Abstract
Objective
A previous multidisciplinary pilot study based on computer simulations for the geriatric population showed that a dose of 0.5 mg/kg/h of propofol could sedate patients older than 65 for pacemaker implantation. The present study validates that the pacemaker implantation can be done in the elderly using 0.5–1 mg/kg/h of propofol with hemodynamic stability.
Methods
66 patients from 65 to 88 years old scheduled for pacemaker implantation were randomly assigned one of three doses of propofol. The first group received 2 mg/kg/h of propofol (P2) that is within normal range of the sedation dose. The second group received 1 mg/kg/h (P1) dose and the third group received the dose of 0.5 mg/kg/h (P0.5) according to the simulation-predicted dose for geriatric populations.
Results
All patients kept MAP between 76 and 85 mmHg, with no hypotension episodes in any of the groups; therefore, they were all hemodynamically stable during the procedure. BIS was between 80 and 65 during the pacemaker implantation for the three groups, BIS of group P2 was significantly lower than the other groups. BIS in groups P1 and P0.5 was within the appropriated range for moderate sedation. Brice was positive for auditory recalls only when there was arousing noise in the operating room.
Conclusions
Moderate sedation, adequate for pacemaker implantation, can be achieved infusing 0.5–1 mg/kg/h of propofol in elderly patients when the patient has proper analgesia management at the device implantation site. The second important condition is to avoid unnecessary and alerting auditory and mechanical stimuli in the operating room, so that the patient will remain calm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global average life expectancy duration increased by 20 years from 1960 to 2014; consequently, there are more elective cardiac surgeries scheduled for patients 65 and older. Pacemaker implantation is one of the most common procedures in geriatrics, but there is no consensus on the anesthetic strategy for the implantation of cardiovascular electronic devices. Some approaches include only local anesthesia, while others include sedation [1]. Sedation is naturally preferred over general anesthesia, because it reduces the total procedure time up to \(21\%\) and reduces concomitant risk of adverse events. Sedation also reduces cost up to \(72\%\) over general anesthesia [2].
There are three levels of sedation, (1) mild, in which the patient still responds normally to verbal stimulation. (2) Moderate sedation, when patients follow simple orders or respond to tactile stimulation, no ventilation support is required, cardiovascular functions are normal, and BIS values are between 80 and 70. Finally, (3) deep sedation occurs when the patient responds only to painful stimulation, a situation that may require airway intervention and usually BIS index values fall to a range of 60–70 [3].
Propofol is used for sedation in minimally invasive cardiac procedures, because it offers rapid onset, and offset; it is associated with lower incidence of postoperative delirium in elderly [4]; however, it also may cause bradycardia, respiratory depression, and hypotension in a dose-dependent manner. Dehydration is a common condition in elderly patients, and it makes them more susceptible to hypotension, because propofol decreases heart contractibility [5, 6]. Therefore, it is important to calculate a minimal optimal dose for the elderly population.
The standard propofol dose for sedation for geriatric patients is 1.2–3.6 mg/kg/h which represents \(80\%\) of the younger adult dose [7, 8]. A recent study reported that in propofol sedation, procedural-directed nurse-administrated techniques (PDNA) during implantation of electrotherapeutic cardiovascular devices, the administered propofol dose was 6–9 mg/kg/h of propofol during 3–5 min to induce sedation. Infusion rates were 1.5–4.5 mg/kg/h, compared to doses of 1.2–3.6 mg/kg/h in patients older than 55. Most frequent adverse events, such as hypoxemia and hypotension, were associated with higher doses of propofol [9].
In a study with 1000 patients undergoing auricular ablation for atrial fibrillation with deep sedation induced by propofol infusion, \(13.6\%\) of patients developed hypotension and \(1.9\%\) respiratory depression. There was a positive correlation between age and blood pressure reduction [6].
The elderly demonstrate greater risk of presenting propofol adverse effects because of the decline in the functional reserve of multiple organs and systems and the co-morbidities highly prevalent in this population [10, 11].
In 2010, Sieber et al. demonstrated that the standard propofol dose used for sedation in the elderly frequently induced hypnosis levels of general anesthesia, this increases the risk of severe adverse events in simple procedures [12]. Considering that the adverse events of propofol are dose-dependant, it is necessary to establish optimal doses for elderly patients.
