Abstract
Purpose
To describe the features of obstructive sleep apnoea (OSA) and its association with arterial hypertension (HT), coronary artery disease (CAD), and arrhythmias in elderly (≥65 years) versus younger patients.
Methods
All adult patients referred to our Sleep Research Unit for suspected OSA were included and underwent a thorough medical examination and an in-laboratory polysomnography. The severity of OSA was defined by the apnoea–hypopnoea index (AHI) as mild [5–15/h), moderate [15–30/h), and severe (≥30/h).
Results
Elderly patients (n = 136) and really old patients (>75 years) had higher prevalence of OSA (89 %) and severe OSA (36.8 %) as compared to younger patients (n = 439; 79.5 and 27.6 %, respectively, p < 0.05). In patients with OSA, the elderly group had a poorer sleep quality and more severe nocturnal oxygen desaturation than the younger group. Elderly patients presented higher percentages of HT (47.8 %), CAD (19.8 %), and arrhythmias (16.2 %) as compared to younger patients (p < 0.01). The odds ratio (OR) for HT increased with OSA severity from 1.0 to 1.65 (95 % confidence interval 0.83–3.27), 1.0 to 2.5 (95 % CI 1.25–5.00), and 1.0 to 3.77 (1.95–7.29) in younger patients, but not in elderly ones where the OR increased from 1.0 to 0.6 (0.17–2.04), 1.0 to 1.14 (0.34–3.82), and 1.0 to 1.46 (0.46–4.63), respectively.
Conclusion
Stronger relation of HT and OSA severity in younger patients should encourage us to screen OSA in these patients at very young age. Increased OSA severity without obesity in very old patients needs to be confirmed and further studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnoea (OSA) is characterised by partial or complete periods of upper airway occlusion, during which increased respiratory efforts occurred, leading to intermittent oxygen desaturation, hypoxemia, hypercapnia, and repeated arousals. These phenomena result in endothelial dysfunction, vascular and systemic inflammation, oxidative stress, sympathetic nervous system activation with subsequent vasoconstriction and hemodynamic alterations that contribute to cardiovascular consequences [1–3]. Recent evidences showed that moderate-to-severe OSA increases the risk of fatal and non-fatal cardiovascular events, especially in men [4, 5]. Therefore, it is important to well characterise patients with OSA in order to identify those with a higher risk of developing cardiovascular complications.
The prevalence of OSA in middle-age populations was estimated at 4 % in men and 2 % in women [6]. Prevalence estimates from studies using two-stage stratified probability sampling method were generally higher, from 3 to 28 % for OSA of at least mild severity [Apnoea–hypopnoea index (AHI) ≥5 events/h] [7]. The AHI was defined as the addition of apnoea episodes and hypopnoea episodes per hour during sleep. It has been now well established that men are at higher risk for OSA (2- to 10-fold greater) [8, 9] and more severe OSA (8-fold greater) than women [10]. In elderly persons, the OSA prevalence was usually increased, ranging 5.6–70 % depending on the sampling methods for patients recruitment [11–13]. Thus, age has become a risk factor for OSA as well as cardiovascular diseases.
In this clinic-based study, we aimed to describe features of sleep quality and OSA parameters in elderly (≥65 years) patients compared to those in young and middle-aged patients, especially concerning the risk of hypertension (HT), coronary artery disease (CAD), and cardiac arrhythmias.
Subjects and methods
Population
We enrolled consecutively 575 subjects, 18–86 years old, who were referred to our Sleep Research Unit for suspicion of obstructive sleep apnoea (OSA). All subjects underwent thorough clinical examination and medical history, including cardiovascular morbidities. Arterial HT was reported if a patient had a systolic blood pressure (BP) ≥140 mm Hg and/or a diastolic BP ≥90 mm Hg, or if he (or she) was taking BP-lowering medication(s). Myocardial ischemia and cardiac arrhythmias were previously confirmed by cardiologist. Cigarettes and alcohol consumption’s information was collected in the medical files. Patients were classified as smokers or non-smokers; alcohol or non-alcohol users. We evaluated subjective daytime sleepiness using the Epworth Sleepiness Scale (ESS) as previously described [14].
