Abstract
Backgrounds
In non-critical hospitalized patients with diabetes mellitus, guidelines suggest subcutaneous insulin therapy with basal-bolus regimen, even in old and vulnerable inpatients.
Aim
To evaluate safety, efficacy, and benefit on clinical management of the GesTIO protocol, a set of subcutaneous insulin administration rules, in old and vulnerable non-ICU inpatients.
Methods
Retrospective, observational study. Patients admitted to Geriatric Clinic of Padua were studied. 88 patients matched the inclusion criteria: type 2 diabetes or hospital-related hyperglycemia, ≥65 years, regular measurements of capillary glycemia, and basal-bolus subcutaneous insulin regimen managed by “GesTIO protocol” for five consecutive days. Main outcome measures: ratio of patients with blood glucose (BG) <3.9 mmol/l; number of BG per patient in target range (5–11.1 mmol/l); daily mean BG; and calls to physicians for adjusting insulin therapy.
Results
Mean age was 82 ± 7 years. 9.1% patients experienced mild hypoglycaemia, and no severe hypoglycaemia was reported. The median number of BG per patients in target range increased from 2.0 ± 2 to 3.0 ± 2 (p < 0.001). The daily mean BG decreased from 11.06 ± 3.03 to 9.64 ± 2.58 mmol/l (−12.8%, p < 0.005). The mean number of calls to physicians per patient decreased from 0.83 to 0.45 (p < 0.05).
Conclusions
Treatment with GesTIO protocol allows a safe and effective treatment even in very old and vulnerable inpatients with a faster management insulin therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing evidence indicates that the development of hyperglycemia during acute medical or surgical illness is a marker of poor hospital outcome [1, 2]. The presence of hyperglycemia was associated with prolonged hospital stay, infection, disability after hospital discharge, and death [2–6]. The previous studies indicate that improved glucose control reduces the risk of multiorgan failure, systemic infections, and mortality [7, 8].
Clinical guidelines from the American Diabetes Association (ADA) and the Endocrine Society recommend for patients in non-critical care setting the use of insulin therapy with the following targets: pre-meal blood glucose <140 mg/dl (7.8 mmol/l) and random blood glucose <180 mg/dl (10.0 mmol/l). Less stringent targets may be appropriate in patients with severe comorbidities, such as older inpatients [9]. The ADA task force suggests that multidisciplinary teams develop hyperglycemia management protocols [10]. Structured subcutaneous insulin order sets and insulin management protocols have been advocated as a method to enhance glycemic control and encourage the use of basal-bolus regimen. Subcutaneous insulin protocol should include target glucose levels, basal, nutritional and supplemental insulin, and daily dose adjustments [5]. Recent trials have indicated that basal-bolus is superior to sliding scale (SSI) approach in hospitalized patients [11, 12].
Protocol for in hospital insulin administration should be safe and effective. Moreover this instrument should permit to determine pre-prandial insulin dose adjustments so that insulin therapy could be managed by nurses. Unfortunately, protocols and order sets for scheduled insulin administration are not available in many hospitals and the use of SSI persists [13].
Despite the known benefits of a basal-bolus scheduled insulin therapy, many hospitalist do not use it, probably due to its complexity and fear of hypoglycemia [14, 15]. This is especially true in aged patients that are much more vulnerable to hypoglycaemia due to a progressive decrease in beta-adrenergic receptor function, impairment in counterregulatory hormone responses and decline in renal and liver function. In addition, older adults usually have a polypharmacy and a high number of comorbidities which can increase the hypoglycemic risk [16–26]. Finally, cognitive and executive dysfunction may interfere with the ability to perform self-care activities and follow the treatment regimen [21]. In these conditions, guidelines allow a less stringent target of HbA1c (7.5–8.5% or 58–69 mmol/mol) to avoid hypoglycemic events [9, 19]. Therefore, in hospitalized frail patients, maintenance of a reasonable degree of glycemic control, such as blood glucose less than 200 mg/dl (11.1 mmol/l), is suggested [19, 25].
In this paper, we analyzed safety and efficacy of a basal-bolus-correction insulin order set named GesTIO (management of insulin therapy in hospital) in patients with diabetes mellitus admitted to a geriatric ward.
