Abstract
Background
Although low muscle function/strength is an important predictor of poor clinical outcome in older patients, information on its impact on mental health in clinical practice is still lacking.
Aims
The aim of this report is to measure the impact of low muscle function measured by handgrip strength on mental health of older people during both acute illness and recovery.
Methods
Four hundred and thirty-two randomly selected hospitalized older patients had their baseline demographic and clinical characteristics assessed within 72 h of admission, at 6 weeks and at 6 months. Low muscle strength-handgrip was defined using the European Working Group criteria. Mental health outcome measures including cognitive state, depression symptoms and quality of life were also measured.
Results
Among the 432 patients recruited, 308 (79%) had low muscle strength at baseline. Corresponding figures at 6 weeks and at 6 months were 140 (73%) and 158 (75%). Patients with poor muscle strength were significantly older with increased disability and poor nutritional status compared with those with normal muscle strength. After adjustment for age, gender, disability, comorbidity including severity of acute illness and body mass index patients with low muscle strength had worse cognitive function, quality of life and higher depression symptoms compared with those with normal muscle strength over a 6-month period (p < 0.05).
Conclusion
Poor muscle strength in older people is associated with poor cognitive state and quality of life and increased depression symptoms during both acute illness and recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of older people is growing rapidly worldwide and looks set to continue to increase further in the future. Ageing in man is associated with physiological and pathological changes some of which known to influence future risk of disease and the recovery from it. For example, both muscle strength and mass deteriorate with ageing and are known to be associated with disability and poor outcome [1,2,3]. Muscle function tests represent the newest approach for evaluating nutritional status and include measuring grip strength. Loss of muscle function/strength over time is known to be greater than loss of muscle mass [4,5,6]. Furthermore, evidence from longitudinal studies showed that age-related decline in muscle strength far exceeds the observed changes in muscle mass or size and that interventions that maintain or even increase muscle mass may not necessarily decrease or prevent muscle weakness in older adults [6, 7]. A number of cross-sectional and prospective studies revealed that muscle strength is a prognostic indicator of functional decline [8,9,10]. Recognizing underlying causes and health impact of poor muscle strength is expected to help guide treatment and, therefore, minimize adverse outcomes [7, 11]. Furthermore, older people are at risk of repeated ill health and during acute illness a series of metabolic events are activated that leads to a state of negative nitrogen balance and significant loss of lean body mass. The loss of lean body mass if significant may lead to adverse clinical outcome [3].
Although poor muscle strength has emerged as an important predictor of frailty and disability not many data on hospitalized patients are available. Specifically little is known about the impact of poor muscle strength on mental health of older people during both acute illness and recovery [12, 13]. The aim of this study is to measure the impact of poor muscle function measured by handgrip strength on mental health of older patients during both acute illness and recovery.
Methods
Subjects
Four hundred and thirty-two unselected acutely ill hospitalized older patients with complete data were included [14]. All subjects admitted to Barnsley District General Hospital 7 days a week were considered for the study. Barnsley District General Hospital serves a total population of 234,000. It has 650 beds, the medical unit has 250 beds for acute medical admissions. Patients admitted for medical emergencies and elective orthopaedic surgeries were recruited. Subjects were first identified through the computerised databases of all patients in hospital. When first admitted all patients have an individualized computerised plan created. This allowed all patients to be screened for suitability including those admitted over the weekend. The medical notes of those identified from the database were examined and eligible patients were approached. Common admission diagnoses of study population include ischaemic heart disease, chest infection, chronic obstructive lung disease, heart failure, falls, stroke, syncope, urinary tract infection, anaemia, septicaemia, diabetes, osteoarthritis, rheumatoid arthritis and fractured limbs. Inclusion criteria were: age ≥65 years; stable medical condition and able to sign an informed written consent form. Patients excluded from the study were those with severe medical or psychiatric illness including those with malignancy, severe dementia and living in institution. The study received local research ethics committee approval. All patients had clinical and nutritional baseline assessment within 72 h of admission in hospital and at 6 weeks and 6 months either in hospital or in the community for those discharged earlier than 6 weeks. Clinical assessment included demographic and medical data, current diagnosis, history of chronic illnesses, smoking, alcohol and drug intake, nutritional status and disability measured using the Barthel score. The Barthel scores 10 functions on a scale 0 (fully dependent) to 20 (independent). The Barthel score posses certain advantage, including completeness, sensitivity to change suitability for statistical manipulation and greater familiarity due to more widespread use. It is also a more reliable and less subjective score for assessing disability [15]. Nutritional status was assessed from anthropometric, haematological and biochemical data [14]. All anthropometric measurements were performed using standard methods with intra-observer’s differences assessed prior to the commencement of the study. Mid-arm circumference (MAC) and triceps skin folds (TSF) were measured by a flexible tape and Harpenden Skinfold calipers accurate to 0.2 mm (Practical Metrology, Sussex, UK), respectively, and the mean of three measures was recorded. The local pathology laboratory performed routine tests including haemoglobin, albumin and transferrin measurements. C-reactive protein (CRP) concentration, a marker of tissue inflammation (severity of illness) was measured by a modified latex-enhanced immuno-turbidimetric assay (normal range ≤10 mg/L). The inter-assay coefficient of variation (CV) was 3.9%.