This study had been approved by the Ethics Committee of our institution and all patients have given their informed consent.
Polysomnography
All patients underwent overnight polysomnography using a Medcare data-acquisition system (Monet, REMbrandt PSG Analysis Manager) with standard electrodes and sensors. Briefly, electroencephalography electrodes were applied at A2-C4, C4-C3, C3-A1, and C3-O1. Two electro-oculography were applied at the sides of both eyes to record horizontal and vertical eyes movements. Submentalis and anterior tibialis muscles electromyography were recorded. Chest and abdominal respiratory movements were measured by strain gauges. Thermistors and nasal pressure cannulas were used to detect airflows. Arterial oxygen saturation was recorded using pulse oximeters.
To establish sleep stages, recorded nocturnal polysomnographies were visually scored on the basis of 30-s epochs, using Rechtschaffen and Kales criteria [15]. A single polysomnographic study conducted during an entire night (for at least 6 h) was used to establish the presence of OSA, based on the recommendations of the American Academy of Sleep Medicine Task Force [16].
Briefly, apnoea was defined as a complete cessation of oronasal airflow of at least 10 s. Hypopnoea was defined as an important reduction in airflow (≥50 %) lasting at least 10 s or moderate reduction (≥30 %) associated with EEG arousal and/or significant oxygen desaturation (≥4 %) [17]. Sleep apnoea group was defined as having an apnoea–hypopnoea index of at least 5/h (AHI ≥ 5/h), cause all subjects were symptomatic for OSA. The OSA severity was classified as mild (5 ≤ AHI < 15), moderate (15 ≤ AHI < 30), and severe (AHI ≥ 30) [16]. Individuals with AHI < 5 were included in the control group. Sleep efficiency was computed as a ratio of time spent asleep (total sleep time) to the amount of time spent in bed.
Statistical analysis
Data were analysed using SPSS 16.0.0. (SPSS Inc. Chicago, IL, USA). Values were expressed as mean ± standard deviation (SD) for continuous variables and number (percentage) for categorical ones. Comparisons were performed by Student’s t test or Chi-squared test as appropriate. Correlations between continuous variables were computed using Pearson’s method. Odd ratios and risk differences of arterial hypertension, coronary artery disease, and cardiac arrhythmias between the groups of elderly patients (≥65 years) versus non-elderly or young-middle-aged patients (<65 years) were calculated using Chi-squared test. The aging difference in the association between OSA severity and HT was determined by a binary logistic regression model with HT as dependent variable and with age, gender, and BMI (with or without tobacco use and alcohol consumption) as covariates. All tests were two-sided and a p value < 0.05 was considered to be statistically significant.
Results
Demographic characteristics and sleep apnoea in the study population according to groups of age (Table 1)
We enrolled 575 subjects (age 54.3 ± 13, 403 men), including 136 elderly persons (≥65 years) and 439 non-elderly ones (<65 years). Only 466 patients (124 elderly and 342 non-elderly), provided information about smoking and alcohol status. There were slightly more smokers in the elderly than in the younger group (p < 0.05). However, there was no significant difference (p > 0.05) in the percentages of men, alcohol consumers, obese patients (BMI ≥ 30), and BMI between the two groups.
In the whole population, 470 persons (81.7 %) completed criteria of OSA (AHI ≥ 5/h and symptomatic), 171 patients (29.7 %) had severe OSA (AHI ≥ 30/h). Elderly patients had more OSA (89 %) and severe OSA (36.8 %) than young-middle-aged patients (79.5 and 27.6 %, respectively, p < 0.05). Very old patients (>75 years) had similar prevalence of OSA (88 %, p > 0.05) but higher prevalence of severe OSA (52 %, p < 0.05) and higher AHI (33.6 ± 20.3 events/h, p < 0.01) than the younger group (≤75 years, 81.5, 28.9 %, and 21.9 ± 20.4 events/h, respectively). They had lower BMI (26.8 ± 4.3 kg/m2) than the younger group (29.2 ± 6.4 kg/m2, p < 0.05) (Table 1).
