extremity function is essential for regaining functional independence [32]. Further, in a prospective study in geriatric patients admitted to an internal medicine ward, each point increase in patients’ SPPB score during acute care was associated with a 17% reduction in risk of falling, a 42% reduction in risk of loss of mobility, and a 28% reduction in risk of death within 1 year after acute care [33]. This was also confirmed by another prospective study in 506 patients aged 70 + years which showed that SPPB score at discharge from acute care predicted mortality (Hazard ratio 0.86; 95% CI 0.78–0.95), functional decline (OR 0.8; 95% CI 0.70–0.96), but not the risk of rehospitalization within 1 year [27]. On the other side, DEMMI’s admission scores (by a 155% increased odd of returning home for each 10 points higher DEMMI score), but not an improvement in DEMMI from admission to discharge, predicted discharge destination. Previous studies have shown that DEMMI scores at discharge were higher in patients discharged home than patients discharged to inpatient rehabilitation [19, 29]. In hip fracture patients, a DEMMI score ≥ 20 upon admission was related to a 4.3-times higher chance of returning home after acute care [21].
Our results need to be interpreted with some caution as the study sample was small. Larger prospective studies are required to confirm our findings. In addition, the results of the current study can only be generalized to patients who have been referred to early geriatric rehabilitation due to fall-related injuries. Further, discharge location is influenced by other factors that have not been taken into account in this study (e.g., social factors, health insurance situation), and we cannot completely rule out that knowing the SPPB mobility scores at entry has also influenced the physicians’ decisions. However, as the decision has been made before the discharge assessment of SPPB and DEMMI, the performance upon discharge and also the change in SPPB, DEMMI performance had no influence on physicians’ decisions regarding the discharge location. Besides these limitations, however, we want to highlight the strength of a continuous prospective data collection from electronic medical records of all seniors admitted to the geriatric ward after a fall-related injury. Patients were included in an inpatient rehabilitation program with a standardized mobility assessment upon admission and at discharge from acute care. Also, our patient group represents a wide range of common trauma injuries among older adults.
Together, these findings are important because they allow clinicians to gain more insight into patients’ characteristics regarding their probable discharge destination. In this way, more realistic rehabilitation goals can be established, and patients and their caregivers can be prepared for probable changes in their living arrangement after discharge [9].
Early geriatric rehabilitation during acute care is a valuable clinical strategy to improve an older patient’s chance to maintain autonomy by regaining mobility skills. This study has identified the SPPB and the DEMMI as valid measures of mobility in acute rehabilitative hospital setting in senior trauma patients. SPPB score change from admission to discharge had the additional advantage of serving as a predictor for discharge destination.
References
Fried TR, Tinetti ME, Iannone L et al (2011) Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med 171:1854–1856. https://doi.org/10.1001/archinternmed.2011.424
Covinsky KE, Palmer RM, Fortinsky RH et al (2003) Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc 51:451–458
Patrick DL, Kinne S, Engelberg RA et al (2000) Functional status and perceived quality of life in adults with and without chronic conditions. J Clin Epidemiol 53:779–785
Moreland JD, Richardson JA, Goldsmith CH et al (2004) Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc 52:1121–1129. https://doi.org/10.1111/j.1532-5415.2004.52310.x
McCloskey R (2004) Functional and self-efficacy changes of patients admitted to a geriatric rehabilitation unit. J Adv Nurs 46:186–193. https://doi.org/10.1111/j.1365-2648.2003.02978.x
Gaugler JE, Duval S, Anderson KA et al (2007) Predicting nursing home admission in the US: a meta-analysis. BMC Geriatr 7:13. https://doi.org/10.1186/1471-2318-7-13
Zisberg A, Shadmi E, Sinoff G et al (2011) Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc 59:266–273. https://doi.org/10.1111/j.1532-5415.2010.03276.x
Campbell SE, Seymour DG, Primrose WR et al (2004) A systematic literature review of factors affecting outcome in older medical patients admitted to hospital. Age Ageing 33:110–115. https://doi.org/10.1093/ageing/afh036
Everink IH, van Haastregt JC, van Hoof SJ et al (2016) Factors influencing home discharge after inpatient rehabilitation of older patients: a systematic review. BMC Geriatr 16:5. https://doi.org/10.1186/s12877-016-0187-4
Kool J, Oesch P, Bachmann S (2017) Predictors for living at home after geriatric inpatient rehabilitation: a prospective cohort study. J Rehabil Med 49:185–190. https://doi.org/10.2340/16501977-2182