Methods
In this retrospective, observational, cross-sectional study, we analyzed the medical records of 132 diabetic patients treated with insulin therapy with a subcutaneous insulin basal-bolus regimen admitted to the Geriatric Clinic of the Department of Medicine (DIMED) at Padua University from march 2009 and October 2011. We selected patients with either a diagnosis of Diabetes Mellitus or hospital-related hyperglycemia (at least a random laboratory glucose >200 mg/dl or a fasting blood glucose >140 mg/dl), aged 65 years or older, having regular measurements of capillary glycemia (almost four times every day) and subcutaneous insulin therapy managed by “GesTIO protocol” for at least five consecutive days (Supplementary Appendix I). We focused the analysis only during 5 days to minimize the “drop-out effect” due to hospital discharge. We excluded patients receiving corticosteroid therapy, with parenteral and/or enteral nutrition and patients receiving palliative care or with limited life expectancy. All selected patients followed a standardized diet: 1500 Kcal/die, 68 g (18%) protein, 50 g (30%) lipids, 212 g (52%) glucides, and 18 g fibers.
GesTIO protocol is a subcutaneous insulin order set for the management of a basal-bolus-correction insulin regimen developed by a multidisciplinary team of diabetologists, internal medicine, and geriatrics specialist physicians of DIMED at Padua University. It is based on ADA guidelines and Trence’s insulin order form [5, 9, 27]. The protocol, shown in Fig. 1 and in Fig. 2, consisted in a single A4 paper (double sided) with a set of specific treatment recommendations, including: (1) method to estimate the total daily dose insulin requirements (TDD); (2) section for prescribing type and scheduled doses of basal and pre-meal (nutritional) insulin; (3) glycemic goals and alarm levels for risk of hypoglycemia or hyperglycemia; (4) the algorithms for supplemental correction-dose insulin to be administered by nurses at pre-meal time; (5) instructions for physicians about how to calculate and use the insulin correctional factor in particular situation; and (6) table for the standardized management of hypoglycemia. All physicians and nurses in the Geriatric Clinic were trained on how to use the protocol through specific educational courses given by a team of specialists.
We recorded detailed demographic and clinical information, including age, gender, body weight, comorbidity, and severity index (CIRS-CI and CIRS-SI) calculated with geriatric cumulative illness rating scale (CIRS-G) [17], estimated creatinine clearance (eCrCl) according to the Cockcroft–Gault formula, HbA1c level (standardized IFCC) on admission. During the five consecutive days analysis, we considered also total insulin daily dose (TDD) and daily glycemic patterns (in each patient glycaemia was measured as capillary glucose at least four times/day: before each meal, at bedtime and when requested by the physician) and number of medical intervention (calls to physicians) required by nurses for adjusting insulin therapy.
Outcome measures
The primary endpoint of the study was to determine the safety of GesTIO protocol when applied in older and frail adults. The secondary endpoint was to evaluate its efficacy on glycemic control and the possible benefit on clinical management of insulin therapy.
To determine the safety of GesTIO protocol, we analyzed the proportion of patients with mild (<70 mg/dl or 3.9 mmol/l) and severe (<40 mg/dl or 2.2 mmol/l) hypoglycemic events.
To evaluate the efficacy of GesTIO protocol, we analyzed:
-
1.
Number of BG per patients below 89 mg/dl (4.9 mmol/l), between 90 and 200 mg/dl (5–11.1 mmol/l), and above 200 mg/dl (11.1 mmol/l) throughout observation time. Range 90–200 mg/dl was selected as an acceptable glycemic target for elderly;
-
2.
Mean daily BG throughout observation time;
-
3.
Glucose variability as standard deviation (±SD) of mean daily BG throughout observation time.
To evaluate the benefit on clinical management, we analyzed the number of calls to physicians per patient throughout observation time.
To determine number of BG, mean daily blood glucose and SD, we evaluated only pre-prandial (8–12–18 h) and bed-time (22 h) BG measurement, because these were the only recorded for all patients.