In 2016, a pilot study based on computer simulation of a pharmacokinetic–pharmacodynamic model (PK–PD model) of propofol designed to fit specifically the elderly was published. The study used an automatic control algorithm to calculate the dose of propofol based on the bispectral index response (BIS) of every in silico patient. The range of propofol calculated to reach moderate sedation was 0.42–0.96 mg/kg/h, and the dose was validated with a pilot clinical study with 30 patients [13].
The aim of this study is to validate the minimal dose of propofol suitable to reach moderate sedation in patients undergoing pacemaker implantation, and not only to achieve adequate hypnosis, but also to ensure hemodynamic stability and avoid traumatic experiences during the procedure.
Methods
This study protocol was approved by the Institutions’ Ethical and Research Committee. All participants provided signed informed consent. After enrollment, no patients were excluded.
The study was designed as an equivalence test to assess if a 1 and 0.5 mg/kg/h dose had the same clinical effect on the elderly, as the 2 mg/kg/h dose. Since the Bispectral Index (BIS) for optimal sedation is in the 80–70 range, 75 was selected as the target for moderate sedation [14] with a tolerance of \(\delta =13\), type 1 error \(2\alpha = 0.5\), and type 2 error \(\beta = 0.2\) [15]. Each group included 22 patients with a total of 66 patients.
Inclusion criteria included 66 patients 65 years and older scheduled for pacemaker implantation, with a physical status ASA II-IV. Exclusion criteria were: patients with ejection fractions lower than \(30\%\), known allergy to propofol, as well as intubated patients.
The patients were randomized using a closed envelope method into one of three groups:
-
1.
P2 Propofol dose of 2 mg/kg/h
-
2.
P1 Propofol dose of 1 mg/kg/h
-
3.
P0.5 Propofol dose of 0.5 mg/kg/h
Patients were blinded with respect to the group that they were assigned, but the anesthesiologist and surgical team were not. Upon the patient arrival to the operating room, 300 ml of Ringer’s lactate solution was intravenously infused to pre-empt any effect of dehydration. The patients were monitored with non-invasive blood pressure and heart rate monitors, pulse oximetry, and bispectral index (BIS). All readings were recorded every 5 min. Low flow oxygen via nasal cannula (2 l/min) was administered to each patient. After basal vital signs were established, propofol infusion was started at one of the three predetermined levels.
Patients received propofol doses by intravenous infusion according to the group to which they were assigned, propofol was not diluted and was injected to the catheter port nearest the patient after the Ringer’s lactate solution was infused. No loading dose was administered to avoid transitory rapid decreased in blood pressure and respiratory rates. Longer periods of time were expected, to reach sedation, because no loading dose was given. Five minutes after propofol infusion was started, local anesthesia was injected subcutaneously in the pectoral area: 10 ml lidocaine \(2\%\) with epinephrine (1:200,000) and 10 ml ropivacaine \(0.7\%\), where a subcutaneous pocket for the pacemaker is made. Verbal and tactile stimulation were avoided during sedation and throughout out the rest of the procedure. Propofol infusion stopped at the end of the procedure.
BIS was used only as an outcome measure and the propofol infusion rate was fixed by the protocol group allocation. Anesthesiologists were advised to look for clinical signs of inadequate sedation, like blood pressure increase \(20\%\) over the basal value, and voluntary movements due to pain or discomfort. If the clinical signs were consistent with inadequate sedation, the patient would be treated using a propofol infusion guided by BIS having 70 as the target.
In case of low cardiac function, the patients would receive 0.02–0.2 mcg/kg/min of norepinephrine until the patient was hemodynamical stable.
After pacemaker implantation, in the recovery room, intraprocedural awareness was assessed using the Brice questionnaire [16]. The patient answered five questions:
-
1.
What is the last thing you remember before going to sleep for the operation?
-
2.
What is the first thing you remember after waking after the operation?
-
3.
Do you remember anything in between?
-
4.
Did you have any dreams?
-
5.
What was the most unpleasant thing you remember from your operation and anesthesia?
Aldrete’s Score was assessed when the patients arrived to recovery room, and at minutes 5, 30, and 60. If after 1 h, the Aldrete’s Score remained 10, then 100 mg of lysine clonixinate was administered intravenously, and the patient was discharged. The home analgesia recommendation was 125 mg of oral lysine clonixinate every 12 h for 3 days if required.