Sleep characteristics in OSA group (AHI ≥ 5/h) (Table 2)
There were 470 patients with OSA (121 elderly, including 22 patients >75 years). We found no significant difference (p > 0.05) in gender, percentages of smokers and alcohol consumers, BMI, Epworth Sleepiness Scale, and AHI. The elderly group had more severe desaturation as assessed by mean nocturnal oxygen saturation (SpO2, p < 0.01) and the sleeping time with SpO2 below 90 % (p < 0.001). About PSG parameters, the elderly group had lower total sleep time (TST, p < 0.05) and sleep efficiency (p < 0.01), and higher percentages of intra-hypnic arousals (p < 0.05) and stage 1 slow wave sleep time to TST (p < 0.001) to the detriment of stage 2 slow wave sleep and REM sleep (p < 0.001 and p = 0.01, respectively). To better illustrate the sleep architecture characteristics evolution with age, we included two representative hypnograms of two patients belonging to different groups of age and having comparable AHI and BMI in the Fig. 1.
Compared with the younger group, “really” old patients (>75 years) had significantly higher AHI (38 ± 17.5 versus 26.4 ± 20.1 events/h, p < 0.01), lower BMI (26.9 ± 4.6 versus 29.7 ± 6.5 kg/m2, p < 0.05), higher stage 1 slow wave sleep time to TST (40.6 ± 17.1 versus 29.2 ± 11.8 %, p < 0.001), and lower stage 2 slow wave sleep time to TST (34.4 ± 11.1 versus 42 ± 10.3 %, p < 0.01). Other characteristics and parameters were statistically comparable between these two groups (Table 2).
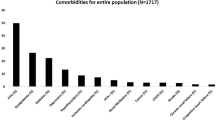
Cardiovascular comorbidities in OSA patients and control subjects, stratified by their age
The Table 1 summarised the prevalence of cardiovascular diseases in overall population. The prevalences of HT (47.8 %), CAD (19.8 %), and cardiac arrhythmias (16.2 %) in the elderly group were significantly higher than those from the young-middle-aged group (30.1, 9.1, and 7.5 %, respectively, p < 0.01) (Table 1).
Arterial hypertension
The risk of hypertension (HT) was significantly increased in patients with OSA as compared to that in control subjects in the whole population (p = 0.001) and in the young-middle-aged group (p = 0.002). However, there was no significant difference in HT risk between OSA and non-OSA patients in the elderly group (p = 0.92) (Fig. 2). The risk difference (RD) of HT between the OSA group and control group was 16.3 % in the whole population, 16.9 % in young-middle-aged patients, but insignificant in elderly ones (RD = 1.3 %) (Table 3).
Arterial hypertension risks in OSA patients and control subjects, according to groups of age. All patients with suggestive symptoms of sleep apnoea underwent an in-laboratory polysomnography (type I PSG). Patients with an AHI < 5/h were included in the control group. Patients with OSA (AHI ≥ 5/h) or without OSA were then divided into the young-middle-aged group (<65 years) and the elderly group (≥65 years). The relation between obstructive sleep apnoea (OSA) and arterial hypertension was determined using Chi-squared test (p < 0.05 as significant). **p < 0.01
The odds ratio (OR) for HT increased with OSA severity from 1.0 to 1.65 in mild OSA (95 % confidence interval 0.83–3.27; p > 0.05), from 1.0 to 2.5 in moderate OSA (95 % CI 1.25–5.00; p < 0.01), and from 1.0 to 3.77 in severe OSA (1.95–7.29; p < 0.002) in young-middle-aged patients, but not in elderly patients where the OR increased from 1.0 to 0.6 (0.17–2.04), 1.0 to 1.14 (0.34–3.82), and 1.0 to 1.46 (0.46–4.63), respectively (p > 0.05) (Fig. 3).
