Abstract
Adequate calcium intake during childhood is necessary to achieve optimal peak bone mass and this has the potential by increasing bone reserves, to modulate the rate of age-associated bone loss. However, data regarding the efficacy of calcium obtained either through the diet or in the form of medicinal supplementation, for prevention of bone loss and osteoporotic fractures in the elderly is conflicting. Calcium alone is unlikely to be of benefit for this purpose though the co-administration of calcium and vitamin D may have modest fracture risk benefits. Supplemental calcium with or without vitamin D has recently come into the spotlight after the publication of the findings from a controversial randomized controlled trial that associated calcium supplementation with an increased risk of myocardial infarction. Since then, multiple studies have explored this potential link. The data remains conflicting and the potential mechanistic link if any exists, remains elusive. This review examines the relationship between supplemental calcium intake and skeletal and cardiovascular health in the aging individual through an appraisal of studies done on the subject in the last three decades. It also briefly details some of the studies evaluating fractional absorption of calcium in the elderly and the rationale behind the current recommended dietary allowances of calcium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
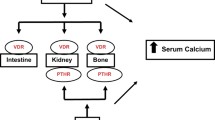
The adult human body contains about 1200 g of calcium of which 99% is present in the bones and the teeth. This contributes to the rigidity and structure of these mineralized tissues. The remaining 1% is found in blood, extracellular fluid and muscles, and plays a vital role in mediating vascular function, muscle contraction and nerve transmission. The concentration of calcium in the plasma is kept within a tightly regulated range through the complex interaction of the hormones; parathyroid hormone (PTH) and 1,25 dihydroxycolecalciferol (1,25(OH2) D3) with the kidneys, bones and the intestine. Calcium is required for normal skeletal growth and development. Until early adulthood, calcium accrual in the skeleton occurs at a steady rate. In humans, bone mass is believed to peak in early adulthood (Fig. 1). The timing of the peak is thought to vary by gender at different skeletal sites. By the time adulthood is reached, the skeleton is in calcium equilibrium. Later, as aging sets in; at the time of menopause in women and about age 55 in men, bone balance starts becoming negative with bone loss occurring at all skeletal sites resulting in calcium losses of approximately 15 g per year [1]. Adequate calcium intake during childhood is essential to achieve optimal peak bone mass and this has the potential by increasing bone reserves to modulate the rate of bone loss that is associated inevitably with aging.
The role that supplemental calcium plays in improving bone mass and/or preventing bone loss and reducing fracture risk in older populations is hotly debated and in recent years this role has come under scrutiny because of studies suggesting potential adverse cardiovascular effects with supplemental calcium use. The purpose of this narrative review is to examine the relationship between supplemental calcium intake and skeletal and cardiovascular health in older individuals (defined arbitrarily as being more than 50 years of age) through an appraisal of studies done on the subject in the last three decades. The review also describes the rationale behind the current recommended dietary allowance of calcium and also provides a short description of the studies evaluating fractional absorption of calcium in the elderly since this has important implications on the efficacy of calcium.
Bioavailability of calcium in the elderly
Calcium retention in the body is determined by the relationship between calcium intake and calcium excretion and absorption. Calcium is absorbed in the small intestine by two general mechanisms: a transcellular active transport process, located largely in the duodenum and upper jejunum; and a paracellular, passive process that functions throughout the length of the intestine [2]. Calcium absorption efficiency influences calcium balance. Calcium absorption decreases with age [3, 4]. An additional decrease is documented in women at the time of menopause [5]. Inefficient absorption or malabsorption of calcium from the gastrointestinal tract can be due to a reduction in renal 1,25-dihydroxyvitamin D synthesis, a decline in intestinal vitamin D receptors or resistance to 1,25 D, 25-hydroxyvitamin D deficiency, or due to a decrease in active calcium transport [3, 4, 6, 7]. Ethnic variations in fractional absorption of calcium exist. White postmenopausal women have been shown to have significantly lower fractional absorption of calcium compared to Hispanic and Black women on dual isotope studies [8]. The average fractional calcium absorption amongst Chinese adults was 33% at an intake of 600 mg/day of calcium [9]. This is approximately 10% higher than Caucasian adults in the United States in whom the average intake of calcium is approximately 800 mg/day [10]. There may thus be a difference in the efficiency of utilization of dietary calcium between races. Fractional absorption of calcium has been shown to remain at a high level with little changes when calcium intake ranges between 300 and 1000 mg/day in Chinese adults [9]. This contrasts with that reported in Caucasians in whom fractional absorption reaches a peak of approximately 35% at a intake of about 400 mg and then falls off as intake increases further to a plateau of 20% with an intake of approximately 1000 mg [10]. Higher fractional absorption and retention of calcium reported in Asian Chinese [11] may thus be a physiological adaptation to low calcium intake that is characteristically seen amongst this population. It is probable the dietary calcium may be more efficiently utilized (with better fractional absorption and less excretion) in Chinese compared to Caucasians.
It is important to note that calcium absorption, metabolism and excretion is a collaborative process which is dependent upon a complex interaction with other nutrients such as dietary protein [12], vitamin D [13] and phosphorous [14]. High dietary protein intakes are well known to increase urinary calcium [15] excretion and some studies have concluded that dietary protein is an even more important regulator of urinary calcium than calcium intake [16]. This hypercalciuria was earlier thought to be exclusively secondary to increased bone resorption. However, recent studies suggest that alterations in dietary protein can profoundly affect intestinal calcium absorption. Increased dietary protein has been shown to be accompanied by a significant increase in intestinal calcium absorption in dual stable isotope studies [15]. Dietary protein intakes at and below 0.8 gm/kg were associated with reductions in intestinal calcium absorption sufficient to cause secondary hyperparathyroidism in the same study [15]. The long-term consequences of these low-protein induced changes in calcium metabolism are not known fully, but it may be detrimental to skeletal health especially in the elderly who are likely to have low protein diets. This needs to be elucidated further.
Calcium and bone health in the elderly
Peak bone mass in adulthood is predictive of subsequent bone density and, therefore, osteoporosis risk in later life [17, 18]. Short-term longitudinal studies appear to suggest that increasing consumption of calcium rich foods or supplementation with calcium in early life is associated with increased peak bone mass [19].Whether it can result in less loss of bone in later life is unclear. In the setting of chronic calcium deficiency due to habitual inadequate intake or poor intestinal absorption of calcium due to vitamin D deficiency, circulating calcium concentration is likely to be maintained at the expense of skeletal mass in the elderly unlike in the case of adolescents and young adults in whom circulating calcium levels can be maintained by increasing intestinal calcium absorption. The cumulative effect of calcium depletion on the skeleton over several years can contribute to the development of osteoporotic fractures with aging [4, 20]. Theoretically, a high calcium intake can decrease bone loss in the elderly by impacting remodeling. Bone remodeling is a sequential process involving bone resorption by osteoclasts followed by osteoblasts forming new bone. Bone loss occurs when bone resorption exceeds bone formation in each remodeling unit. Calcium by decreasing bone turnover and the number of bone remodeling units, can potentially result in a decrease in bone loss.