Statistical analysis
Continuous variables are reported as mean ± standard deviation, and categorical variables as n. Kruskal–Wallis tests were performed for comparing continuous variables and Chi-square test was performed to compare categorical variables.
Results
Demographic data of the studied population are shown in Table 1. There was no significant difference in male/female ratio, age, weight, and body mass index (BMI) between the three groups. There was significant difference in procedure time but not in the total sedation time between the groups. None of the patients presented any adverse events or clinical signs of awareness, and the propofol doses were kept for every patient until the end of procedure. There was no significant difference in basal values of BIS, heart rate, and SpO\(_2\) between the three groups.
Figure 1 shows that BIS values do not drop to the sedation range (80–70) until minute 10, because no loading propofol bolus was administered. During the rest of the procedure, all patients remained in the moderate sedation rage of BIS. None of the patients required a change of propofol dose, because even the minimal dose used provided adequate sedation.
There was a statistically significant difference between groups in the basal Mean Arterial Pressure (MAP), \(p = 0.041\), but it was not clinically relevant, as can be seen in Fig. 2. The group with the lowest basal MAP was the P0.5. During the procedure, MAP remained between 72 and 85 mmHg. There was no statistically significant difference at minutes 5 and 10 across the groups (\(p_{5\text {min}}=0.08\), \(p_{10\text {min}}=0.3\)). Thus, the induction of sedation resulted in similar MAP for the three groups. At minute 30, and at the end of surgery, there was statistically significant difference between the groups. The group with the lowest MAP was P2, but there was no clinical difference as can be seen in Fig. 2. All patients kept adequate blood pressure values, and P0.5 group had the highest MAP during the procedure. It was not necessary to use norepinephrine in any of the patients.
The heart rate is plotted in Fig. 3, it can be seen that the three groups had very similar results. The basal SpO\(_2\) was between 95 and \(97\%\) for all groups, before placement of nasal cannula, but for the rest of the procedure, SpO\(_2\) values remained above \(98\%\) due to the low flow oxygen, as shown in Fig. 4.
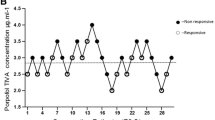
Awareness during procedure was assessed with the Brice questionnaire after the procedure, two patients had auditory recalls in group P2, one patient had auditory recalls in group P0.5, and no patient had recall in group P1. The recalls coincide with moments when there was arousing noise outside the operating room.
Aldrete’s Score is reported in Table 1. By minute 30, every patient had a stable Aldrete’s Score of 10 and 60 min after arriving to the recovery room they were discharged. The patients were encouraged to report any pain during their stay in recovery room and none of them reported any pain or discomfort.
Discussion
Current sedation guidelines for elderly patients indicate that sedation is induced with a bolus of 4.8–7.2 mg/kg/h of propofol to reach peak drug effect. Maintenance dose is 1.2–3.6 mg/kg/h. These doses correspond to \(80\%\) of the doses for adult patients under 65 years old [7, 9]. Propofol dose reduction is justified, because geriatric patients have increased sensitivity to propofol [17, 18]. Sieber et al. demonstrated that the dose used for sedation frequently induce BIS levels corresponding to general anesthesia that increased the risk associated with anesthesia [12].
In a study based on a PK–PD model, optimal doses were calculated for the elderly using a closed loop algorithm. The calculated doses were 0.42–0.96 mg/kg/h [13]. Since the deleterious effects of propofol on blood pressure and respiratory rate are dose-dependent, the aim of this study was to determine if pacemaker implantation could be performed under reduced doses of propofol to maintain moderate sedation and hemodynamic stability. The doses tested in this study were 2, 1, and 0.5 mg/kg/h.
Propofol sedation has several advantages, namely, it avoids post-anesthetic nausea, rapid emergence if neurological evaluation is necessary, as well as fast sedation and anesthesia induction; however, caution is advised for cardiovascular procedures, since ventricular performance is affected. Patients undergoing pacemaker implantation present bradycardia, and therefore, it is necessary to achieve optimal propofol dosing to avoid severe hypotension secondary to propofol administration during the procedure.
We did not see any of the negative effects of propofol in any of the sedated patients in this study. While the P0.5 group’s MAP rose slightly during the procedure, this did not represent discomfort or increased awareness as determined by clinical observations, BIS values, and the Brice questionnaire. None of the patients required a change of propofol dose (protocol contingency). No loading dose was used, because the elderly population usually has reduced blood volume and this would produce a higher initial propofol plasma concentration that could induce a transitory hypotensive event [11].
