Abstract
Sarcopenia encompasses the loss of muscle mass and strength/function during aging. Several methods are available for the estimation of muscle or lean body mass. Popular assessment tools include body imaging techniques (e.g., magnetic resonance imaging, computed tomography, dual X-ray absorptiometry, ultrasonography), bioelectric impedance analysis, anthropometric parameters (e.g., calf circumference, mid-arm muscle circumference), and biochemical markers (total or partial body potassium, serum and urinary creatinine, deuterated creatine dilution method). The heterogeneity of the populations to be evaluated as well as the setting in which sarcopenia is investigated impacts the definition of “gold standard” assessment techniques. The aim of this article is to critically review available methods for muscle mass estimation, highlighting strengths and weaknesses of each of them as well as their proposed field of application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcopenia involves the loss of muscle mass and strength or function during aging. The bidimensional nature of the condition implies that both its quantitative and qualitative domains should be objectively assessed [1]. Hence, distinct tools are necessary for the correct identification of sarcopenia [2]. At the same time, diagnostic procedures need to be valid, standardized, repeatable, reliable, accurate, and cost-effective [3].
Although a wide range of imaging, functional and biological markers for sarcopenia has become available, the objective measurement of the sarcopenia domains is hampered by limitations intrinsic to the assessment tools [1]. Furthermore, the heterogeneity of the populations to be evaluated (e.g., healthy vs. multimorbid, severely disabled vs. mildly functionally impaired, bedridden vs. ambulating) as well as the setting in which sarcopenia is being investigated (e.g., community, hospital, research laboratory) impacts the definition of “gold standard” assessment techniques to be systematically adopted.
This review provides an outline of the currently available methods for muscle mass estimation, highlighting their strengths and weaknesses. For each method, the purpose (e.g., screening vs. diagnosis), setting (clinical vs. research), and the population for which it is suitable are also proposed.
Body imaging techniques
Magnetic resonance imaging (MRI)
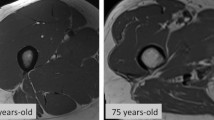
Data acquisition is achieved through the generation of a magnetic field that directs the alignment of hydrogen nuclei. The presence of gradient coils creates a smaller identification field, known as gradient field. The presence of the external gravitational field in combination with the gradient field produces a net external magnetic field. The resulting radio frequency coil provides the force necessary to rotate nuclear spins away from the direction of the external magnetic field. As the nuclear spins process back towards the direction of the external magnetic field, they emit radio frequency signals (i.e., T1 and T2), which are combined to form an image. The differentiation of the various tissues and organs is based on their specific magnetic resonance properties, such as the density of hydrogen atoms and relaxation time. For the study of body composition, variations in the radio frequency pulse sequence are used to make determinations about adipose tissue and fat-free mass. For example, a short T1 and a long T2 proton relaxation time are indicative of adipose tissue [4]. Notably, MRI scans provide information on muscle quality through the assessment of intermuscular and intramyocellular lipid depots via magnetic resonance spectroscopy analysis [5, 6]. MRI may also be used for the noninvasive estimation of fiber type composition [7].
Although MRI does not involve radiation exposure and has excellent accuracy in measuring quantitative and qualitative parameters of skeletal muscle, its high costs, technical difficulties and space requirement limit availability to well-equipped clinical and research centers. The time needed for the acquisition of high-quality scans and post-acquisition processing further impedes the large-scale implementation of MRI. Finally, the existence of multiple protocols for data acquisition impacts the standardization of this technique for the study of muscle mass and quality. Indeed, while most protocols focus at the mid-thigh level [8], others are based on the acquisition of single abdominal images that are subsequently used to estimate whole-body muscle and adipose tissue [9]. Bearing all these considerations in mind, MRI is presently better suited to small-scale research studies in which accurate measurements of muscle quantity and quality are needed.
Computed tomography (CT)
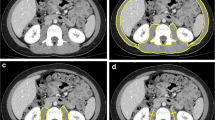
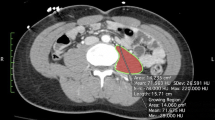
Together with MRI, CT is presently considered to be the gold standard method for the assessment of body composition [3]. Indeed, CT is highly reliable in quantifying both adipose tissue and fat-free mass [10]. Furthermore, CT allows differentiation of total fat content into subcutaneous and visceral components [10].
The method is based on X-ray attenuation measured by a computer program that reconstructs cross-sectional images represented by a 2-D map of pixels [8]. The pixels are given a numerical value (Hounsfield unit, HU), based on tissue attenuation (related to electron density) that are colored white [most dense (i.e., water)] and black [least dense (i.e., air)]. Bone, skeletal muscle, and adipose tissue, as well as visceral organs, have specific HU ranges, allowing for their identification in the cross-sectional images [11, 12]. The tissue area (cm2) of the cross-sectional image is subsequently calculated by multiplying the number of pixels for a given tissue by its surface area.