Odds ratio for hypertension in patients with OSA versus control subjects in two groups of age. All patients with suggestive symptoms of sleep apnoea underwent an in-laboratory polysomnography (type I PSG). Patients with an apnoea–hypopnoea index (AHI) <5/h were included in the control (Non-OSA) group. Patients with OSA (AHI ≥ 5/h) or without OSA were then divided into elderly group (≥65 years) and non-elderly group. Relations between the severity of obstructive sleep apnoea (OSA) and arterial hypertension risk in patients with mild (5 ≤ AHI < 15), moderate (15 ≤ AHI< 30), and severe OSA (AHI ≥ 30) were determined using chi-squared test with odds ratio calculation versus control subjects (AHI <5). The value of p < 0.05 was considered as statistically significant). **p < 0.01; ***p < 0.001. OR odds ratio, CI confidence interval
Conversely, overweight and obesity as assessed by body mass index (BMI ≥ 25 and ≥30, respectively) increased significantly the odds ratios of HT in both elderly and non-elderly groups even though the RD was higher in the young-middle-aged group (27.1 %) than that from the elderly group (19.8 %) (Table 4).
In a logistic regression analysis of the association between OSA severity (AHI as continuous variable) and HT, adjustment for differences in age, gender, and BMI resulted in a weakening of the OR in the younger group to 1.011 (95 % CI 1.000–1.023; p = 0.045). However, the association remained insignificant in the elderly group (OR: 1.002; 95 % CI 0.984–1.021; p = 0.8). The role of gender in this association was weak (p = 0.5 and p = 0.9, respectively). The results were similar when adding tobacco use and alcohol consumption into covariates for adjustment (data not shown).
Coronary artery disease
The risk difference of CAD between the OSA group and the control group was 10.8 % (p = 0.002) in the whole population, and 8.7 % (p = 0.011) in the non-elderly group but here again, the RD was not statistically significant in the elderly group (p = 0.3) (Table 5). In addition, there was no correlation between the CAD risk and the severity of OSA as evaluated by AHI in the whole population (p = 0.07), in elderly (p = 0.25), and non-elderly (p = 0.297) groups.
Cardiac arrhythmias
The risk difference for cardiac arrhythmias between OSA and control groups was not statistically significant (p > 0.05; χ 2 test) in the whole population and in every group of age.
Discussion
Our main results showed an increased prevalence of OSA and severe OSA in elderly persons and more importantly in the “really” old group of patient who were more than 75 years, as compared to the younger group. We also observed the higher prevalence of cardiovascular comorbidities as hypertension, myocardial ischemia, and cardiac arrhythmia in elderly persons as compared to younger ones. However, the increased risks of HT and CAD in aged patients were not linked to OSA severity as observed in the young-middle-aged group. In the logistic regression analysis, the significant association of HT and OSA in the younger group was attenuated essentially by overweight (BMI ≥ 25 kg/m2), but not by gender or tobacco or alcohol consumption. Furthermore, elderly patients presented a disruption of sleep quality irrespective of the presence of OSA. Nocturnal oxygen desaturation worsened in younger patients (<65 years) than that in elderly ones.
The prevalence of OSA was relatively high in our study (Table 1), at least partially due to the institution-based recruitment of patients. They were all symptomatic, most usually with daytime sleepiness, non-restorative sleep, and loud snoring. Patients were often referred to our Sleep Research Unit by their family doctor who has previously performed thorough medical examination, the fact that might account for the high probability of positive OSA diagnosis and the high Epworth score in the elderly group as well as in the younger one. Our results were consistent with those from Ancoli-Israel et al. [11] showing that 81 % elderly patients (≥65 years) had a respiratory disturbance index (RDI) ≥ 5/h in a randomly selected population-based study. In another study on 233 elderly persons living in a nursing home, the prevalence of OSA (defined as AHI ≥ 5/h) was 70 % in the whole population, slightly higher in men (76 %) than in women (68 %) [18]. Other reports found that the increase of OSA prevalence across the age happened essentially before the age of 65 with the peak prevalence at about 50–55 years in men, and 60–65 in women [19, 20]. This effect is probably due to the confounding factors, especially obesity [20]. Our data found no significant difference of BMI between the elderly versus younger patients in the whole population as well as in patients with OSA (Tables 1, 2). Therefore, it seems unlikely that overweight and obesity account for this discrepancy in our observation.