While available data to support optimizing this nutrient in growing children for bone accrual has been consistent, its effectiveness in adults and specifically the elderly is fraught with controversy. There have been numerous studies exploring the efficacy or lack thereof of calcium supplementation on bone health in elderly adults (Table 1). The results have been inconsistent and this is not unexpected given that these studies vary in design and the study populations and outcomes have been very heterogenous. There are several reasons why generalizing the results obtained from these studies is difficult. These include baseline differences in the study population’s dietary calcium intake, i.e., whether it is sufficient or insufficient and difficulty in separating out the effect of calcium versus vitamin D, since in most cases, they are co-administered. It is difficult to delineate doses that may be harmful or beneficial and, because the type, and doses of calcium and vitamin D vary amongst the trials, it is also difficult to isolate the effect of dietary calcium versus the effects of supplementation. It is also important to consider whether it was bone mineral density gains or fracture reduction that was the end point in the studies and important to consider the hormonal status of the subjects involved since the biological effects of the mineral may vary between pre and post-menopausal women. Lastly, adherence to the supplementation can also affect the outcomes studied.
Effects of dietary calcium and tablet supplementation on bone mineral density in the elderly
Studies of calcium supplementation in post-menopausal women have been mostly of short duration ranging from 1 to 5 years. These studies in general show that the effectiveness of calcium differs by skeletal site, by duration of menopause and that it also is dependent upon the quantity of habitual calcium intake.
Calcium supplementation during the first 5 years after menopause (when typically, bone loss is most rapid) was not shown to be effective in preventing bone loss from trabecular regions of the skeleton though there was a trend towards a slowed loss in cortical bone in one study [21]. Similar results of a lack of effect on slowing of trabecular bone loss, but with effect on slowing of cortical BMD loss with calcium supplementation in post-menopausal women has been shown in other studies also [22, 23]. The significance of this reduction in the rate of cortical bone loss noted in these studies and whether this reduction is sustained remains a topic of debate, however.
Calcium supplementation was found to increase BMD by 0.7–1.8% at all skeletal sites assessed at one, two and over two and a half years but the size of the increase in BMD at later time points was similar to the increase at 1 year in a random effects meta-analysis of 59 randomized controlled trials which included both studies exploring dietary sources of calcium as well as those that studied calcium supplements [24]. In a meta-analysis conducted to assess whether the assumption that the effects of calcium supplementation is the same year after year, it was found that the reduction in bone loss with calcium supplementation was significant only in the first year of supplementation [25].
The influence of gender on the efficacy of calcium on bone health is not well studied. A large cross-sectional study in Korea exploring associations between calcium intake with metabolic syndrome and bone mineral density in 14,705 men and women found that in men and in post-menopausal women, dietary calcium intake between 400 to ≤ 800 mg daily was associated with significantly increased BMD at both the femoral neck and lumbar spine [26].
Calcium supplementation appears to be more effective in maintaining bone density or retarding its loss in women who are more than 5 years postmenopausal [22, 27,28,29,30] and in women who have lower intakes of calcium at baseline [22]. The investigators of this latter randomized double-blind placebo-controlled trial surmised that healthy older women who had their menopause 6 years or longer before and had a daily calcium intake of less than 400 mg /day can significantly reduce bone loss by increasing their calcium intake to 800 mg /day [22].
The relative contributions of calcium and vitamin D to bone mineral density in the elderly have been explored in large cross-sectional studies such as the National Health and Nutrition Examination Survey (NHANES) III [31]. In NHANES III, it was found that the BMD of men and women increased with increasing serum 25(OH)D concentration independent of calcium intake. In the women, calcium intake was significantly and positively associated with femoral neck BMD in those with serum 25(OH)D concentrations below 50 nmol/L, but not in those with higher 25(OH)D concentrations. The investigators concluded that in both men and women, 25(OH)D status appeared to be the dominant predictor of BMD and that higher calcium intake was beneficial only in those women with 25(OH)D concentrations less than 50 nmol/L [31]. In men, there was no association of calcium intake with femoral neck BMD at any serum 25(OH)D concentrations. However, the mean calcium intake in the NHANES III cohort was over 700 mg/d in women and over 800 mg/d in men. These intakes are considerably higher than calcium intakes in many parts of the world, including Asian countries such as China, India, Japan, and Korea [32]. Whether the impact of calcium as shown above to be confined to those with low serum 25(OH)D concentrations, would be similarly restricted in populations with lower mean calcium intakes was explored in a study amongst South Korean adults aged 50 years and older with a mean calcium intake of only 485 mg/day in the Korean National Health and Examination Survey (KNHANES) [33]. In this study when associations of quintiles of calcium intake with bone mineral density (BMD) within three categories of serum 25(OH)D levels: < 50, 50–75, and > 75 nmol/L were explored, within each category of serum 25(OH)D, higher calcium intake was significantly positively associated with BMD of the femoral neck [33]. Within the lower two 25(OH)D categories, calcium intake was positively associated with BMD of the spine. In this low-intake population, calcium intake was found to be a significant determinant of BMD at higher as well as lower 25(OH)D levels. This finding indicates that low calcium intake cannot be compensated for with higher 25(OH)D levels alone. The investigators concluded that a calcium intake of at least 668 mg/d and a serum 25(OH)D level of at least 50 nmol/L may be needed to maintain bone mass in this calcium deficient population.
Effects of dietary calcium and tablet supplementation on fracture risk in the elderly
Though there have been several randomized control trials, systematic reviews and meta-analyses evaluating the benefit of calcium and vitamin D supplementation on osteoporotic fracture risk [34,35,36,37] the effectiveness of isolated calcium supplementation in the prevention of these fractures remains controversial. To study the association between dietary intake of calcium and fractures is even more challenging.