For the study of muscle, CT scanning is usually performed at 130-kVp exposure [13]; exposure and scanning times are 0.1 and 0.4 s, respectively. Slice thickness is comprised between 6 and 10 mm. After the identification of the appropriate HU for non-bone non-adipose tissue (primarily skeletal muscle and skin), the entire area with these HUs is measured for each slice. Subsequently, the skin is eliminated from the image to assess the amount of skeletal muscle in each CT image. To more easily “erase” skin (which has the same HU as skeletal muscle), specific software programs are available to automatically remove the outer two-voxel layers (1.835–2.62 mm) of the air–tissue interface.
Due to its high-resolution, CT allows muscle quantity to be measured accurately. CT also provides valuable information on muscle quality by evaluating muscle density, a parameter related to intra- and extramyocellular lipid deposition. The degree of muscle attenuation, in turn, has been associated with muscle strength and adverse health outcomes independent of muscle cross-sectional area [14, 15]. Similar to MRI, the widespread implementation of CT in the field of sarcopenia is hampered by the high cost of equipment, space requirement and need of highly qualified personnel. Although the time necessary for image acquisition is shorter than with MRI, it should be considered that CT involves radiation exposure (up to 30 mrem per scan) [16]. Similar to MRI, the field of application of CT in the context of sarcopenia is currently limited to small-scale research studies.
An appealing alternative to traditional CT scanning, is peripheral quantitative CT (pQCT), which was originally developed for bone mass quantification. The method is based on the same technical premises of standard CT, but it offers the advantages of portability and lower radiation exposure (1–3 mrem). Similar to CT, pQCT allows quantitative and qualitative assessments of muscle. However, pQCT is limited to scanning the upper and lower extremities, and does not provide enough contrast to distinguish individual muscles [17]. At present, pQCT may be used for research purposes when equipment portability is needed (e.g., community-based studies) and high accuracy is not requested.
Dual energy X-ray absorptiometry (DXA)
DXA is the most popular technique for body composition estimation. The principle of DXA relies on the property of X-rays to be attenuated in proportion to the composition and thickness of the material the beam is passed through. The DXA scanner emits two different energy beams (40 and 70 keV). As a result of the interaction within the human body, the energy of the incident X-ray photon is exponentially attenuated. By knowing how many photons are transmitted with respect to the number detected, the amount of bone mineral and soft tissue (fat and fat-free mass) can be determined. Skeletal muscle and adipose tissue mostly comprise water and organic compounds, which restrict the flux of X-rays less than bone [18].
The radiation dose varies depending on models and manufacturers, but it is typically small (<1 mrem for whole-body scans), which makes DXA a safe option for repeated body composition measurements [16]. Indeed, albeit only rarely necessary, DXA scans may be acquired also in pregnant women [19]. Other notable advantages of DXA include its relatively low cost and speed of measurement (whole-body scans require less than 20 min). Furthermore, DXA allows measurement of three body composition compartments and can provide regional estimates of each of them. This latter property has been harnessed to estimate appendicular skeletal muscle (ASM) mass by measuring the amount of lean soft tissue in the upper and lower extremities, which is mainly muscle [20]. The ASM has been widely used in the study of sarcopenia; indeed, low ASM is one of the parameters on which all the available definitions of sarcopenia rely.
The main disadvantage of DXA is that the equipment is not portable, which may preclude its use in large-scale epidemiological studies. In addition, DXA scanners are not typically available in primary care setting. Furthermore, body thickness, hydration status, and diseases with water retention (e.g., heart, kidney or liver failure) can affect DXA results [8]. Indeed, DXA may overestimate muscle mass in persons with extracellular fluid accumulation, due to its inability to differentiate between water and bone-free lean tissue [21]. In contrast, DXA underestimates trunk and thigh fat mass, and overestimate thigh muscle mass in obese persons, and this bias increases in heavier individuals [22]. In addition, DXA is unable to measure intramuscular adipose tissue, which impedes muscle quality estimation. Finally, hardware and software packages differ substantially between manufacturers. This inconsistency limits the ability to compare measurements obtained with different scanners and prompts the introduction of a unique standard.
Taking all strengths and weaknesses into consideration, DXA may be considered the current reference technique for assessing muscle mass and body composition in research and clinical practice. Indeed, DXA will be used for the measurement of ASM in the “Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies” (SPRINTT) clinical trial, the largest study on physical frailty and sarcopenia [23]. Given the multicenter nature of the trial, special procedures have been put in place to ensure a high standard of quality and consistency of DXA scans results obtained across the study sites, using different scanners and software. To minimize this potential bias, a centralized DXA reading unit has been set up at the Catholic University of the Sacred Heart (Rome, Italy). A cross-calibration plan has also been developed, through the use of a special phantom for the calibration of the DXA instrument. The data obtained from scanning this phantom will allow the central reading unit to analyze the scans from each study site with better accuracy.
Muscle ultrasonography
The use of ultrasounds (US) in body composition assessment relies on their ability to quantify tissue thickness. The US beam propagates through the tissues and is partially reflected back to the transducer by which it is detected as an echo [24]. The echo is then converted into electric signals to form a two-dimensional image [24]. The degree of US reflection by tissues varies according to their acoustic impedance. When performed by an experienced operator, muscle ultrasonography provides information on thickness and cross-sectional area of individual muscles with high test–retest reliability [25]. An excellent intra- and inter-rater reliability for US measurements of quadriceps muscle layer thickness has recently been reported [25]. Being radiation-free, muscle ultrasonography may be serially acquired. In addition, the equipment is portable, which allows muscle mass to be estimated bedside.