To determine other outcomes, any BG measurement recorded during the observation was considered.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences V.20.0 ITA for Windows®-SPSS Inc, Chicago, IL, USA. The data are presented as mean ± standard deviations (SD) for continuous variables and median ± IQR (Inter Quartile Range) or number (N and %) for categorical variables. To analyze differences within group, we used ANOVA for repeated measurement and Friedman test with pairwise comparison with a Bonferroni correction for multiple comparisons. p value of <0.05 was considered significant.
Results
88 patients (mean age 82.0 ± 6.8 years) matched the inclusion criteria. The main clinical and demographic characteristics of enrolled patients are listed in Table 1. All patients were affected by Type 2 DM (86/88 pts, 97.7%) or by DM due to pancreatic diseases (2/88 pts, 2.3%), none was affected by Type 1 DM. The more common diagnosis were: heart failure (27/88, 30.7%) and DM with poor glycaemic control (15/88 patients, 17%). 9/88 (10.2%) patients were admitted for infective diseases (respiratory and urinary). The mean total insulin dose (TDD) was 0.42 ± 0.19 units/kg/day at the first day and 0.44 units/kg/day at fifth day with no statistical difference. The majority of patients were treated with insulin before admission (51/88, 58%), while 16/88 (18.2%) patients started it during hospital stay (we found lack of data about 21/88 patients).
80.7% (71/88) of patients started insulin therapy guided by GesTIO between the first and third days of hospital stay. Only 59 out of 1760 sticks (minimum needed to manage insulin therapy: four sticks per patients every day during 5 days = 1760 sticks) equal to 3.35% were missing.
During the 5 days of GesTIO protocol, mild hypoglycaemia occurred in 9.1% of patients (8/88) [95% CI 3–15.2%]. No one experienced severe hypoglycaemia (BG <40 mg/dl or 2.2 mmol/l).
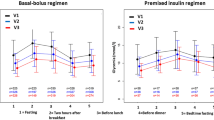
At day 1, there was a median of 2.0 ± 2 sticks per patient between 90 and 200 mg/dl (5–11.1 mmol/l) and 3.0 ± 2 at day 5: number of BG in target range was significantly different throughout observation time, χ 2(4) = 25.055, p < 0.001. Post hoc analysis revealed statistically significant differences from first (Mdn = 2) to third, fourth, and fifth (Mdn = 3) days (p < 0.05). At day 1, there was a median of 2.0 ± 2 sticks per patient above 200 mg/dl (11.1 mmol/l) and 1.0 ± 1 at day 5, χ 2(4) = 21.271, p < 0.001.
The mean BG, over all 5 days, was 182.9 ± 39.4 mg/dl (10.2 ± 2.2 mmol/l). The daily mean BG decreased from 199.1 ± 54.5 (11.06 ± 3.03 mmol/l) at day 1 to 173.6 ± 46.4 mg/dl (9.64 ± 2.58 mmol/l) at day 5, with a 25.5 mg/dl (1.4 mmol/l) decrease, F (2.67, 232.67) = 7.42, p < 0.005 (corrected with ε calculated according to Greenhouse and Geisser), see Table 2. Post hoc analysis with a Bonferroni adjustment revealed that mean BG was significantly decreased only from the first to third–fourth–fifth days.
The standard deviation of mean BG decreased from 49.44 ± 30.0 mg/dl (2.8 ± 1.7 mmol/l) at day 1 to 36.13 ± 19.5 mg/dl (2.0 ± 1.1 mmol/l) at day 5, F (3.37, 293.21) = 6.98, p < 0.005 (corrected with ε calculated according to Greenhouse and Geisser), see Table 2. Post hoc analysis with a Bonferroni adjustment revealed that SD of mean BG was statistically significantly decreased only from the first to all other days.
The mean number of calls to physicians during the observation time was 2.51 per patients and decreased from 0.83 (73/88) at day 1 to 0.45 (40/88) at day 5, χ 2(4) = 26.134, p < 0.001, see Table 2. Post hoc analysis revealed statistically significant differences from the first to second and fourth days (p < 0.05) but not to other days.