Pandya et al. demonstrated in 2009 that propofol used with midazolam and fentanyl could be used for cardiac device implantation avoiding hypotension episodes; when the total dose of propofol was less than 203 mg [19], in our study, the average total propofol dose was 155 mg for the P2 group, 84.6 mg for P1 group and 43.9 mg for the P0.5 group. There is a significant difference between the dose used in both studies, even though the procedure time is similar. Dr. Pandya’s study was not specifically focused on an elderly population; however, the doses used in our study can be compared, because the mean age of the patients is comparable, 69 versus 71 years, respectively.
In 2009, Keyl et al. used deep sedation with target control infusion (TCI) of propofol with the Schnider Model, and used BIS as the outcome measure. The dose was manually adjusted every 3 min until achieving the desired level of sedation assessed by a score 1 of the Observer’s Assessment of Alertness/Sedation (OAA/S) [20]. This process is time-consuming and can become risky. Some of their patients had BIS values lower than 30 that is below the ideal BIS for general anesthesia of 50. In our study, we demonstrate that moderate sedation (BIS = 75) is sufficient to perform the pacemaker implantation procedure and the minimal dose of propofol avoids hemodynamic instability, which is the chief unwanted side effect that has limited the use of propofol in these kinds of procedures. The main difference in the methodology is that, in our study, the dose was calculated for the population and desired level of sedation and predicted with computer simulations [13]. This precalculation allowed us to efficiently achieved adequate level of sedation while avoiding the undesirable effects of propofol; furthermore, our study did not used any opioids or benzodiazepines.
Other approaches for sedation of cardiac patients include the use of dexmedetomidine, because it has rapid onset and offset with minimal respiratory depression, but caution is advised in patients with predisposition to cardiac electrical conduction abnormalities, and patients with pacemakers, because dexmedetomidine can cause elevation of pacing threshold resulting in noncapture of the pacing impulses and arrhythmias[21, 22].
The sedation target level for pacemaker implantation is moderate sedation, because in this stage, the patient is calm and motionless, and usually maintains spontaneous ventilation, deeper sedation would increase the risk of respiratory depression, that could require mechanical ventilation that also increases the risk of adverse events. The negative implication of having only moderate sedation is that the patient can purposefully response to auditory stimulation; therefore, it is important to keep the level of noise minimal.
Adequate analgesia at the surgical site is key to reduce the propofol dose and thus avoid unwanted secondary effects. In this study, a combination of lidocaine and ropivacaine was used, because it provides a rapid action onset, and analgesia up to 6 h postprocedure [23, 24].
Three patients had auditory recalls, none of them complain of pain or discomfort. Selecting moderate sedation, awareness due to auditory stimulation may increase, making it necessary to reduce the level of noise in close proximity to the patient. A simple countermeasure could be noise cancelling patient headphones to allow usual operating room behavior.
The minimal optimal dose was calculated via a model-based approach to find the optimal minimal dose and then pre-validated via simulation to avoid a time-consuming and risky titration procedure [13]. The three doses tested in this study were safe and effective in all patients, but two requirements had to be met (1) avoiding loud alerting noise in the operating room and (2) adequate local anesthesia. The minimal propofol dose calculated (0.5 mg/kg/h) provided a pain free procedure and it is seven times lower than the standard propofol dose for elderly population. Safe sedation with minimal doses of propofol could also be used for other minimal invasive in elderly patients.