However, muscle and adipose tissue have similar acoustic impedance, which may limit the interpretation of muscle-fat interfaces. Another source of potential error with muscle ultrasonography is related to the compression of muscle when excessive pressure is applied on the skin with the transducer [26].
In conclusion, the use of US for muscle mass estimation in clinical and research settings is promising, because the technique is safe, noninvasive, repeatable, portable, and allows for simplicity of measurements.
Bioelectric impedance analysis (BIA)
The use of BIA for measurement of body composition is based on the notion that electrolyte-rich fluids such as body water pose the least impedance to the passage of an electrical current than those enriched with lipid, such as the adipose tissue. Whole-body bioelectrical impedance analysis measurements are taken between the right wrist and ankle with the subject in a supine position [27]. Muscle mass is calculated using the equation developed by Janssen et al. [28]:
where height is in cm; BIA resistance is in ohms; for gender, men = 1 and women = 0; and age is in years. The skeletal muscle index [SMI ([kg/m2)] is subsequently obtained by dividing absolute muscle mass by squared height [29].
Using the cut-off points indicated in the European Working Group on Sarcopenia in Older People (EWGSOP) consensus paper [30], low muscle mass is defined as an SMI lower than 8.87 and 6.42 kg/m2 in men and women, respectively. These cut-off points are similar to those obtained among 2276 older (≥60 years) women and 2223 older men enrolled in the Third National Health and Nutrition Examination Survey (NHANES III) [31].
BIA is inexpensive, easy to use, readily reproducible and appropriate for both ambulatory and bedridden patients. In addition, prediction equations have been validated for multiethnic adults and reference values established for adult white men and women, including older adults [30].
BIA should be acquired under standard conditions to limit the possible variability arising from body position, hydration status, consumption of food and beverages, ambient air and skin temperature, recent physical activity, and conductance of the examining table [32]. Although BIA could be an inexpensive, safe, and portable alternative to whole-body imaging, its results may easily be altered by fluid retention and health status in general [33]. Its use is therefore limited to the screening of sarcopenia in community settings and bedridden patients.
Other techniques for muscle mass estimation
Neutron activation (NAA)
NAA is an imaging technique used for determining the concentrations of elements in a vast amount of materials. NAA relies on the atom excitation by neutrons so that the treated sample emits gamma rays. Such a process allows the precise identification and quantification of the elements in a given sample. NAA has a large field of applications including chemistry, geology, archeology, etc.
The technique is based on a sequence of events, starting from the interaction of a neutron with an atomic nucleus resulting in the transformation of the target nucleus into an unstable isotope or nuclear state. The nucleus almost instantaneously de-excites into a more stable configuration through the emission of one or more characteristic prompt gamma rays. In many cases, this new configuration yields a radioactive nucleus which also de-excites (or decays) by emission of one or more characteristic delayed gamma rays, but at a much lower rate according to the unique half-life of the radioactive nucleus. The emitted gamma ray has an energy signature of the decaying isotope and, therefore, serves as an identifier of the atomic composition of the irradiated sample.
For body composition analysis, the subject is positioned on a bed and passed over a stationary neutron beam. The gamma detection system is positioned above the subject.
Although this technique is able to give a very accurate estimation of overall skeletal muscle mass, high costs, radiation exposure, and technical difficulty limit substantially the implementation of NAA. As such, NAA is not currently recommended for application in clinical or research settings.
Electrical impedance myography (EIM)
EIM is a noninvasive, localized tetrapolar bioimpedance-based and painless technique that is effective in evaluating muscle composition and architecture. Remarkable parameters that can be captured include reductions in myocyte number or size, edema, re-innervation, and deposition of endomysial connective tissue and fat. EIM has been used for the assessment of neuromuscular diseases [34, 35], but it may also find applications in the characterization of muscle dysfunction in older people.
In its most basic form (i.e., linear EIM), a low-intensity, 50-kHz alternating current is applied through widely separated electrodes, and the evoked surface voltage pattern is evaluated via a second set of electrodes placed in a linear array over the muscle of interest [33, 36, 37].
The parameters obtained include the resistance (R), which measures the impedance associated with the passage of current through intra- and extra-cellular fluids, and the reactance (X), which reflects the impedance of cell membranes. All tissues underlying the voltage electrodes could theoretically contribute to the impedance signature, including subcutaneous fat and bone, but the inherent resistivity of muscle is an order of magnitude lower than that of fat and two orders of magnitude lower than that of bone [38]. Consequently, virtually all electrical current in the region of interest travels through the muscle, with changes in X and R reflecting variations in the muscle tissue itself. Both R and X depend on the shape and cross-sectional area of the muscle, but these dependences tend to cancel in the calculation of the phase, θ, obtained from the equation θ = arctan X/R.
The acquisition of EIM requires trained personnel. Furthermore, the equipment for EIM is not widely available. Therefore, the technique is not presently suitable for clinical application, although it is able to provide potentially important information for research.