Muscle strength–handgrip [2, 3]
This was measured using a handgrip dynamometer (Practical Metrology, Sussex, UK). Subjects had three measurements using their dominant hand unless this was unusable (arms in plaster, recent stroke weakness). Using the cut-off points of the European Working Group on sarcopenia in older people, low muscle strength was classified as muscle strength–handgrip less than 30 and 20 kg in men and women, respectively.
Cognitive function [15]
Cognitive state was assessed by the abbreviated mental test questionnaire (AMT). It consists of ten questions: age, year, date of birth (day and month), name of institution, immediate re-call, recognition of two persons, year of First World War, name of present monarch and counting backward (20–1). The maximum score is 10. A score of 6 or less indicates cognitive deficit.
Depression [15]
Depressive symptoms were assessed using the 15 item Geriatric Depression questionnaire (GDS). The 15-item GDS is suitable as a screening test for depressive symptoms in the elderly and ideal for evaluating the clinical severity of depression. It is easy to administer, needs no prior psychiatric knowledge and has been well validated in many environments. The GDS maximum score is 15. In clinical practice a score of 0–4 = no depression; 5–10 = mild depression; ≥11 = severe depression.
Quality of life was assessed using the validated Medical Outcomes Study 36-item (SF-36) General Health Survey questionnaire [16]. The questionnaire consists of 36 questions forming eight multi-item scale including physical functioning, role limitations—physical, role limitation—emotional, bodily pain, general health, vitality, social functioning and mental health. Its validity is now well established and it has been used in several large studies. It has been adapted for use with older adults and this was the version used in this study [16]. The questionnaire is measured on a 0–100 (good health) scale, self-administered with help provided when needed and took about 10 min to complete.
Statistical analyses
Statistical analyses were performed with SPSS software, version 22 (SPSS Inc., Chicago). Descriptive tests [mean (SD)] were used to describe the baseline characteristics of the subjects. Independent student t test or the nonparametric Mann–Whitney U test was used depending on data distribution to test between group differences with a p value of <0.05 regarded as statistically significant. A repeated-measure analysis of variance was performed to determine the influence of admission muscle strength on mental health measured using AMT, GDS and SF-36 at baseline, 6 weeks and 6 months after adjusting for a number of covariates including age, gender, disability, comorbidity (previous illnesses and drugs), body mass index (BMI) and severity of acute illness (inflammation) measured using CRP. Kruskal–Wallis analysis of variance was also used.
Results
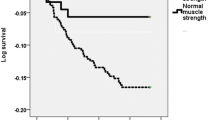
All 432 acutely ill older patients admitted to hospital and followed up for a period of 6 months were included in this analysis. Among the 432 patients recruited 308 (79%) had low muscle strength at baseline. Corresponding figures at 6 weeks and at 6 months were 140 (73%) and 158 (75%). Exclusions were due to early discharge, death or inability to provide outcome data at follow-up visits. Table 1 shows baseline characteristics of study population. Patients with poor muscle strength were significantly older with increased disability and poor nutritional status compared with those with normal muscle strength (Table 1). Patients with low muscle strength at admission and at 6-week and 6-month follow-up had significantly poor cognitive function, quality of life and increased depression symptoms compared with those with normal muscle strength (Table 2). SF-36 Quality of life multi-item scores were significantly better in patients with normal muscle strength compared with those with low strength (Table 3). Table 4 summarizes results of the multiple regression analysis for the association between age, gender, disability, comorbidity including severity of acute illness (tissue inflammation), body mass index and handgrip strength on AMT, GDS and SF-36. The analysis revealed significant and independent association between handgrip strength and AMT, GDS and SF-36. However, gender, disability and chronic illness were primarily associated with depression symptoms and quality of life. Tissue inflammation was also associated with AMT and GDS. Furthermore, patients with low muscle strength at admission to hospital had poorer cognitive function and quality of life scores and higher depression symptoms compared with those with normal muscle strength throughout the study period (p = 0.047, p = 0.256, p = 0.087, respectively) (Figs. 1, 2, 3).