References
Trouvé-Buisson T, Arvieux L, Bedague D et al (2013) Anaesthesiological support in a cardiac electrophysiology laboratory: a single-centre prospective observational study. Eur J Anaesthesiol 30:658–663
Stix G, Anvari A, Podesser B et al (1999) Local anaesthesia versus general anaesthesia for cardioverter–defibrillator implantation. Wiener klinische Wochenschrift 111:406–409
Furniss SS, Sneyd JR, Veasey RA (2015) Safe sedation in modern cardiological practice. Heart 101:1526–1530
Ishii K, Makita T, Yamashita H et al (2016) Total intravenous anesthesia with propofol is associated with a lower rate of postoperative delirium in comparison with sevoflurane anesthesia in elderly patients. J Clin Anesth 33(Supplement C):428–431. https://doi.org/10.1016/j.jclinane.2016.04.043. http://www.sciencedirect.com/science/article/pii/S0952818016301556
Ozaki M (2002) The effects of propofol and midazolam on canine left ventricular contractility. Masui Jpn J Anesthesiol 51:611–619
Thomas SP, Thakkar J, Kovoor P et al (2014) Sedation for electrophysiological procedures. Pacing Clin Electrophysiol 37:781–790
App Pharmaceuticals (2008) Diprivan (propofol) injectable emulsion for iv administration prescribing information. Schaumburg, IL
Baxter (1999) Propofol injectable emulsion for iv administration prescribing information. Wilmington, DE
Sayfo S, Vakil KP, Alqaqa’a A et al (2012) A retrospective analysis of proceduralist-directed, nurse-administered propofol sedation for implantable cardioverter-defibrillator procedures. Heart Rhythm 9:342–346
Lovett P, Gómez V, Hodge DO, Ladlie B (2017) Propofol versus midazolam/fentanyl sedation for colonoscopy in the elderly patient population. J PeriAnesth Nurs 32:210–214
Priebe H (2000) The aged cardiovascular risk patient. Br J Anaesth 85:763–778
Sieber FE, Gottshalk A, Zakriya KJ et al (2010) General anesthesia occurs frequently in elderly patients during propofol-based sedation and spinal anesthesia. J Clin Anesth 22:179–183
Gallardo-Hernandez A, Hernandez-Perez A, Ordoñez Espinosa G, Sanchez-Lopez A, Revilla-Monsalve C, Islas-Andrade S (2016) Clinical testing of propofol geriatic dose for sedation designed via in silico trial. Comput Methods Prog Biomed. https://doi.org/10.1016/j.cmpb.2016.04.019
Aspect Medical System (2008) BIS VISTA\(^{TM}\) Monitoring System, Operating Manual
Christensen E (2007) Methodology of superiority vs. equivalence trials and non-inferiority trials. J Hepatol 46:947–954
Brice D, Hetherington R, Utting J (1970) A simple study of awareness and dreaming during anaesthesia. Br J Anaesth 42:535–542
Schnider TW, Minto CF, Shafer SL et al (1999) The influence of age on propofol pharmacodynamics. Anesthesiology 90:1502–1516
Sepúlveda P, Abadía L (2013) Total intravenous anaesthesia in geriatrics: the example of propofol. Revista Española de Anestesiología y Reanimación 60:327–335
Pandya K, Patel MB, Natla J et al (2009) Predictors of hemodynamic compromise with propofol during defibrillator implantation: a single center experience. J Intervent Cardiac Electrophysiol 25:145–151
Keyl C, Trenk D, Laule S et al (2009) Predicted and measured plasma propofol concentration and bispectral index during deep sedation in patients with impaired left ventricular function. J Cardiothorac Vasc Anesth 23:182–187
Shah AN, Koneru J, Nicoara A et al (2007) Dexmedetomidine related cardiac arrest in a patient with permanent pacemaker: a cautionary tale. Pacing Clin Electrophysiol 30:1158–1160
Shepard SM, Tejman-Yarden S, Khanna S et al (2011) Dexmedetomidine-related atrial standstill and loss of capture in a pediatric patient after congenital heart surgery. Crit Care Med 39:187–189
Golzari SE, Soleimanpour H, Mahmoodpoor A et al (2014) Lidocaine and pain management in the emergency department: a review article. Anesthesiol Pain Med 4:e15444. https://doi.org/10.5812/aapm.15444
Kuthiala G, Chaudhary G (2011) Ropivacaine: a review of its pharmacology and clinical use. Indian J Anaesth 55:104–110
Acknowledgements
This work was supported by Fondo de Investigación en Salud of Instituto Mexicano del Seguro Social (IMSS), Grant FIS/IMSS/PROT/G15/1419.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Statement of human rights
This study protocol was approved by the Institutions’ Ethical and Research Committee. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Hernandez-Perez, A.L., Gallardo-Hernandez, A.G., Ordoñez-Espinosa, G. et al. Significant and safe reduction of propofol sedation dose for geriatric population undergoing pacemaker implantation: randomized clinical trial. Aging Clin Exp Res 30, 1233–1239 (2018). https://doi.org/10.1007/s40520-018-0914-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-018-0914-0