Anthropometry
Imaging equipments is typically unavailable in primary care settings (e.g., the general practitioner’s office), which represent the first access point for the majority of sarcopenic older adults [39]. In such situations, the estimation of body composition and muscle mass through anthropometric measurements may allow an initial assessment of sarcopenia [40, 41]. Anthropometry offers the most portable, commonly applicable, inexpensive and noninvasive technique for assessing the size, proportions and composition of the human body. For these reasons, anthropometric measures are utilized in many contexts to screen for or monitor diseases among children and young people. Anthropometry is relatively less frequently used and thus less standardized among older adults. Nevertheless, mid-arm muscle circumference (MAMC) and calf circumference (CC) have been shown to reflect both health and nutritional status and predict performance and survival in older people [40, 41]. They may therefore be used for the screening of sarcopenia in primary care as well as in community-based epidemiological studies.
Calf circumference
CC is commonly measured using a non-elastic flexible plastic tape, on the left leg (or the right leg for left-handed persons) at the point of greatest circumference with the person in a sitting position with the knee and ankle at a right angle and feet resting on the floor. The subject should remove his/her clothing on the lower body so that the measurement can be taken on bare skin. CC is taken at the nearest 0.1 cm. Caution should be observed not to compress the subcutaneous tissue. CC has shown an excellent reliability when performed by experienced personnel [42]. As described by Rolland et al. [43], a CC smaller than 31 cm is considered to be indicative of low muscle mass. This cut-off point has been recommended in older people by the World Health Organization (WHO) Expert Committee [44].
Mid-arm muscle circumference
MAMC is calculated using a standard formula [45]: MAMC = mid-arm circumference–(3.14 × triceps skinfold thickness).
The mid-arm circumference is measured using a standard flexible measuring tape on the left upper arm, at the mid-point between the olecranon process of the shoulder and the acromion, with the subject in a seated position. The measure should be approximated to the nearest 0.1 cm. The triceps skinfold thickness is measured using a calibrated skinfold caliper (range 0.00–50.00 mm; minimum graduation 0.2 mm). Skinfold thickness is recorded to the nearest 0.2 mm. For both these parameters, the average of three measurements is usually computed. Like CC, MAMC measurement has an excellent reliability when performed by well-trained personnel [42].
Biochemical markers for muscle mass estimation
As previously elaborated, imaging techniques, especially MRI, CT and DXA, are the most accurate methods for assessing body composition. However, these measurements require equipment and well-trained personnel, which may not be available in primary care settings. The estimation of muscle mass via BIA, ultrasonography or anthropometry is either error-prone or not sufficiently standardized to be used as a diagnostic procedure. The development and validation of biomarkers of muscle mass that can be measured in biofluids and used in a cost-effective manner to identify and monitor sarcopenia would mark a substantial step forward in the management of the condition [1, 46]. Such biomarkers would also increase healthcare professionals’ awareness on sarcopenia, eventually promoting its incorporation in standard practice [46].
Surrogate biochemical markers of muscle mass have been developed over the years. Though, each of them presents important limitations that have prevented their inclusion in the routine evaluation of sarcopenia. However, the research on sarcopenia biomarkers is highly active and new molecules are currently under investigation. For a detailed description of biomarkers for sarcopenia the reader is referred to specialized reviews on the topic (for instance, [46]).
Total or partial body potassium
Elemental potassium (K) is the most abundant intracellular cation. Because the skeletal muscle contains approximately 60% of the total body potassium (TBK) pool, TBK quantification has been proposed as a means for muscle mass estimation [47]. TBK measurement is based on the notion that the isotope 40K isotope exists at a known and constant natural abundance of 0.0012%. 40K can therefore be measured by whole-body scintillation counter. The body cell mass is subsequently derived from TBK using standard equations. However, calculations are based on several assumptions (e.g., constant intracellular potassium content, nitrogen content, hydration coefficient of lean body mass) which may not hold in advanced age or in the setting of a variety of disease conditions [48]. Partial body potassium (PBK) of the arm or the leg has been proposed as a simpler alternative to TBK [49, 50]. Although both TBK and PBK are safe and relatively inexpensive, they are not recommended for the estimation of muscle mass in older adults [48].
Serum and urinary creatinine
Creatinine is a breakdown product of creatine phosphate in muscle; its serum levels are therefore proportional to muscle mass. Indeed, creatinine is produced at a relatively constant rate by the body. Hence, in the presence of stable renal function (and meat intake), circulating creatinine concentrations can be assumed to be proportional to the absolute amount of muscle mass [51]. Studies have shown that, under steady-state conditions, serum creatinine levels may serve as a reliable muscle mass biomarker, also in persons with chronic kidney failure or on hemodialysis [52–54]. Given the fact that creatinine is eliminated in the urine, 24-h creatinine excretion also reflects muscle mass in the presence of stable renal function and constant meat intake [51, 55]. Equations have been developed to predict 24-h creatinine excretion from sex, weight, race, and age [56]. This prediction allows avoiding possible biases related to day-by-day variations in creatinine excretion and diet as well as to incomplete urine collection. However, the lack of reference value for both serum creatinine and 24-h creatinine excretion in older people makes this method currently unsuitable to the study of sarcopenia.