Abbreviated mental test score (AMT) over 6-month period for study patients with low baseline handgrip-muscle strength compared with those with normal handgrip-muscle strength, mean (SD). Abbreviated mental test (AMT) maximum score is 10. A score of 6 or less indicates cognitive deficit. p = 0.047 for adjusted differences in cumulative changes
Geriatric depression score (GDS) over 6-month period for study patients with low baseline handgrip-muscle strength compared with those with normal handgrip-muscle strength, mean (SD). Geriatric depression (GDS) score 0–4 no depression; 5–10 mild depression; ≥11 severe depression. p = 0.256 for adjusted differences in cumulative changes
Quality of life scores (SF-36 global) over 6-month period of study patients with low baseline handgrip-muscle strength compared with those with normal handgrip-muscle strength, mean (SD). Quality of life general health survey (SF-36) measured on a 0 to 100+ (good health). p = 0.087 for adjusted differences in cumulative changes
Discussion
In this study, we found poor muscle strength in older patients is associated with poor cognitive state and quality of life and increased depression symptoms during both acute illness and recovery. Because all patients with severe medical and psychiatric illnesses or living in an institution were excluded from the study, those excluded were more likely to have low muscle strength and this might have, therefore, underestimated the prevalence of poor mental health in this cohort.
Well-recognized determinants of poor muscle strength in older patients during both acute illness and after recovery include age, gender, chronic diseases, disability and tissue inflammation [17]. We have adjusted for most of these poor prognostic indicators and it was possible, therefore, to identify a potential independent association between poor muscle strength on patient’s mental health and quality of life. In addition, all patients with severe medical and psychiatric illnesses such as liver, gastrointestinal, kidney or neoplasm were excluded from this study.
Grip strength is now an important marker of sarcopenia and been proposed as a useful single marker of generalized frailty and biological ageing [18]. Although a number of studies have identified a relationship between poor muscle strength and clinical outcome very few studies have addressed its impact on mental health. Furthermore, most of these studies were either cross-sectional or involved older people in the community. For example, a cross-sectional study on community-dwelling men and women aged 59–73 years showed that lower handgrip strength is associated with poor health-related quality of life [19]. Another cross-sectional analysis of 3025 women aged 75 years and over reported no significant association between different operative definitions of sarcopenia and cognitive impairment [12]. A recent prospective study from Japan investigated the relationship between baseline handgrip strength and the risk of depressive symptoms in community-based individuals aged 40–79 years with 1-year follow-up reported a significant association between lower handgrip strength and depressive symptoms [20]. To our knowledge, our study is the first to examine the relationship between handgrip strength and cognitive function, depression symptoms and quality of life in older people during both acute illness and recovery period.
A relationship between changes in body composition and mental health parameters in older people has been proposed, specifically, a common underlying pathophysiological mechanism linking changes in lean and fat mass with cognitive decline; however, supporting data are still lacking [21]. For example, age-related low-grade inflammation and increased oxidative stress have been postulated as mechanisms linking low muscle mass and sarcopenia with cognitive impairment and higher depressive symptoms [6]. A cross-sectional study on 672 women aged 65 and older reported an independent association between oxidative protein damage and low grip strength suggesting an involvement of increased oxidative stress in loss of muscle strength in older people [22]. Another recent cross-sectional survey reported an association between C-reactive protein a marker of inflammation and low handgrip strength in men and women aged 65–74 years [23]. Finding a plausible underlying mechanism linking muscle function with mental health is clearly an area for future research.
Muscle function tests represent the newest approach for evaluating nutritional status and include measuring grip strength [24]. Change in physical activity and body composition deserves special attention in older people with poor muscle function following acute illness. First, because acutely ill older patients particularly those with poor muscle strength are more likely to have premorbid decrease in physical activity and function and, therefore, poor muscle strength. Their muscle strength is likely to deteriorate further as the result of the catabolism associated with the acute illness because of loss of lean body mass [3, 25]. This is compounded further by the demands of the prolonged rehabilitation period in some patients. Second, following acute illness older people tend to slow down and many will not regain their premorbid physical activity levels for some time after recovery from acute illness. This is clinically relevant because physical activity is an important aspect of health and confers benefit on most risk factors of ageing including muscle function.
This study lacked information on premorbid and long-term post-discharge dietary intake. Another important limitation is the number of exclusion at follow-up visits and inherent difficulties in measuring anthropometric and biochemical nutritional indices in ageing patients. The purpose of assessing intra-observer’s differences on anthropometeric measurements, the longitudinal design of the study and the use of a number of analyses to adjust for poor prognostic clinical indicators was to overcome some of these weaknesses.
In conclusion, this study shows that poor muscle strength is associated with poor cognitive state and quality of life and increased depression symptoms in hospitalized older patients over a 6-month period. Research combining human clinical trials with molecular and cellular investigations is needed to fully understand the relationship between muscle and mental functions and also explore the role of optimizing dietary intake including protein and increase physical activity particularly following acute illness on muscle and mental functions in ageing patients.