Some studies suggested current cigarette smoking [21, 22] and alcohol consumption [23–25] could increase the relative risk of OSA and worsen nocturnal oxygen desaturation and sleep-disordered breathing. In our report, we obtained information about smoking and alcohol uses in more than 80 % patients. Elderly patients were more likely smokers (62.9 %) and consumed more cigarettes (mean of package years of smoking: 17.3) than younger persons (51.5 % and 13.2 package-years, respectively) in overall population, probably due to accumulative effect. These differences were, however, not significant in the group of OSA patients (AHI ≥ 5/h) nor in control subjects (AHI < 5/h). The results on alcohol consumption were also not different between the elderly and younger groups (Table 1). Overall, our data did not support the role of cigarette smoke or alcohol use in the increase of OSA prevalence in elderly subjects.
The prevalence of severe OSA (AHI ≥ 30/h) [16], was higher in elderly (36.8 %) than non-elderly (27.6 %, p < 0.05) patients. Other criteria of OSA severity such as AHI and nocturnal hypoxia (mean nocturnal SpO2 and sleeping time with SpO2 <90 % on TST) were also more severe in the elderly group than in the young-middle-aged group (Table 2). A community-based cross-sectional study in a healthy elderly population (827 subjects) in France observed the same prevalence of severe OSA (37 %) [26]. In addition, the proportions of patients having moderate-to-severe OSA (AHI ≥ 15/h) were about 1.7- to 4-fold higher in older (≥60 years) than in younger ones [19, 20, 27]. In these patients, the cardiovascular disease comorbidities and overall mortality increased significantly in cross-sectional [28–31] and prospective cohort studies [5, 32, 33] as compared to subjects without OSA (AHI < 5/h).
In our report, the prevalence of arterial hypertension (HT) was significantly higher in OSA group than that from control group with the risk difference of 16.2 %. However, the correlation of HT and OSA severity was only found in the young and middle-aged group but not in the elderly one (Fig. 1; Table 3). The association of sleep-disordered breathing, sleep apnea, and hypertension was observed in a large number of community-based multicenter studies in 2000s such as the Sleep Heart Health Study (SHHS) [29], the Wisconsin Sleep Cohort Study [30], the Pennsylvania Sleep Study [31], and the sleep clinic-based study in Toronto [28]. After adjusting for the most frequent confounding factors, especially BMI, age, sex, alcohol intake, and smoking, the OR for HT varied from 1.37 [95 % confidence interval (CI): 1.03–1.83] [28], 2.89 (95 % CI 1.46–5.64) [29], to 6.85 (95 % CI 2.02–26.36) [31]. Especially, in a cohort of 2,677 patients aged 20–85 years, Lavie et al. [28] nicely showed that each additional apneic event per hour of sleep increased the OR for HT by about 1 %, and that each 10 % decrease in nocturnal oxygen saturation increased the odds by 13 %. However, the association of HT and OSA stratified by age (middle-age and elderly) has shown discrepancy among studies. In the SHHS, the association of HT and OSA was significant in the middle-aged (40–64 years) as well as in older individuals (≥65 years) [29]. Another report [34] did not support the association of AHI and systolic/diastolic HT in older patients (≥60 years), findings that were in line with our results. This phenomenon might be due to the physiological mechanism of aging that is strong enough to induce endothelial dysfunction and increase arterial stiffness contributing to the high prevalence of HT, even in the absence of OSA [35]. Longitudinal prospective study also confirmed the association of HT and OSA. After a follow-up of 3 years, patients with AHI ≥ 30/h had an odds ratio of new-onset hypertension of 1.8 (95 % CI 1.1–2.8; p = 0.02) after adjusted for usual confounding factors [36].