Discussion
The true incidence and prevalence of hypoglycaemia among hospitalized patients with diabetes are not well known. In two retrospective studies in younger hospitalized patients (respectively mean aged 61 ± 17.8 and 65.2 ± 18.3 years), a hypoglycaemia rate of 10.5% [20] and a severe hypoglycemia rate of 1.9% [21] were reported. Frail patients, like elderly, have an increased risk of hypoglycemia and related complications [21, 24, 27]. The rate of hypoglycemia in our study was lower but similar to the previous studies: only 8 (9.1%) out of 88 patients experienced a BG <70 mg/dl (< 3.9 mmol/l) and no one had a value <40 mg/dl (<2.2 mmol/l). Therefore, the GesTIO protocol seems to be safely applicable even in such frail diabetic patients.
A comprehensive definition of frailty requires an assessment of physical performance [28]. However, older adults with complex care needs, multiple comorbidities, and increased mortality are generally considered as a vulnerable or “frail” patients [29]. Our patients could be considered vulnerable: they were very old, they had a reduced capability of self-management (67% of them had a Barthel Index ≤40) and they had a relevant number of comorbidities (see Table 1). Furthermore, they had a compromised cognitive function: 24.9% of patients has a severe cognitive impairment and 18.2% had a moderate ones. In these patients, guidelines suggest both a prudential starting dose of insulin and less stringent glycaemic targets [9, 19]. Our patients had a mean insulin TDD pro kilos of 0.43 ± 0.17 per day, similar to the values suggested by published studies: 0.2 to 0.4 UI/Kg/die [5, 7, 19]. There are not specific glycemic target indications in clinical guidelines and most of them suggest to use the standard targets [9]. However, less stringent targets may be more appropriate for our patients: some previous studies suggest to maintain blood glucose below 200 mg/dl or 11.1 mmol/l [19, 26]. For these reasons in our study we considered 90 to 200 mg/dl (5–11.1 mmol/l) as an acceptable range of glycaemia: the proportion of glycemic sticks in this range increased from 52.3 to 70.1%. Moreover, we found a significant reduction both of mean daily BG and standard deviation of mean BG. With the GesTIO protocol, we reached in only 5 days a mean BG of 173.6 mg/dl (9.64 mmol/l) that could be considered close to the target of random BG <180 mg/dl (<10.0 mmol/l) [9] and an SD of 36.13 mg/dl (22.7 mmol/l) that is less than 1/3 of mean BG as Hirsh defined for a good glycaemic variability [30]. Although we cannot establish GesTIO protocol clinical efficacy, due to retrospective design of our study, we believe that these are indicators of the efficacy of insulin therapy guided by GesTIO protocol even in frail diabetic inpatients.
GesTIO protocol appears to be a reliable instrument for applying an early insulin protocol in elderly diabetic patients during hospital stay. Moreover, the application of such protocol has shown to reduce the number of medical intervention to adjust insulin therapy of about 50% in 5 days. This is an interesting indicator of GesTIO’s protocol ability to self-adjust pre-prandial insulin dosage without a strict control by physicians. Therefore, the GesTIO protocol reduces the workload and demonstrated to be useful in clinical management of insulin therapy.
The main limitation of this study lies in its retrospective and observational design without a similar control group due to large application of GesTIO protocol (107 over 132 patients treated with basal-bolus insulin regimen followed GesTIO protocol). Therefore, a larger prospective, multicentric, randomized clinical trial in general medicine and surgery setting is certainly advisable to address differences between basal-bolus insulin therapy guided by GesTIO and any other basal-bolus guided insulin protocol.
In summary, we can confirm that applying a standardized order set for insulin therapy, such as the GesTIO protocol, is safe and allows an acceptable glycemic control in only 5 days of observation also in elderly hospitalized people without increasing commitment for the physicians.