Deuterated creatine (D3-creatine) dilution method
Creatine is a guanidino compound that is produced endogenously in the liver and kidney or introduced with foods. Creatine is actively taken up by muscle cells (>95%) and neurons (<5%) and converted into the high-energy metabolite phosphocreatine in a reaction catalyzed by the enzyme creatine-kinase. Because creatine is converted to creatinine through an irreversible reaction and excreted in urine, the ingestion of an oral dose of deuterate creatine (D3-creatine), enriches urine with D3-creatinine. In turn, D3-creatinine quantification provides a measure of whole-body creatine pool size and, thus, skeletal muscle mass [57]. The estimation of muscle mass via D3-creatine dilution has shown excellent concordance with MRI measurements of whole-body muscle mass in both rats [58, 59] and humans [57]. However, urinary sample processing and D3-creatinine determination are quite laborious, besides requiring sophisticated laboratory equipment (i.e., isotope ratio mass spectrometry or liquid chromatography/tandem mass spectrometry) and experienced personnel. For these reasons, the D3-dilution method is currently better suited for epidemiological studies than for clinical applications.
Definition of low muscle mass in the SPRINTT trial
The recent reports by the Foundation for National Institutes of Health (FNIH) Sarcopenia Project [60–62] have motivated a reevaluation of previously existing operational definitions of sarcopenia that were largely based on expert consensuses. Indeed, the cut-points for appendicular lean mass (aLM) [61] and muscle strength [62] proposed by the FNIH were identified through ad hoc analyses of multiple cohort studies of older persons. The FNIH project has indicated two alternative gender-specific measures to define low muscle mass [61]. The first criterion (i.e., aLM-to-body mass index [BMI] ratio, aLMBMI) is the one recommended by the FNIH project, while the second (i.e., crude aLM) is proposed as an alternative.
Given the relevance of the FNIH initiative and the adopted approach, these definitions may be considered the current “best practice” for defining low muscle mass in the elderly. In SPRINTT, it was therefore decided to follow the FNIH recommendations. Thus, each potential participant is considered “eligible” only if presenting an aLMBMI below the gender-specific cutpoints indicated in the FNIH reports [61]. If this first recommended criterion is not fulfilled, the participant is tested with the alternative criterion (based on the crude aLM) to verify the true absence of a sarcopenic phenotype. As previously mentioned, DXA will be used for the assessment of aLM. This approach will not only facilitate the recruitment of the participants in the clinical trial, but will also allow the conduction of pre-planned and post hoc analyses aimed at refining the operational definition of physical frailty and sarcopenia at the end of the SPRINTT project on the basis of the collected data. In fact, the combination of the two criteria will lead to the recruitment of participants with a wide spectrum of body composition profiles. Within this wider range, it will then be possible to establish if specific phenotypes show differential responses to the interventions.
Conclusions
Over the last years, sarcopenia has gained its spotlight in biogerontology and clinical research. The recent assignment of a specific code for sarcopenia in the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10), valid as of October 1st 2016, will likely provide further impetus to the development of standards for the screening, diagnosis and monitoring of the condition. In such a context, the establishment of an international consensus on an accurate, reliable, and cost-effective method to assess muscle mass across research and clinical settings is of utmost importance. Biomedical research is now progressing towards portability and miniaturization of imaging equipment and the identification of surrogate biomarkers for muscle mass. These advancements, together with a clear operationalization of sarcopenia, will allow clinicians, regulators, and policy-makers to overcome existing obstacles to the development of new treatments for sarcopenia and its negative outcomes.