Dietary supplementation of calcium and its effect on fracture risk
The optimal amount of calcium needed (if at all there is such) to prevent fractures is not clear. Elderly subjects with higher milk or milk and yoghurt intakes were found to be at lower risk of hip fractures when assessed 10 years later in a subset of men and women from the Framingham Cohort study [38]. In this study, participants with medium (> 1 and < 7 servings/week) or higher (≥ 7 servings/week) milk intake tended to have lower hip fracture risk than those with low (≤ 1 serving/week) intake [38]. It was postulated that this effect was partially mediated by the effect of these dairy products on bone mineral density. A 30% reduced risk of fractures was reported in subjects with the highest quartile of calcium intake when compared to those in the lowest quartile in another study in which participants were drawn from the Melbourne Collaborative Cohort Study [39]. Increased fracture risk has been reported in women with daily calcium intakes less than 525 mg/day [40]. However, this association was more marked in younger women below the age of 50 than in older women and was not noted in men [40] and in a comprehensive systematic review and meta-analysis of studies that explored associations between calcium intake (from diet and/or supplementation) and risk of fracture in participants 50 years of age and older, most reported no associations between milk and dairy intake and fractures [36].
Non-dietary calcium supplementation and its effect on osteoporotic fracture risk
The efficacy of calcium supplementation in the form of tablets in the prevention of osteoporotic fractures remains controversial. In a large meta-analysis of pooled cohort studies and randomized controlled trials that included 170,991 women and 68,606 men, calcium intake was not significantly associated with any reduction in hip fracture risk and in fact, a potentially increased risk of hip fracture with calcium supplementation alone albeit in a relatively low number of participants was seen [35]. In the randomized trials that were included in the analysis there was a neutral effect of calcium supplementation on any non-vertebral fractures [35]. In another meta-analysis of controlled trials that randomized post-menopausal women to calcium supplementation or usual calcium intake and included 1806 participants who were followed for at least 1 year, there was no meaningful reduction in the incidence of non-vertebral fractures despite a notable increase in BMD [41]. The RECORD (Randomised Evaluation of Calcium Or Vitamin D) study which was a secondary prevention trial that evaluated the efficacies of supplementation with calcium, vitamin D or both in the elderly concluded that none of these methods were effective in reducing the risk of secondary low impact fractures [42]. The authors concluded that the findings did not support routine oral supplementation with calcium and vitamin D3, either alone or in combination, for the prevention of further fractures in previously mobile elderly people. Similar findings were noted in a recent meta-analysis in which it was noted that calcium with or without vitamin D supplementation did not decrease fractures in community dwelling older adults. Neither the incidence of hip, non-vertebral or vertebral or total fractures were lower with calcium supplementation when compared to placebo in this meta-analysis [43]. It has to be noted, however, that in the RECORD study, compliance with the supplements was only moderate with only 63% of study subjects taking the supplements at 2 years [42] and this thus raises questions on the generalizability of the findings noted. The findings from the RECORD study and the meta-analysis mentioned above are contrary to that reported in another meta-analysis in which combined supplementation with calcium and vitamin D resulted in a reduction in the risk of fractures in patients 50 years and older [34]. In this analysis which identified 17 trials reporting fracture as the outcome and included 52,625 subjects, studies of calcium, or calcium and vitamin D, were analysed separately. The majority of patients considered were included in trials of calcium and vitamin D supplementation (n = 46,108), with only 6517 subjects included in trials of calcium supplementation alone. The relative risk (RR) of any fracture with calcium and vitamin D supplementation was 0.87 (95% CI: 0.77, 0.97), and with calcium alone it was 0.90 (95% CI: 0.80, 1.00), i.e., it was of borderline statistical significance only. In a comprehensive systematic review and meta-analysis of associations between calcium intake (from diet and/or supplementation) and risk of fracture in which 26 trials (n = 69,107) of calcium supplementation, calcium and vitamin D supplementation or factorial designs using both approaches were identified, calcium supplements reduced the risk of total fracture [RR: 0.89, (95% CI: 0.81,0.96)] and vertebral fracture [RR: 0.86 (95% CI: 0.74, 1.00)], but not hip [RR: 0.95 (95% CI: 0.6,1.18)] or forearm fracture [RR: 0.96 (95% CI: 0.85, 1.09)] [36]. In the 4 randomized controlled trials included in the meta-analysis that had the lowest risk of bias, however, there was no effect of calcium supplementation alone on risk of fracture at any site. The authors concluded that the evidence that calcium supplements prevent fractures is weak and inconsistent [36].
Putting all the evidence together, majority of the randomized controlled trials and cohort studies suggest that the use of calcium in combination with vitamin D supplementation rather than its use as a sole agent may be more efficacious in reducing fracture risk. In an individual patient data (IPD) meta-analysis of seven individual randomized controlled trials with 68,500 subjects drawn from both residential/nursing homes and from the community and with duration of follow-up varying from 18 to 85 months, a modest reduction in all fractures [HR: 0.92 (95% CI: 0.86, 0.99)] and hip fractures [HR: 0.83 (95% CI: 0.69, 0.99) was noted for the combined use of calcium and vitamin D [44].Combined calcium and vitamin D administration was associated with a risk reduction for all fractures [RR: 0.92 (95% CI: 0.86, 0.99)] as well as hip fractures [RR: 0.84 (95% CI: 0.74, 0.96)] in the pooled random effects meta-analysis study described earlier [36]. More than 75% of the participants in the 4 trials that had the least risk of bias included in the above meta-analysis were contributed by the WHI trial where participants were allowed personal consumption of calcium and vitamin D supplements.
Fracture risk reduction with calcium and /or calcium with vitamin D supplementation has been noted to be significantly greater in those trials included in the meta-analysis in which the compliance rate was high. Treatment effect has also been noted to be better with calcium doses of 1200 mg or more than with doses less than 1200 mg and with vitamin D doses of 800 IU or more than with doses less than 800 IU [34]. In the Women’s Health Initiative calcium and vitamin D trial, where the adherence was only 59% at the end of the 7 year follow-up, calcium supplementation did not reduce hip fracture rates [45]. However, censoring data from women in the study when they ceased to adhere to the study medication reduced the hazard ratio for hip fracture to 0.71 (95% CI: 0.52, 0.97) [45]. Reduced risk of osteoporotic fractures and improvements in parameters related to bone density such as quantitative ultrasonography findings of the heel, femoral neck and whole-body dual X-ray absorptiometry data, and bone strength were observed in women who consumed more than 80% of their calcium supplements per year over 5 years compared with placebo-treated patients in a double-blind placebo-controlled trial of elderly women over the age of 70 years [46]. Adherence to the supplements thus appear to play a critical role in the efficacy of calcium supplements with or without vitamin D to reduce osteoporotic fracture risk. It is likely that calcium like vitamin D is a threshold nutrient. Patients who are deficient in the nutrient are at risk and may benefit from supplementation. Nutritional status and supplementation with nutrients including calcium and vitamin D have been shown to be related to outcomes and mortality in older adults with hip fracture who are likely to be deficient in these nutrients [47]. However, patients who have adequate intake of calcium would not benefit from taking calcium supplementation and the efficacy of calcium with or without vitamin D supplementation in healthy postmenopausal women at low risk of fractures is equivocal [45].
An expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF) concluded that though calcium and vitamin D supplementation leads to a modest reduction in fracture risk, supplementation with calcium alone for fracture reduction is not supported by the literature and that routine supplementation of calcium and vitamin D as a population-level intervention strategy is not recommended [48]. They have, however, opined that it appears reasonable to recommend calcium with concomitant vitamin D supplementation, for patients at high risk of calcium and vitamin D insufficiency, and in those who are receiving treatment for osteoporosis. This is supported by consensus guidelines as in the European guidance for the diagnosis and management of osteoporosis in postmenopausal women [49].
Calcium supplementation and cardiovascular risk in the elderly
The safety profile of calcium supplementation and its potential effects on especially the cardiovascular system have been of significant concern recently. The link between calcium supplementation and cardiovascular risk is controversial. The controversy started with the publication of a randomized controlled trial in 2008 in which an increased risk of self-reported myocardial infarction that was subsequently adjudicated by review of medical records was noted [RR: 2.24 (95% CI: 1.20, 4.17)] in patients who received calcium citrate supplementation [50]. The composite endpoint of myocardial infarction, stroke or sudden death was also statistically higher in the calcium supplemented group [RR: 1.66 (95% CI: 1.15,2.40)]. It is worth noting that calcium was not administered with vitamin D in this study and that the studied group received high calcium doses (1000 mg/day). This exceeded the RDA when the subjects’ average dietary calcium intakes (860 mg/day) was included. The same group of researchers then performed a meta-analysis of 16 studies that evaluated cardiovascular outcomes of patients who received ≥ 500 mg of calcium/day and that had included cardiovascular events obtained from self-reports, hospital admissions, and death certificates. What was found was a statistically significant difference in the incidence of myocardial infarction in the calcium treated group as compared to placebo in both the analysis of patient level data [HR: 1.31(95% CI: 1.02, 1.67, P = 0.035)] and in the analysis of trial level data [pooled RR 1.27 (95% CI: 1.01, 1.59, P = 0.038)]. There was a statistically significant interaction between treatment and baseline dietary calcium intake [51] with the association between supplemental calcium intake and myocardial infarction stronger when the median calcium intake was more than 825 mg per day. This interaction was observed only for the outcome of myocardial infarction. To counter the criticism that these meta-analyses only evaluated studies with isolated calcium supplementation and to explore further the interaction between treatment and baseline calcium intake, the same investigators extended their original analysis by including a re-analyses of the WHI calcium and vitamin D (WHI CaD) study public access dataset into the meta-analysis with eight other studies [52]. When the analysis was stratified according to personal use of calcium and vitamin D supplements, in those subjects not taking personal supplements at baseline it was found that there was an increased risk of myocardial infarction [RR:1.24 (95% CI: 1.07, 1.45), p = 0.004] and the composite of myocardial infarction or stroke [RR: 1.15 (95% CI: 1.03, 1.27, p = 0.009]. It must be noted that in the initial meta-analysis by this group, the increased risk of myocardial infarction was seen in those subjects with higher median baseline calcium (though dietary) intake as mentioned earlier whilst in this latter meta-analysis, it was in those who were not taking personal calcium supplements at baseline.
The original randomized controlled trial and the two meta-analysis have come under criticism for several reasons including the heterogeneity of event reporting and multiple end point testing. Other flaws that have been pointed out include the fact that majority of the myocardial infarctions were self-reported, cardiovascular events were not the primary outcome of either the former randomized trial or of the included studies in the meta-analyses, the potentially beneficial effects of supplementation were not emphasized [amongst those who were using personal calcium supplementation in the WHI study, death was actually greater in the placebo group (p = 0.01)] and the risk of stroke and the composite outcome of clinical myocardial infarction or stroke were less frequent in those who were taking personal supplements and that in the meta-analyses, studies that did not record myocardial infarction were not included [48, 53, 54]. Notwithstanding the above criticisms, some other studies have also reported an increased MI risk in adult men and women [55] and increased risk of death due to cardiovascular disease in men with calcium supplementation [56, 57].
The relationship between calcium supplementation and CVD risk and the plausible mechanism behind any potential effect of calcium on the cardiovascular system is unclear and tenuous at best. Calcium is not only required for contraction and relaxation of heart muscles, but it also serves as a second messenger in signal transduction pathways of the cardiovascular system. Transient receptor potential vanilloid 2 (TRPV2), a stretch-mediated channel and regulator of calcium homeostasis has been shown to be an important modulator of cardiomyocyte hypertrophy in response to cardiac aging in mice and it may play an important role in humans also [58]. The main hypothesis behind a possible mechanistic link for any deleterious effect of calcium on the cardiovascular system is that an acute elevation in serum calcium levels after supplementation may cause increased vascular resistance, calcification and thrombogenesis [28]. Acute increases in serum calcium have been observed after oral intake of calcium supplements in healthy male volunteers [59]. However, endothelial and myocardial perfusion parameters measured before and 3 h after supplementation with 1000 mg of calcium citrate have shown that arterial constriction decreases and myocardial perfusion increases with elevated serum calcium levels suggesting that it is unlikely that calcium supplementation is associated with adverse changes in cardiovascular function [60]. Observational studies suggest that serum calcium and/or phosphate concentrations may be related to ischemic cardiac events. However, evidence to suggest a link between serum calcium concentrations and coronary calcification is less clear A sex discordance appears to exist in the association between serum calcium and coronary artery calcification with serum total calcium concentrations positively associated with coronary artery calcification score on CT in men, but not amongst women in one study [61]. However, in another large prospective data base study- the AMORIS Swedish cohort, serum corrected calcium concentrations were associated with increased risk of incident myocardial infarction, non-fatal cardiovascular disease and stroke in women at a greater magnitude than in men [62]. The reason for this apparent conflicting sex discordance is unclear. Serum calcium levels in a multi-ethnic population of older men and women have been found to be associated with carotid plaque thickness; a predictor of clinical coronary and cerebrovascular events [63]. However, reassuringly, no increases in the risk of atherosclerotic plaques or carotid intima-media thickness was seen in elderly women who received calcium supplementation for 3 years in an ancillary study of the CAIFOS randomized controlled trial [64]. Several other studies also confirm this finding with no difference in calcium coronary scores noticed between women who received calcium and vitamin D supplementation for 7 years versus those who did not in the Women’s Health Initiative [65] and no increase in calcification noted in the carotid and coronary arteries or abdominal aorta of diabetic patients with either dietary intake or medicinal supplementation of calcium in another study [66]. Though no convincing benefit of either dietary or supplemental calcium intake on blood pressure (another possible ischemic heart disease risk factor) has been observed, there is no evidence of any deleterious effect either [67,68,69,70,71].