Most of studies analysing the association of HT and OSA adjusted the results with usual confounding factors such as age, sex, BMI, alcohol and smoking status. Using multiple logistic regression analysis for arterial HT, we found that the presence of obesity (BMI ≥ 30 kg/m2) modified considerably the association of HT and OSA irrespectively of age, gender, and alcohol and cigarette consumptions. Our results were consistent with those from other reports [28, 31, 37].
Patients with OSA might be predisposed to cardiac arrhythmias resulted from sympathetic nervous system stimulation due to hypoxemia and respiratory acidosis secondary from apnoeic events [1–3]. Cross-sectional analysis of OSA patients in the SHHS showed that patients with severe OSA syndrome (AHI ≥ 30/h) had 2–4 times higher odds of complex arrhythmias (atrial fibrillation, non-sustained ventricular tachycardia, and premature ventricular complexes) than those without OSA (AHI < 5/h) [38]. The same cohort of patients had been followed up for a median of 8.7 years in a prospective longitudinal study, demonstrating that men 40–70 years old with severe OSA were 68 % more likely to develop coronary arterial disease (CAD) and chronic heart failure than those without OSA (AHI < 5/h) [39]. In our study, patients with OSA had higher prevalence of CAD (13.6 %) and arrhythmias (10.2 %) than non-OSA individuals (2.9 and 6.7 %, respectively). The risk difference between elderly and young-middle-aged groups was only significant for CAD but not for cardiac arrhythmias. There was no significant relation between these cardiovascular pathologies and the severity of OSA, probably due to the scarce distribution of cases in the non-OSA group. Another reason was probably owed to one of the confounding factors, dyslipidemia, that we did not include in our adjusted-data analysis [38, 39].
Our study had some limitation. The sleep clinic-based recruitment of patients might increase the likelihood of diagnosed OSA cases. Some potential confounders for cardiovascular risks were not included in the analysis (hyperlipidemia, diabetes). Despite these limitations, our results confirm an association between OSA and arterial hypertension as well as coronary ischemia disease in the studied population. This relation was stronger in young and middle-aged patients with OSA (than elderly subjects), inciting us to diagnose these patients as soon as possible in the youngest age, as it was recommended by other authors [40]. Increased OSA severity without obesity in very old patients (>75 years) needed to be confirmed since we only had 25 persons including 22 with OSA. The physiopathological mechanism(s) might be interesting to further study in a larger population.
References
Shamsuzzaman AS, Gersh BJ, Somers VK (2003) Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 290:1906–1914
McNicholas WT, Bonsigore MR (2007) Management Committee of EU COST ACTION B26. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J 29:156–178
Lavie L, Lavie P (2009) Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J 33:1467–1484
Marin JM, Carrizo SJ, Vicente E, Agusti AG (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365:1046–1053
Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ et al (2008) Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 31:1071–1078
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235
Young T, Peppard PE, Gottlieb DJ (2002) Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165:1217–1239
Lin CM, Davidson TM, Ancoli-Israel S (2008) Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev 12:481–496
Mohsenin V, Yaggi HK, Shah N, Dziura J (2009) The effect of gender on the prevalence of hypertension in obstructive sleep apnea. Sleep Med 10:759–762
O’Connor C, Thornley KS, Hanly PJ (2000) Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med 161:1465–1472
Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O (1991) Sleep-disordered breathing in community-dwelling elderly. Sleep 14:486–495
Chung S, Yoon IY, Lee CH, Kim JW (2009) Effects of age on the clinical features of men with obstructive sleep apnea syndrome. Respiration 78:23–29
Pływaczewski R, Bednarek M, Jonczak L, Zieliński J (2008) Sleep-disordered breathing in a middle-aged and older Polish urban population. J Sleep Res 17:73–81