References
Marzetti E (2012) Editorial: imaging, functional and biological markers for sarcopenia: the pursuit of the golden ratio. J Frailty Aging 1:97–98
Cruz-Jentoft AJ, Landi F (2014) Sarcopenia. Clin Med (Lond) 14:183–186. doi:10.7861/clinmedicine
Cesari M, Fielding RA, Pahor M, Goodpaster B, Hellerstein M, van Kan GA, Anker SD, Rutkove S, Vrijbloed JW, Isaac M, Rolland Y, M’rini C, Aubertin-Leheudre M, Cedarbaum JM, Zamboni M, Sieber CC, Laurent D, Evans WJ, Roubenoff R, Morley JE, Vellas B; International Working Group on Sarcopenia (2012) Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J Cachexia Sarcopenia Muscle 3:181–190. doi:10.1007/s13539-012-0078-2
Houmard JA, Smith R, Jendrasiak GL (1995) Relationship between MRI relaxation time and muscle fiber composition. J Appl Physiol (1985) 78;807–809
White LJ, Ferguson MA, McCoy SC, Kim H (2003) Intramyocellular lipid changes in men and women during aerobic exercise: a (1)H-magnetic resonance spectroscopy study. J Clin Endocrinol Metab 88:5638–5643. doi:10.1210/jc.2003-031006
Marzetti E, Lees HA, Manini TM, Buford TW, Aranda JM Jr, Calvani R, Capuani G, Marsiske M, Lott DJ, Vandenborne K, Bernabei R, Pahor M, Leeuwenburgh C, Wohlgemuth SE (2012) Skeletal muscle apoptotic signaling predicts thigh muscle volume and gait speed in community-dwelling older persons: an exploratory study. PLoS One 7:e32829. doi:10.1371/journal.pone.0032829
Kuno S, Katsuta S, Akisada M, Anno I, Matsumoto K (1990) Effect of strength training on the relationship between magnetic resonance relaxation time and muscle fibre composition. Eur J Appl Physiol Occup Physiol 61:33–36. doi:10.1007/BF00236690
Prado CM, Heymsfield SB (2014) Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr 38:940–953. doi:10.1177/0148607114550189
Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, Heshka S (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 97:2333–2338. doi:10.1152/japplphysiol.00744.2004
Ferland M, Després JP, Tremblay A, Pinault S, Nadeau A, Moorjani S, Lupien PJ, Thériault G, Bouchard C (1989) Assessment of adipose tissue distribution by computed axial tomography in obese women: association with body density and anthropometric measurements. Br J Nutr 61:139–148. doi:10.1079/BJN19890104
Mattsson S, Thomas BJ (2006) Development of methods for body composition studies. Phys Med Biol 51:R203–R228. doi:10.1088/0031-9155/51/13/R13
Hangartner TN, Warner S, Braillon P, Jankowski L, Shepherd J (2013) The Official Positions of the International Society for Clinical Densitometry: acquisition of dual-energy X-ray absorptiometry body composition and considerations regarding analysis and repeatability of measures. J Clin Densitom 16:520–536. doi:10.1016/j.jocd.2013.08.007
Levine JA, Abboud L, Barry M, Reed JE, Sheedy PF, Jensen MD (2000) Measuring leg muscle and fat mass in humans: comparison of CT and dual-energy X-ray absorptiometry. J Appl Physiol (1985) 88:452–456
Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB (2001) Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 90:2157–2165
Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB; Health ABC Study (2010) Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res 25;513–519. doi:10.1359/jbmr.090807
Damilakis J, Adams JE, Guglielmi G, Link TM (2010) Radiation exposure in X-ray-based imaging techniques used in osteoporosis. Eur Radiol 20:2707–2714. doi:10.1007/s00330-010-1845-0
Frank-Wilson AW, Johnston JD, Olszynski WP, Kontulainen SA (2015) Measurement of muscle and fat in postmenopausal women: precision of previously reported pQCT imaging methods. Bone 75:49–54. doi:10.1016/j.bone.2015.01.016
Lustgarten MS, Fielding RA (2011) Assessment of analytical methods used to measure changes in body composition in the elderly and recommendations for their use in phase II clinical trials. J Nutr Health Aging 15:368–375. doi:10.1007/s12603-011-0049-x
Damilakis J, Perisinakis K, Vrahoriti H, Kontakis G, Varveris H, Gourtsoyiannis N (2002) Embryo/fetus radiation dose and risk from dual X-ray absorptiometry examinations. Osteoporos Int 13:716–722. doi:10.1007/s001980200098
Heymsfield SB, Adamek M, Gonzalez MC, Jia G, Thomas DM (2014) Assessing skeletal muscle mass: historical overview and state of the art. J Cachexia Sarcopenia Muscle 5:9–18. doi:10.1007/s13539-014-0130-5
Proctor DN, O’Brien PC, Atkinson EJ, Nair KS (1999) Comparison of techniques to estimate total body skeletal muscle mass in people of different age groups. Am J Physiol 277:E489–E495
Bredella MA, Ghomi RH, Thomas BJ, Torriani M, Brick DJ, Gerweck AV, Misra M, Klibanski A, Miller KK (2010) Comparison of DXA and CT in the assessment of body composition in premenopausal women with obesity and anorexia nervosa. Obesity (Silver Spring) 18:2227–2233. doi:10.1038/oby.2010.5
Marzetti E, Calvani R, Landi F, Hoogendijk EO, Fougère B, Vellas B, Pahor M, Bernabei R, Cesari M; SPRINTT Consortium (2015) Innovative Medicines Initiative: The SPRINTT Project. J Frailty Aging 4:207–208. doi:10.14283/jfa.2015.69