In contrast to the studies by Bolland et al. other investigators have failed to find an association between calcium supplementation and myocardial infarction or other cardiovascular complications. Calcium with vitamin D supplementation was shown to neither increase nor decrease coronary or cerebrovascular risk in generally healthy postmenopausal women over a 7-year use period in an initial assessment of the WHI trial in which cardiovascular disease was a prespecified secondary efficacy outcome [72]. It must be noted, however, that subgroup analysis was not performed in this study, and instead subjects who were or were not using personal supplements were analysed together, contrary to Bolland’s study that analysed subjects who were not on personal supplementation separately. The WHI study participant data were also re-evaluated with a focus on whether the participants were or were not using calcium supplements at the time of enrolment. No increased risk of myocardial infarction with calcium and vitamin D supplementation in either the entire trial population [HR: 1.03 (95% CI: 0.90, 1.19] or in those who were or were not on personal supplements [HR: 1.11 (95% CI: 0.90, 1.37)] was seen [73]. In an analysis of 20,024 participants of the third National Health and Nutrition Examination Survey (NHANES), no increase in cardiovascular mortality with either dietary or supplemental calcium intake was observed [74]. In a similar meta-analysis to that of Bolland et al. but using only trials in which coronary heart disease end point were validated and used ICD-based definitions, overall there was no effect of calcium or calcium and vitamin D supplementation on myocardial infarction [RR: 1.08 (95% CI: 0.93, 1.25)], angina pectoris [RR: 1.09 (95% CI: 0.95, 1.24)] or chronic heart disease [RR: 0.92 (95% CI: 0.73, 1.15)] was seen [75]. In the UK Biobank; a large prospective cohort comprising 502,637 men and women aged 40–69 years at recruitment, supplementation with calcium/vitamin D was self-reported, and information on incident hospital admission for ischemic heart disease myocardial infarction, and subsequent death was obtained from linkage to national registers. In crude and adjusted analyses, there were no associations between use of calcium supplements and risk of incident hospital admission with either ischemic heart disease or subsequent death. Results were similar for vitamin D and combination supplementation [76].
In summary (Table 2), the evidence for adverse cardiovascular effects with calcium supplementation remains inconclusive. The fact that cardiovascular events were not the primary outcome in any of the studies, were not ascertained and validated homogenously, the interactions between baseline calcium supplement intake and trial medications and the lack of a convincing mechanistic link to explain the adverse outcomes described, all raise doubts on whether truly a detrimental effect of calcium supplementation on the cardiovascular system exists. On the other hand, the signal of an increased risk of myocardial infarction repeatedly seen in several of the studies is not something that can be ignored. A large properly conducted randomized double blinded controlled trial with properly adjudicated and or ascertained cardiovascular end points is needed to convincingly answer this concern though the ethical issues that would surround such a trial would make it problematic to perform it.
Dietary calcium intake and its effect on cardiovascular outcomes in the elderly
There have been several studies that have specifically explored the influence of dietary calcium on cardiovascular outcomes. In the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition Study (EPIC-Heidelberg) where the associations of dietary calcium intake and calcium supplementation with MI and stroke risk and overall CVD mortality was assessed, compared with the lowest quartile, the third quartile of total dietary and dairy calcium intake had a significantly reduced MI risk with a HR of 0.69 (95% CI 0.50, 0.94) and 0.68 (95% CI 0.50, 0.93) respectively while only users of calcium supplement had a pronounced increase in MI risk [55]. Associations for stroke risk and CVD mortality were overall null with the dietary intake group. The authors surmised that though increasing calcium intake from the diet may not confer significant cardiovascular benefit, calcium from supplements may raise MI risk. It has to be noted, however, that this was a prospective cohort study and there were only 7 cases of myocardial infarctions in the calcium supplement only group [55]. The highest quartile (median of 1348 mg/day) of dietary calcium intake when compared to the lowest quartile (473 mg/day) was associated with a lower risk of non-fatal CVD [OR: 0.84 (95% CI 0.70, 0.99, p = 0.04)] and all-cause mortality [HR: 0.86 (95% ci [CI] 0.76–0.98, p = 0.01); in the population-based prospective Melbourne Collaborative Cohort Study [39]. A potential benefit of dietary rather than medicinal supplementation, is the minimization of the transient abrupt increases in serum calcium that has been described in patients taking calcium supplements [60] and what has been hypothesized to be the cause of increased cardiovascular risk in the latter group of patients.
Calcium intake and mortality in the elderly
Reassuringly, no study to date has demonstrated an association between supplemental calcium intake and increased mortality. In the meta-analysis by Bolland et al. that included the WHI CaD study, no effect on mortality was noted amongst the participants not using calcium/vitamin D personal supplementation [RR: 0.99 (95% CI: 0.86, 1.14)] and in those using personal supplementation at baseline, in fact a reduced risk of death [RR:0.84 (95% CI: 0.73, 0.97)] was seen [52]. In another meta-analysis of randomized controlled trials in which seventeen trials contributed all-cause mortality data, the pooled relative risk of mortality was 0.96 (95% CI: 0.91, 1.02; p = 0.18) [75] providing reassuring evidence that calcium with or without vitamin D supplementation does not appear to increase mortality risk in elderly individuals. In a study using pooled data from randomized controlled trials to assess mortality amongst participants assigned to vitamin D alone or vitamin D with calcium, when individual patient data (IPD) and trial level meta-analyses were performed, risk of death was reduced if vitamin D was given with calcium (HR: 0.91; 95% CI, 0.84, 0.98) in the IPD analysis while vitamin D alone did not reduce this risk [77]. Trial level meta-analysis (24 trials with 88,097 participants) also showed similar results, i.e., mortality was reduced with vitamin D plus calcium (odds ratio, 0.94; 95% CI, 0.88, 0.99), but not with vitamin D alone [OR: 0.98 (95% CI: 0.91, 1.06)] [77].