Eagles D, Yadav K, Perry JJ et al (2017) Mobility assessments of geriatric emergency department patients: a systematic review. CJEM. https://doi.org/10.1017/cem.2017.46
Soares Menezes KVR, Auger C, de Souza Menezes WR et al (2017) Instruments to evaluate mobility capacity of older adults during hospitalization: a systematic review. Arch Gerontol Geriatr 72:67–79. https://doi.org/10.1016/j.archger.2017.05.009
Guralnik JM, Simonsick EM, Ferrucci L et al (1994) A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49:M85–M94
Fisher S, Ottenbacher KJ, Goodwin JS et al (2009) Short physical performance battery in hospitalized older adults. Aging Clin Exp Res 21:445–452
Guralnik JM, Ferrucci L, Pieper CF et al (2000) Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 55:M221–M231
Volpato S, Cavalieri M, Guerra G (2008) Performance-based functional assessment in older hospitalized patients: feasibility and clinical correlates. J Gerontol A Biol Sci Med Sci 63:1393–1398
Volpato S, Cavalieri M, Sioulis F et al (2011) Predictive value of the short physical performance battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci 66:89–96. https://doi.org/10.1093/gerona/glq167
de Morton NA, Davidson M, Keating JL (2008) The de Morton Mobility Index (DEMMI): an essential health index for an ageing world. Health Qual Life Outcomes 6:63. https://doi.org/10.1186/1477-7525-6-63
de Morton NA, Davidson M, Keating JL (2010) Validity, responsiveness and the minimal clinically important difference for the de Morton Mobility Index (DEMMI) in an older acute medical population. BMC Geriatr 10:72. https://doi.org/10.1186/1471-2318-10-72
de Morton NA, Brusco NK, Wood L et al (2011) The de Morton Mobility Index (DEMMI) provides a valid method for measuring and monitoring the mobility of patients making the transition from hospital to the community: an observational study. J Physiother 57:109–116. https://doi.org/10.1016/S1836-9553(11)70021-2
Hulsbæk S, Larsen RF, Rosthøj S et al (2018) The Barthel Index and the Cumulated Ambulation Score are superior to the de Morton Mobility Index for the early assessment of outcome in patients with a hip fracture admitted to an acute geriatric ward. Disabil Rehabil. https://doi.org/10.1080/09638288.2018.1424951
Fried LP, Tangen CM, Walston J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Guigoz Y (2006) The mini nutritional assessment (MNA) review of the literature—what does it tell us? J Nutr Health Aging 10:466–485 (discussion 485–467)
Husted JA, Cook RJ, Farewell VT et al (2000) Methods for assessing responsiveness: a critical review and recommendations. J Clin Epidemiol 53:459–468
Norman GR, Sloan JA, Wyrwich KW (2003) Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 41:582–592. https://doi.org/10.1097/01.MLR.0000062554.74615.4C
Corsonello A, Lattanzio F, Pedone C et al (2012) Prognostic significance of the short physical performance battery in older patients discharged from acute care hospitals. Rejuvenation Res 15:41–48. https://doi.org/10.1089/rej.2011.1215
Dasenbrock L, Berg T, Lurz S et al (2016) The De Morton Mobility Index for evaluation of early geriatric rehabilitation. Z Gerontol Geriatr 49:398–404. https://doi.org/10.1007/s00391-016-1061-x
de Morton NA, Nolan J, O’Brien M et al (2015) A head-to-head comparison of the de Morton Mobility Index (DEMMI) and Elderly Mobility Scale (EMS) in an older acute medical population. Disabil Rehabil 37:1881–1887. https://doi.org/10.3109/09638288.2014.982832
Perera S, Mody SH, Woodman RC et al (2006) Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 54:743–749. https://doi.org/10.1111/j.1532-5415.2006.00701.x
Bachmann S, Finger C, Huss A et al (2010) Inpatient rehabilitation specifically designed for geriatric patients: systematic review and meta-analysis of randomised controlled trials. BMJ 340:c1718
Guralnik JM, Ferrucci L, Simonsick EM et al (1995) Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 332:556–561. https://doi.org/10.1056/NEJM199503023320902
Quadri P, Tettamanti M, Bernasconi S et al (2005) Lower limb function as predictor of falls and loss of mobility with social repercussions one year after discharge among elderly inpatients. Aging Clin Exp Res 17:82–89
Funding
This study was performed without external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The local ethics committee has approved the study.
Informed consent
We only included data of patients who gave written general consent to use their data for research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gazzotti, A., Meyer, U., Freystaetter, G. et al. Physical performance among patients aged 70 + in acute care: a preliminar comparison between the Short Physical Performance Battery and the De Morton Mobility Index with regard to sensitivity to change and prediction of discharge destination. Aging Clin Exp Res 32, 579–586 (2020). https://doi.org/10.1007/s40520-019-01249-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-019-01249-9