Wagner DR (2013) Ultrasound as a tool to assess body fat. J Obes 2013:280713. doi: 10.1155/2013/280713
Tillquist M, Kutsogiannis DJ, Wischmeyer PE, Kummerlen C, Leung R, Stollery D, Karvellas CJ, Preiser JC, Bird N, Kozar R, Heyland DK (2013) Bedside ultrasound is a practical and reliable measurement tool for assessing quadriceps muscle layer thickness. JPEN J Parenter Enteral Nutr 38:886–890. doi:10.1177/0148607113501327
Mayans D, Cartwright MS, Walker FO (2012) Neuromuscular ultrasonography: quantifying muscle and nerve measurements. Phys Med Rehabil Clin N Am 23:133–148. doi:10.1016/j.pmr.2011.11.009
Lukaski HC, Johnson PE, Bolonchuk WW, Lykken GI (1985) Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am J Clin Nutr 41:810–817
Janssen I, Heymsfield SB, Baumgartner RN, Ross R (2000) Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol (1985) 89:465–471
Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R (2004) Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 159:413–421
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423. doi:10.1093/ageing/afq034
Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnson CL, Heymsfield SB, Lukaski HC, Friedl K, Hubbard VS (2002) Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord 26:1596–1609. doi:10.1038/sj.ijo.0802167
NIH Expert Panel (1996) Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference Statement. Am J Clin Nutr 64(3 Suppl):524S–532S
Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJ, Cummings SR, Evans WJ, Fearon K, Ferrucci L, Fielding RA, Guralnik JM, Harris TB, Inui A, Kalantar-Zadeh K, Kirwan BA, Mantovani G, Muscaritoli M, Newman AB, Rossi-Fanelli F, Rosano GM, Roubenoff R, Schambelan M, Sokol GH, Storer TW, Vellas B, von Haehling S, Yeh SS, Anker SD; Society on Sarcopenia, Cachexia and Wasting Disorders Trialist Workshop (2011) Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc 12:403–409. doi:10.1016/j.jamda.2011.04.014
Rutkove SB, Aaron R, Shiffman CA (2002) Localized bioimpedance analysis in the evaluation of neuromuscular disease. Muscle Nerve 25:390–397. doi:10.1002/mus.10048
Rutkove SB (2009) Electrical impedance myography: Background, current state, and future directions. Muscle Nerve 40:936–946. doi:10.1002/mus.21362
Aaron R, Shiffman CA (2000) Using localized impedance measurements to study muscle changes in injury and disease. Ann N Y Acad Sci 904:171–180. doi:10.1111/j.1749-6632.2000.tb06443.x
Shiffman CA, Aaron R, Amoss V, Therrien J, Coomler K (1999) Resistivity and phase in localized BIA. Phys Med Biol 44:2409–2429. doi:10.1088/0031-9155/44/10/304
Faes TJ, van der Meij HA, de Munck JC, Heethaar RM (1999) The electric resistivity of human tissues (100 Hz-10 MHz): a meta-analysis of review studies. Physiol Meas 20:R1–R10. doi:10.1088/0967-3334/20/4/201
Landi F, Martone AM, Calvani R, Marzetti E (2014) Sarcopenia risk screening tool: a new strategy for clinical practice. J Am Med Dir Assoc 15:613–614. doi:10.1016/j.jamda.2014.05.015
Landi F, Russo A, Liperoti R, Pahor M, Tosato M, Capoluongo E, Bernabei R, Onder G (2010) Midarm muscle circumference, physical performance and mortality: results from the aging and longevity study in the Sirente geographic area (ilSIRENTE study). Clin Nutr 29:441–447. doi:10.1016/j.clnu.2009.12.006
Landi F, Onder G, Russo A, Liperoti R, Tosato M, Martone AM, Capoluongo E, Bernabei R (2014) Calf circumference, frailty and physical performance among older adults living in the community. Clin Nutr 33:539–544. doi:10.1016/j.clnu.2013.07.013
Wijnhoven HA, van Bokhorst-de van der Schueren MA, Heymans MW, de Vet HC, Kruizenga HM, Twisk JW, Visser M (2010) Low mid-upper arm circumference, calf circumference, and body mass index and mortality in older persons. J Gerontol A Biol Sci Med Sci 65:1107–1114. doi:10.1093/gerona/glq100
Rolland Y, Lauwers-Cances V, Cournot M, Nourhashémi F, Reynish W, Rivière D, Vellas B, Grandjean H (2003) Sarcopenia, calf circumference, and physical function of elderly women: a cross-sectional study. J Am Geriatr Soc 51:1120–1124. doi:10.1046/j.1532-5415.2003.51362.x
de Onis M, Habicht JP (1996) Anthropometric reference data for international use: recommendations from a World Health Organization Expert Committee. Am J Clin Nutr 64:650–658
Antonelli Incalzi R, Landi F, Cipriani L, Bruno E, Pagano F, Gemma A, Capparella O, Carbonin PU (1996) Nutritional assessment: a primary component of multidimensional geriatric assessment in the acute care setting. J Am Geriatr Soc 44:166–174. doi:10.1111/j.1532-5415.1996.tb02434
Calvani R, Marini F, Cesari M, Tosato M, Anker SD, von Haehling S, Miller RR, Bernabei R, Landi F, Marzetti E; SPRINTT consortium (2015) Biomarkers for physical frailty and sarcopenia: state of the science and future developments. J Cachexia Sarcopenia Muscle 6;278–286. doi:10.1002/jcsm.12051