Dietary calcium intake and its effect on mortality
A study done in Sweden on two large cohorts, one with 61,433 women (39–74 years at baseline) and one with 45,339 men (45–79 years at baseline) had shown an association between dietary and total calcium intake and mortality from all causes, cardiovascular disease and ischemic heart disease, but not from stoke [adjusted hazard ratio of all-cause mortality 1.15 (95% CI: 1.13, 1.17) in women and 1.03 (95% CI: 1.01, 1.04) in men] [78]. It was cautioned that the observational study designs with the inherent possibility of residual confounding and reverse causation phenomena were likely responsible for this unexpected finding. In another Swedish cohort study, intakes of nonfermented milk and butter were found to be associated with higher all-cause mortality, and fermented milk and cheese intakes associated with lower all-cause mortality [79]. To shed more light on these findings a recent random-effect meta-analyses performed that included 29 cohort studies with 938,465 participants for total dairy (high and low fat), milk, fermented dairy, cheese and yoghurt was performed [80]. No associations were found for total dairy and milk with any of the outcomes of mortality, coronary heart disease or cardiovascular disease. A marginally inverse association of fermented dairy and cheese with mortality and CVD risk was attenuated in sensitivity analysis, and therefore, the investigators surmised that dairy products had no significant association with cardiovascular and all-cause mortality.
Defining recommendations for calcium intake in the elderly
In 2011, the Institute of Medicine (IOM), USA published Dietary Reference Intakes (DRIs) for calcium [81]. The IOM used evidence for skeletal health outcomes for basing the reference values for adequacy for calcium as well as vitamin D. Most of the evidence for the recommendations were derived from single dose studies and hence, a dose–response relationship for calcium was not elucidated. Discriminating the effect of calcium alone was not completely possible with the available data since in most of the studies, calcium and vitamin D were co-administered. There was also not enough data to recommend different calcium requirements for different ethnicities. The basis for the recommendation was derived from data obtained from 19 feeding studies undertaken by the USDA (US Department of Agriculture) in 155 American non-pregnant women and men between the ages of 20–75 years and who had daily calcium intakes of 415–1740 mg [10]. An intake of 741 mg/day was found to be sufficient in this analysis to achieve a neutral calcium balance [10]. This value was approximated to 800 mg /day and adopted as the estimated average requirement (EAR - corresponding to the median need of the population) for males above the age of 50 by the IOM, while the upper limit of the 95% prediction interval (1035 mg/day) was rounded to 1000 mg/day and adopted as the estimated average requirements for women above the age of 50. An additional 200 mg/day as extra allowance was given and the total adopted as the recommended dietary allowance (RDA) in men and women between the ages of 51–70 since this would cover the needs of 97.5% or more of the population (corresponding to two standard deviations above the EAR). Therefore, the RDA for men and women between the ages of 51–70 were set at 1000 mg/day and 1200 mg/day respectively. For adults above 70 years (both men and women), levels of 1000 mg/day were arbitrarily adopted as EAR, and therefore, 1200 mg/day was set as the RDA.
Despite these recommendations, it is well known that the intake of dietary calcium varies widely geographically. Across 74 countries that had data and from which studies were included in a systematic review that drew attention to regions where calcium intake needs to be assessed, it was shown that average national dietary calcium intake ranges from 175 to 1233 mg/day. This review formed the basis for the development of the International Osteoporosis Foundation’s Global Calcium Map (https://www.iofbonehealth.org/facts-and-statistics/calcium-map). This map reveals that many countries in Asia have average dietary calcium intake less than 500 mg/day. Countries in Africa and South America mostly have low calcium intake between about 400 and 700 mg/day. Only Northern European countries have national calcium intake averaging greater than 1000 mg/day [32]. This observation could have important implications. Amongst populations such as Caucasians who are at higher risk for osteoporotic fractures, habitual calcium intakes below the UK and EU lower reference value of 400 m/day may be associated with an increased risk of these fractures. In studies from the USA, Italy, UK and Hong Kong, it has been shown that where average dietary intake is low, the risk of hip fracture increases at intakes below the average, but there is no continued risk reduction at intakes higher than the average. In those countries with higher average intakes, there is no evidence of a gradient of fracture risk with calcium intake [82].
Customary dietary calcium intake may also have a bearing on the purported adverse cardiovascular effects of calcium supplementation. As noted previously, in the original randomized controlled trial performed by Bolland et al. [50] the studied group receiving a supplemental intake of 1000 mg of calcium per day had an average dietary calcium intake of 860 mg/day. Whether the same findings would be seen in populations where the customary dietary calcium is much lower is a matter that needs to be elucidated further.
Conclusion
There is no doubt that calcium plays a vital role in human musculoskeletal health. Adequate calcium intake during childhood is essential to achieve optimal peak bone mass and this has the potential by increasing bone reserves to modulate the rate of age-associated bone loss. Although routine supplementation of calcium and vitamin D as a population-level intervention strategy is not recommended based on the evidence, prescribing calcium and vitamin D supplementation only to those individuals who would benefit from such treatment, i.e., those who are at high risk for osteoporosis and fragility fractures and in those who cannot achieve recommended daily requirements appears to be a sensible approach. Dietary intake of calcium has so far not exhibited deleterious cardiovascular effects and intakes as per national guidelines should be followed. The attribution of increased cardiovascular risk to calcium supplement use appears to be restricted to a few studies and they are not convincingly supported by most others. Neither do the plausible mechanisms that are purported to account for the deleterious effects of calcium supplementation on cardiovascular health hold up to rigorous scrutiny. Further studies aimed at assessing the risk–benefit ratios of calcium supplementation in different populations and to delineate the differences in efficiencies in fractional absorption of calcium between different ethnicities and whether this would have a bearing on recommended daily requirements is also essential.