References
Levetan CS, Magee MF (2000) Hospital management of diabetes. Endocrinol Metab Clin North Am 29:745–770
Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE (2002) Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 87:978–982
Falciglia M, Freyberg RW, Almenoff PL, D’Alessio DA, Render ML (2009) Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med 37:3001–3009
Pomposelli JJ, Baxter JK 3rd, Babineau TJ et al (1998) Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr 22:77–81
Clement S, Braithwaite SS, Magee MF et al (2004) Management of diabetes and hyperglycemia in hospitals. Diabetes Care 27:553–591
Krinsley JS (2003) Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc 78:1471–1478
Inzucchi SE (2006) Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med 355:1903–1911
Van den Berghe G, Wilmer A et al (2006) Intensive insulin therapy in the medical ICU. N Engl J Med 354:449–461
American Diabetes Association (2013) Standards of medical care in diabetes—2013. Diabetes Care. 36(Suppl 1):S11–S66.
ACE/ADA Task Force on Inpatient Diabetes (2006) American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control. Diabetes care. 29:1955–1962
Umpierrez GE, Smiley D, Zisman A et al (2007) Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 30:2181–2186
Umpierrez GE, Smiley D, Hermayer K et al (2013) Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care 36:2169–2174
Draznin B, Gilden J, Golden SH et al (2013) Pathways to quality inpatient management of hyperglycemia and diabetes: a call to action. Diabetes Care 36:1807–1814
Umpierrez G, Maynard G (2006) Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity? J Hosp Med 1:141–144
Cook CB, Castro JC, Schmidt RE et al (2007) Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum. J Hosp Med 2:203–211
Seaquist ER, Anderson J, Childs B et al (2013) Hypoglycemia and diabetes: a report of a workgroup of the american diabetes association and the endocrine society. Diabetes Care 36:1384–1395
Parmelee PA, Thuras PD, Katz IR, Lawton MP (1995) Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc 43:130–137
Bremer JP, Jauch-Chara K, Hallschmid M, Schmid S, Schultes B (2009) Hypoglycemia unawareness in older compared with middle-aged patients with type 2 diabetes. Diabetes Care 32:1513–1517
Umpierrez GE, Hellman R, Korytkowski MT et al (2012) Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 97:16–38
Boucai L, Southern WN, Zonszein J (2011) Hypoglycemia-associated mortality is not drug-associated but linked to comorbidities. Am J Med 124:1028–1035
Krinsley JS, Grover A (2007) Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med 35:2262–2267
Chelliah A, Burge MR (2004) Hypoglycaemia in elderly patients with diabetes mellitus: causes and strategies for prevention. Drugs Aging 21:511–530
Bonds ED, Miller ME, Bergenstal RM et al (2010) The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 340:b4909
Turchin A, Matheny ME, Shubina M, Scanlon JV, Greenwood B, Pendergrass ML (2009) Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care 32:1153–1157
Kirkman MS, Briscoe VJ, Clark N et al (2012) Diabetes in older adults. Diabetes Care 35:2650–2664
Franchin A, Corradin ML, Giantin V et al (2012) Diabetes in a geriatric ward: efficacy and safety of new insulin analogs in very old inpatients. Aging Clin Exp Res 24(3 Suppl):17–19
Trance DL, Kelly JL, Hirsch IB (2003) The rationale and management of hyperglycemia for in-patients with cardiovascular disease: time for change. J Clin Endocrinol Metab 88:2430–2437
Fried LP, Tangen CM, Walston J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156
McMillan GJ, Hubbard RE (2012) Frailty in older inpatients: what physicians need to know. Q J Med 105:1059–1065
Hirsch IB (2005) Glycemic variability: it’s not just about a1c anymore! Diabetes Technol Ther 7:780–783
Author contributions
A.F. wrote the research proposal and manuscript. A.F., F.R., M.L.C, and F.Z. collected and researched data. D.B., A.F., A.M., F.R., M.L.C, F.Z., and E.M. contributed to write and to edit manuscript. The GesTIO Group (GM.B., D.B., A.F., A.M., and N.S.) reviewed and edited the research proposal and manuscript and contributed to the discussion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
No funding source is relevant to this paper.
Informed consent
Consent for study has been obtained from all participant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Franchin, A., Maran, A., Bruttomesso, D. et al. The GesTIO protocol experience: safety of a standardized order set for subcutaneous insulin regimen in elderly hospitalized patients. Aging Clin Exp Res 29, 1087–1093 (2017). https://doi.org/10.1007/s40520-017-0728-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-017-0728-5