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14:540–545
Rechtschaffen A, Kales A (eds) (1968) A manual of standardized terminology, techniques and scoring system of sleep stages in human subjects. Brain Information Service/Brain Research Institute, University of California, Los Angeles
(1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 22:667–89
Tsai WH, Flemons WW, Whitelaw WA, Remmers JE (1999) A comparison of apnea-hypopnea indices derived from different definitions of hypopnea. Am J Respir Crit Care Med 159:43–48
Ancoli-Israel S, Klauber MR, Kripke DF, Parker L, Cobarrubias M (1989) Sleep apnea in female patients in a nursing home. Increased risk of mortality. Chest 96:1054–1058
Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A (1998) Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med 157:144–148
Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A et al (2001) Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 163:608–613
Wetter DW, Young TB, Bidwell TR, Badr MS, Palta M (1994) Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med 154:2219–2224
Kashyap R, Hock LM, Bowman TJ (2001) Higher prevalence of smoking in patients diagnosed as having obstructive sleep apnea. Sleep Breath 5:167–172
Taasan VC, Block AJ, Boysen PG, Wynne JW (1981) Alcohol increases sleep apnea and oxygen desaturation in asymptomatic men. Am J Med 71:240–245
Scanlan MF, Roebuck T, Little PJ, Redman JR, Naughton MT (2000) Effect of moderate alcohol upon obstructive sleep apnoea. Eur Respir J 16:909–913
Peppard PE, Austin D, Brown RL (2007) Association of alcohol consumption and sleep disordered breathing in men and women. J Clin Sleep Med 3:265–270
Sforza E, Roche F, Thomas-Anterion C, Kerleroux J, Beauchet O, Celle S et al (2010) Cognitive function and sleep related breathing disorders in a healthy elderly population: the SYNAPSE study. Sleep 33:515–521
Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ et al (2002) Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med 162:893–900
Lavie P, Herer P, Hoffstein V (2000) Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ 320:479–482
Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S et al (2000) Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 283:1829–1836
Peppard PE, Young T, Palta M, Skatrud J (2000) Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342:1378–1384
Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Leiby BE, Vela-Bueno A et al (2000) Association of hypertension and sleep-disordered breathing. Arch Intern Med 160:2289–2295
Lavie P, Lavie L, Herer P (2005) All-cause mortality in males with sleep apnoea syndrome: declining mortality rates with age. Eur Respir J 25:514–520
Chami HA, Resnick HE, Quan SF, Gottlieb DJ (2011) Association of incident cardiovascular disease with progression of sleep-disordered breathing. Circulation 123:1280–1286
Haas DC, Foster GL, Nieto FJ, Redline S, Resnick HE, Robbins JA et al (2005) Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation 111:614–621
Pimenta E, Oparil S (2012) Management of hypertension in the elderly. Nat Rev Cardiol 9:286–296
Guillot M, Sforza E, Achour-Crawford E, Maudoux D, Saint-Martin M, Barthélémy JC et al (2013) Association between severe obstructive sleep apnea and incident arterial hypertension in the older people population. Sleep Med 14:838–842
Hedner J, Bengtsson-Boström K, Peker Y, Grote L, Råstam L, Lindblad U (2006) Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case-control study. Eur Respir J 27:564–570
Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL et al (2006) Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 173:910–916
Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF et al (2010) Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 122:352–360
Lavie P, Herer P, Lavie L (2007) Mortality risk factors in sleep apnoea: a matched case-control study. J Sleep Res 16:128–134
Conflict of interest
There are no conflicts of interest related to the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hua-Huy, T., Rouhani, S., Nguyen, XY. et al. Cardiovascular comorbidities in obstructive sleep apnoea according to age: a sleep clinic population study. Aging Clin Exp Res 27, 611–619 (2015). https://doi.org/10.1007/s40520-015-0318-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-015-0318-3