References
Nordin BE (1997) Calcium and osteoporosis. Nutrition 13:664–686
Bronner F (2003) Mechanisms of intestinal calcium absorption. J Cell Biochem 88:387–393
Pattanaungkul S, Riggs BL, Yergey AL et al (2000) Relationship of intestinal calcium absorption to 1,25-dihydroxyvitamin D [1,25(OH)2D] levels in young versus elderly women: evidence for age-related intestinal resistance to 1,25(OH)2D action. J Clin Endocrinol Metab 85:4023–4027
Nordin BEC, Need AG, Morris HA (2004) Effect of age on calcium absorption in postmenopausal women. Am J Clin Nutr 80:998–1002
Heaney RP, Recker RR, Stegman MR et al. (1989) Calcium absorption in women: relationships to calcium intake, estrogen status, and age. J Bone Miner Res 4:469–475
Tsai KS, Heath H, Kumar R et al. (1984) Impaired vitamin D metabolism with aging in women. Possible role in pathogenesis of senile osteoporosis. J Clin Invest 73:1668–1672
Morris HA, Need AG, Horowitz M, O’Loughlin PD, Nordin BE (1991) Calcium absorption in normal and osteoporotic postmenopausal women. Calcif Tissue Int 49:240–243
Ramsubeik K, Keuler NS, Davis LA et al. (2014) Factors associated with calcium absorption in postmenopausal women: a post hoc analysis of dual-isotope studies. J Acad Nutr Dietetics 114:761–767
Fang AP, Li KJ, Shi HY et al. (2016) Habitual dietary calcium intakes and calcium metabolism in healthy adults Chinese: a systematic review and meta-analysis. Asia Pac J Clin Nutr 25:776–784
Hunt CD, Johnson LK (2007) Calcium requirements: new estimations for men and women by cross-sectional statistical analyses of calcium balance data from metabolic studies. Am J Clin Nutr 86:1054–1063
Kung AW, Luk KD, Chu LW et al. (1998) Age-related osteoporosis in Chinese: an evaluation of the response of intestinal calcium absorption and calcitropic hormones to dietary calcium deprivation. Am J Clin Nutr 68:1291–1297
Kerstetter JE, O’Brien KO, Insogna KL (2003) Dietary protein, calcium metabolism, and skeletal homeostasis revisited. Am J Clin Nutr 78:584S–592S
Heaney RP (2008) Vitamin D and calcium interactions: functional outcomes. Am J Clin Nutr 88:541S–544S
Fenton TR, Lyon AW, Eliasziw M et al. (2009) Phosphate decreases urine calcium and increases calcium balance: a meta-analysis of the osteoporosis acid-ash diet hypothesis. Nutr J 8:41
Kerstetter JE, O’Brien KO, Insogna KL (2003) Low protein intake: the impact on calcium and bone homeostasis in humans. J Nutr 133:855S–861S
Zemel MB (1988) Calcium utilization: effect of varying level and source of dietary protein. Am J Clin Nutr 48:880–883
Hansen MA, Overgaard K, Riis BJ et al. (1991) Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. BMJ 303:961–964
Riis BJ, Hansen MA, Jensen AM et al. (1996) Low bone mass and fast rate of bone loss at menopause: equal risk factors for future fracture: a 15-year follow-up study. Bone 19:9–12
Matkovic V, Goel PK, Badenhop-Stevens NE et al. et al (2005) Calcium supplementation and bone mineral density in females from childhood to young adulthood: a randomized controlled trial. Am J Clin Nutr 81:175–188
Heaney RP (2002) The importance of calcium intake for lifelong skeletal health. Calcif Tissue Int 70:70–73
Riis B, Thomsen K, Christiansen C (1987) Does calcium supplementation prevent postmenopausal bone loss? A double-blind, controlled clinical study. N Engl J Med 316:173–177
Dawson-Hughes B, Dallal GE, Krall EA et al (1990) A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N Engl J Med 323:878–883
Elders PJ, Lips P, Netelenbos JC et al (1994) Long-term effect of calcium supplementation on bone loss in perimenopausal women. J Bone Miner Res 9:963–970
Tai V, Leung W, Grey A et al. (2015) Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ 351:h4183
Mackerras D, Lumley T (1997) First- and second-year effects in trials of calcium supplementation on the loss of bone density in postmenopausal women. Bone 21:527–533
Kim MK, Chon SJ, Noe EB et al (2017) Associations of dietary calcium intake with metabolic syndrome and bone mineral density among the Korean population: KNHANES 2008–2011. Osteoporos Int 28:299–308
Nelson ME, Fisher EC, Dilmanian FA et al. (1991) A 1-y walking program and increased dietary calcium in postmenopausal women: effects on bone. Am J Clin Nutr 53:1304–1311
Reid IR, Ames RW, Evans MC et al. (1993) Effect of calcium supplementation on bone loss in postmenopausal women. N Engl J Med 328:460–464
Chevalley T, Rizzoli R, Nydegger V et al (1994) Effects of calcium supplements on femoral bone mineral density and vertebral fracture rate in vitamin-D-replete elderly patients. Osteoporos Int 4:245–252
Prince R, Devine A, Dick I et al (1995) The effects of calcium supplementation (milk powder or tablets) and exercise on bone density in postmenopausal women. J Bone Miner Res 10:1068–1075
Bischoff-Ferrari HA, Kiel DP, Dawson-Hughes B et al (2009) Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. J Bone Miner Res 24:935–942
Balk EM, Adam GP, Langberg VN et al (2017) Global dietary calcium intake among adults: a systematic review. Osteoporos Int 28:3315–3324
Joo NS, Dawson-Hughes B, Kim YS (2013) Impact of calcium and vitamin D insufficiencies on serum parathyroid hormone and bone mineral density: analysis of the fourth and fifth Korea National Health and Nutrition Examination Survey (KNHANES IV-3, 2009 and KNHANES V-1, 2010). J Bone Miner Res 28:764–770
Tang BMP, Eslick GD, Nowson C et al. (2007) Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet 370:657–666
Bischoff-Ferrari HA, Dawson-Hughes B, Baron JA et al (2007) Calcium intake and hip fracture risk in men and women: a meta-analysis of prospective cohort studies and randomized controlled trials. Am J Clin Nutr 86:1780–1790
Bolland MJ, Leung W, Tai V et al (2015) Calcium intake and risk of fracture: systematic review. BMJ 351:h4580
Weaver CM, Alexander DD, Boushey CJ et al (2016) Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int 27:367–376
Sahni S, Mangano KM, Tucker KL et al (2014) Protective association of milk intake on the risk of hip fracture: results from the Framingham Original Cohort. J Bone Miner Res 29:1756–1762
Khan B, Nowson CA, Daly RM et al (2015) Higher dietary calcium intakes are associated with reduced risks of fractures, cardiovascular events, and mortality: a prospective cohort study of older men and women. J Bone Miner Res 30:1758–1766
Key TJ, Appleby PN, Spencer EA et al (2007) Calcium, diet and fracture risk: a prospective study of 1898 incident fractures among 34 696 British women and men. Public Health Nutr 10:1314–1320
Shea B, Wells G, Cranney A et al (2002) Meta-analyses of therapies for postmenopausal osteoporosis. VII. Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis. Endocr Rev 23:552–559
Grant AM, Avenell A, Campbell MK et al (2005) Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet 365:1621–1628
Zhao JG, Zeng XT, Wang J et al. (2017) Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA 318:2466–2482
DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group (2010) Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ 340:b5463
Jackson RD, LaCroix AZ, Gass M et al (2006) Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 354:669–683