Heymsfield SB, Gallagher D, Visser M, Nuñez C, Wang ZM (1995) Measurement of skeletal muscle: laboratory and epidemiological methods. J Gerontol A Biol Sci Med Sci 50 Spec No:23–29. doi:10.1093/gerona/50A
Kehayias JJ, Fiatarone MA, Zhuang H, Roubenoff R (1997) Total body potassium and body fat: relevance to aging. Am J Clin Nutr 66:904–910
Wielopolski L, Ramirez LM, Gallagher D, Sarkar SR, Zhu F, Kaysen GA, Levin NW, Heymsfield SB, Wang ZM (2006) Measuring partial body potassium in the arm versus total body potassium. J Appl Physiol (1985) 101;945–949. doi:10.1152/japplphysiol.00999.2005
Wielopolski L, Ramirez LM, Spungen AM, Swaby S, Asselin P, Bauman WA (2009) Measuring partial body potassium in the legs of patients with spinal cord injury: a new approach. J Appl Physiol (1985) 106;268–273. doi:10.1152/japplphysiol.90435.2008
Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S (1983) Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr 37:478–494
Keshaviah PR, Nolph KD, Moore HL, Prowant B, Emerson PF, Meyer M, Twardowski ZJ, Khanna R, Ponferrada L, Collins A (1994) Lean body mass estimation by creatinine kinetics. J Am Soc Nephrol 4:1475–1485
Bhatla B, Moore H, Emerson P, Keshaviah P, Prowant B, Nolph KD, Singh A (1995) Lean body mass estimation by creatinine kinetics, bioimpedance, and dual energy X-ray absorptiometry in patients on continuous ambulatory peritoneal dialysis. ASAIO J 41:M442–M446
Patel SS, Molnar MZ, Tayek JA, Ix JH, Noori N, Benner D, Heymsfield S, Kopple JD, Kovesdy CP, Kalantar-Zadeh K (2013) Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross-sectional study and review of literature. J Cachexia Sarcopenia Muscle 4:19–29. doi:10.1007/s13539-012-0079-1
Wang ZM, Gallagher D, Nelson ME, Matthews DE, Heymsfield SB (1996) Total-body skeletal muscle mass: evaluation of 24-h urinary creatinine excretion by computerized axial tomography. Am J Clin Nutr 63:863–869
Gerber LM, Mann SJ (2014) Development of a model to estimate 24-hour urinary creatinine excretion. J Clin Hypertens (Greenwich) 16:367–371. doi:10.1111/jch.12294
Clark RV, Walker AC, O’Connor-Semmes RL, Leonard MS, Miller RR, Stimpson SA, Turner SM, Ravussin E, Cefalu WT, Hellerstein MK, Evans WJ (2014) Total body skeletal muscle mass: estimation by creatine (methyl-d3) dilution in humans. J Appl Physiol (1985) 116:1605–1613. doi:10.1152/japplphysiol.00045.2014
Stimpson SA, Turner SM, Clifton LG, Poole JC, Mohammed HA, Shearer TW, Waitt GM, Hagerty LL, Remlinger KS, Hellerstein MK, Evans WJ (2012) Total-body creatine pool size and skeletal muscle mass determination by creatine-(methyl-D3) dilution in rats. J Appl Physiol (1985) 112:1940–1948. doi:10.1152/japplphysiol.00122.2012
Stimpson SA, Leonard MS, Clifton LG, Poole JC, Turner SM, Shearer TW, Remlinger KS, Clark RV, Hellerstein MK, Evans WJ (2013) Longitudinal changes in total body creatine pool size and skeletal muscle mass using the D3-creatine dilution method. J Cachexia Sarcopenia Muscle. doi:10.1007/s13539-013-0110-1
Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam TT, Vassileva MT (2014) The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 69:547–558. doi:10.1093/gerona/glu010
Cawthon PM, Peters KW, Shardell MD, McLean RR, Dam TT, Kenny AM, Fragala MS, Harris TB, Kiel DP, Guralnik JM, Ferrucci L, Kritchevsky SB, Vassileva MT, Studenski SA, Alley DE (2014) Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci 69:567–575. doi:10.1093/gerona/glu023
McLean RR, Shardell MD, Alley DE, Cawthon PM, Fragala MS, Harris TB, Kenny AM, Peters KW, Ferrucci L, Guralnik JM, Kritchevsky SB, Kiel DP, Vassileva MT, Xue QL, Perera S, Studenski SA, Dam TT (2014) Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci 69:576–583. doi:10.1093/gerona/glu012
Acknowledgements
The present work was funded by a Grant from the Innovative Medicines Initiative—Joint Undertaking (IMI-JU 115621). The work was also partly supported by the “Centro Studi Achille e Linda Lorenzon” (E.M., R.C.), Fondazione Roma (NCDs Call for Proposals 2013; E.M., R.C.), and intramural research grants from the Catholic University of the Sacred Heart (D3.2 2013 and D3.2 2015; E.M., F.L., M.T., R.C.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of the present work are partners of the SPRINTT Consortium, which is partly funded by the European Federation of Pharmaceutical Industries and Associations (EFPIA). E.M. served as a consultant for Huron Consulting Group, Genactis and Novartis. M.C. served as a consultant for and/or received honoraria for scientific presentations from Nestlé.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study informed consent is not required.
Rights and permissions
About this article
Cite this article
Tosato, M., Marzetti, E., Cesari, M. et al. Measurement of muscle mass in sarcopenia: from imaging to biochemical markers. Aging Clin Exp Res 29, 19–27 (2017). https://doi.org/10.1007/s40520-016-0717-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-016-0717-0