References
Sayer AA (2010) Sarcopenia: a research agenda has been set, but recognition in clinical practice is lagging behind. BMJ 341:952
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, European Working Group on Sarcopenia in Older People et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing 39:412–423
Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, Bernabei R, Onder G (2012) Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. doi:10.1016/j.clnu.2012.02.007
Frontera WR, Reid KF, Phillips EM, Krivickas LS, Hughes VA, Roubenoff R et al (2008) Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol 105:637–642
Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV et al (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61:1059–1064
Hughes VA, FronteraWR, Wood M, EvansWJ, Dallal GE, Roubenoff R et al (2001) Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 56:B209–B217
Cesari M, Fielding RA, Pahor M, Goodpaster B, Hellerstein M et al (2012) Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J Cachexia Sarcopenia Muscle 3:181–190
Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L (1999) Midlife hand grip strength as a predictor of old age disability. JAMA 281:558–560
Hicks GE, Shardell M, Alley DE, Miller RR, Bandinelli S, Guralnik J, Lauretani F, Simonsick EM, Ferrucci L (2012) Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 67:66–73
Stenholm S, Rantanen T, Heliovaara M, Koskinen S (2008) The mediating role of C-reactive protein and handgrip strength between obesity and walking limitation. J Am Geriatr Soc 56:462–469
Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB et al (2011) Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12:249–256
Abellan van Kan G, Cesari M, Gillette-Guyonnet S et al (2013) Sarcopenia and cognitive impairment in elderly women: results from the EPIDOS cohort. Age Ageing 42:196–202
Roubenoff R (2000) Exercise, sarcopenia, cognition and mood. In: Rosenberg IH, Sastre A (eds) Nutrition and ageing. Nestle Nutrition Workshop Series Clinical and Performance Program, vol 6. Basel, pp 151–162
Gariballa S, Alessa A (2013) Sarcopenia: prevalence and prognostic significance in hospitalized patients. Clin Nutr 32:772–776
Standardised Assessment Scales for Elderly People (1992) A report of joint workshops of the research unit of the Royal College of Physicians and the British Geriatrics Society. Royal College of Physicans and the British Geriatics Society, London, pp 24–27
Brazier JE, Harper R, Jones NMB, O’Cathain A, Thomas KJ, Usherwood T, Westlake L (1992) Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 305:160–164
Roberts HC, Syddall HE, Sparkes J, Ritchie J, Butchart J, Kerr A, Cooper C, Sayer AA (2014) Grip strength and its determinants among older people in different healthcare settings. Age Ageing 43(2):241–246
Syddall H, Cooper C, Martin F, Briggs R, Aihie SA (2003) Is grip strength a useful single marker of frailty? Age Ageing 32:650–656
Sayer AA, Syddall HE, Martin HJ, Dennison EM, Roberts HC, Cooper C (2006) Is grip strength associated with health-related quality of life? Findings from the Hertfordshire Cohort Study. Age Ageing 35:409–415
Fukumori N, Yamamoto Y, Takegami M, Yamazaki S, Onishi Y, Sekiguchi M, Otani K, Konno S, Kikuchi S, Fukuhara S (2015) Association between hand-grip strength and depressive symptoms: locomotive syndrome and health outcomes in Aizu Cohort Study (LOHAS). Age Ageing 44:592–598
Burns JM, Johnson DK, Watts A et al (2010) Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch Neurol 67:428–433
Howard C, Ferrucci L, Sun K, Fried LP, Walston J, Varadhan R, Guralnik JM, Semba RD (2007) Oxidative protein damage is associated with poor grip strength among older women living in the community. J Appl Physiol (1985) 103:17–20
Sousa AC, Zunzunegui MV, Li A, Phillips SP, Guralnik JM, Guerra RO (2016) Association between C-reactive protein and physical performance in older populations: results from the International Mobility in Aging Study (IMIAS). Age Ageing 45:274–280
Klein, Kinney J, Jeejeebhoy K, Alpers D, Hellerstein M, Murray M et al (1997) Nutrition support in clinical practice: review of published data. Am J Clin Nutr 66:683–706
Gariballa SE (2001) Malnutrition in hospitalised elderly patients: when does it matter? Clin Nutr 20:487–491
Acknowledgements
The index study was funded by The Health Foundation project grant. Thank-you to Dr. Sarah Forster for her help with data collection.
Author contributions
SG is the principal investigator, wrote the first draft, participated in the design of the study and performed the statistical analysis and writing of the final manuscript. AA helped with data entry and analysis and drafting of the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial or non-financial competing interest or conflict of interest.
Ethical approval
The study received local research ethics committee approval.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gariballa, S., Alessa, A. Association between muscle function, cognitive state, depression symptoms and quality of life of older people: evidence from clinical practice. Aging Clin Exp Res 30, 351–357 (2018). https://doi.org/10.1007/s40520-017-0775-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-017-0775-y