Prince RL, Devine A, Dhaliwal SS et al. (2006) Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch Intern Med 166:869–875
Malafarina V, Reginster JY, Cabrerizo S et al (2018) Nutritional status and nutritional treatment are related to outcomes and mortality in older adults with hip fracture. Nutrients 10:E555. https://doi.org/10.3390/nu10050555
Harvey NC, Biver E, Kaufman JM et al (2017) The role of calcium supplementation in healthy musculoskeletal ageing: an expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF). Osteoporos Int 28:447–462
Kanis JA, Cooper C, Rizzoli R et al. (2019) Scientific advisory board of the european society for clinical and economic aspects of osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 30:3–44
Bolland MJ, Barber PA, Doughty RN et al (2008) Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 336:262–266
Bolland MJ, Avenell A, Baron JA et al (2010) Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ 341:c3691
Bolland MJ, Grey A, Avenell A et al. (2011) Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ 342:d2040
Nordin BEC, Lewis JR, Daly RM et al (2011) The calcium scare—what would Austin Bradford Hill have thought? Osteoporos Int 22:3073–3077
Body JJ, Bergmann P, Boonen S et al (2012) Extraskeletal benefits and risks of calcium, vitamin D and anti-osteoporosis medications. Osteoporos Int 23:S1–S23
Li K, Kaaks R, Linseisen J, Rohrmann S (2012) Associations of dietary calcium intake and calcium supplementation with myocardial infarction and stroke risk and overall cardiovascular mortality in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition study (EPIC-Heidelberg). Heart 98:920–925
Pentti K, Tuppurainen MT, Honkanen R et al (2009) Use of calcium supplements and the risk of coronary heart disease in 52-62-year-old women: the Kuopio Osteoporosis Risk Factor and Prevention Study. Maturitas 63:73–78
Xiao Q, Murphy RA, Houston DK, Harris et al (2013) Dietary and supplemental calcium intake and cardiovascular disease mortality: the National Institutes of Health-AARP diet and health study. JAMA Internal Med 173:639–646
Jones S, Mann A, Worley MC et al (2017) The role of transient receptor potential vanilloid 2 channel in cardiac aging. Aging Clin Exp Res 29:863–873
Deroisy R, Zartarian M, Meurmans L et al (1997) Acute changes in serum calcium and parathyroid hormone circulating levels induced by the oral intake of five currently available calcium salts in healthy male volunteers. Clin Rheumatol 16:249–253
Burt MG, Mangelsdorf BL, Srivastava D et al. (2013) Acute effect of calcium citrate on serum calcium and cardiovascular function. J Bone Miner Res 28:412–418
Grønhøj MH, Gerke O, Mickley H et al (2016) Associations between calcium-phosphate metabolism and coronary artery calcification; a cross sectional study of a middle-aged general population. Atherosclerosis 251:101–108
Rohrmann S, Garmo H, Malmström H et al (2016) Association between serum calcium concentration and risk of incident and fatal cardiovascular disease in the prospective AMORIS study. Atherosclerosis 251:85–93
Rubin MR, Rundek T, McMahon DJ et al (2007) Carotid artery plaque thickness is associated with increased serum calcium levels: the Northern Manhattan study. Atherosclerosis 194:426–432
Lewis JR, Zhu K, Thompson PL et al. (2014) The effects of 3 years of calcium supplementation on common carotid artery intimal medial thickness and carotid atherosclerosis in older women: an ancillary study of the CAIFOS randomized controlled trial. J Bone Miner Res 29:534–541
Manson JE, Allison MA, Carr JJ et al (2010) Calcium/vitamin D supplementation and coronary artery calcification in the Women’s Health Initiative. Menopause 17:683–691
Raffield LM, Agarwal S, Cox AJ et al (2014) Cross-sectional analysis of calcium intake for associations with vascular calcification and mortality in individuals with type 2 diabetes from the Diabetes Heart Study. Am J Clin Nutr 100:1029–1035
Chai W, Cooney RV, Franke AA et al. (2013) Effects of calcium and vitamin D supplementation on blood pressure and serum lipids and carotenoids: a randomized, double-blind, placebo-controlled, clinical trial. Ann Epidemiol 23:564–570
Reid IR, Horne A, Mason B et al (2005) Effects of calcium supplementation on body weight and blood pressure in normal older women: a randomized controlled trial. J Clin Endocrinol Metab 90:3824–3829
Reid IR, Ames R, Mason B et al (2010) Effects of calcium supplementation on lipids, blood pressure, and body composition in healthy older men: a randomized controlled trial. Am J Clin Nutr 91:131–139
Pfeifer M, Begerow B, Minne HW et al. (2001) Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab 86:1633–1637
Wang L, Manson JE, Buring JE et al. (1979) Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension (Dallas Tex 51:1073–1079 2008
Hsia J, Heiss G, Ren H et al (2007) Calcium/vitamin D supplementation and cardiovascular events. Circulation 115:846–854
Prentice RL, Jackson RD, Rossouw JE (2013) Calcium supplements and cardiovascular risk in the Women’s Health Initiative: response to Bolland et al. Osteoporos Int 24:2373–2374
Van Hemelrijck M, Michaelsson K, Linseisen J, Rohrmann S (2013) Calcium intake and serum concentration in relation to risk of cardiovascular death in NHANES III. PloS One 8:e61037
Lewis JR, Radavelli-Bagatini S, Rejnmark L et al (2015) The effects of calcium supplementation on verified coronary heart disease hospitalization and death in postmenopausal women: a collaborative meta-analysis of randomized controlled trials. J Bone Miner Res 30:165–175
Harvey NC, D’Angelo S, Paccou J et al (2018) Calcium and Vitamin D Supplementation Are Not Associated With Risk of Incident Ischemic Cardiac Events or Death: Findings From the UK Biobank Cohort. J Bone Miner Res 33:803–811
Rejnmark L, Avenell A, Masud T et al (2012) Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. J Clin Endocrinol Metab 97:2670–2681
Michaëlsson K, Wolk A, Langenskiöld S et al (2014) Milk intake and risk of mortality and fractures in women and men: cohort studies. BMJ 349:g6015
Tognon G, Nilsson LM, Shungin D et al (2017) Nonfermented milk and other dairy products: associations with all-cause mortality. Am J Clin Nutr 105:1502–1511
Guo J, Astrup A, Lovegrove JA et al (2017) Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol 32:269–287
Ross AC, Manson JE, Abrams SA et al (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96:53–58
Prentice A (2004) Diet, nutrition and the prevention of osteoporosis. Public Health Nutr 7:227–243
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Manju Chandran, Donovan Tay and Ambrish Mithal do not have any Conflicts of interest or disclosures.
Statement of human and animal rights
This is a review article and none of the authors have performed any studies with human or animal participants for it.
Informed consent
This is a review paper and does not require informed consent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chandran, M., Tay, D. & Mithal, A. Supplemental calcium intake in the aging individual: implications on skeletal and cardiovascular health. Aging Clin Exp Res 31, 765–781 (2019). https://doi.org/10.1007/s40520-019-01150-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-019-01150-5