Abstract
Bacterial resistance to antibiotic treatment raises serious public health-related concerns, in parallel with increasing efforts to develop efficient and safe therapeutic alternatives. Silver nanoparticles (Ag-NPs) have been synthesized and enhanced to increase their antibacterial properties using two primary types of lasers. These include the Q-switched Nd:YAG laser and the 405 nm diode laser. The former was used to prepare Ag-NPs colloidal solutions that shown effectiveness against sensitive Staphylococcus aureus, whereas the latter was utilized to activate Ag-NPs against methicillin-resistant S. aureus (MRSA). The approach of this work is to enhance the antibacterial potential of Q-switched Nd:YAG synthesized Ag-NPs against both normal and resistant strains of S. aureus, once by using them in combination with antibiotics and another time by exposing them to 405 nm diode laser. The synthesized silver nanoparticles were characterized by different methods such as UV–Visible, TEM, AFM and zeta potential. These characterizations revealed the formation of AgNPs with sizes in the range from 10 to 30 nm in response to pulsed laser ablation of pure Ag metal plates. The NPs efficiently deactivated S. aureus. The minimum inhibitory concentration (MIC) of AgNPs was 60 µg/ml, which caused a growth inhibition zone with a diameter of 12 mm. A remarkable improvement in antibacterial activity was achieved upon the irradiation of AgNPs with 405 nm laser light, causing a reduction of the MIC to the half (30 µg/ml), even when the treated strain is known to be resistant (MRSA). It is concluded that further enhancement of laser-synthesized AgNPs leads to more powerful antimicrobial impacts that even involve antibiotic-resistant bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In spite of being generally effective over several decades, antibacterial drugs have been counteracted by continuously developing bacterial resistance that reduced drug effectiveness in the treatment of infections [11]. Over the last three decades, pharmaceutical industries also committed to producing new antibiotics that have the ability to effectively suppress bacterial cell wall synthesis, protein synthesis, and DNA replication [34]. However, despite these developments, bacterial resistance to conventional antibiotics has continued to pose a crucial issue in health care that has significant impact on communities around the world [22]. Although community-acquired MRSA species are less resistant to antimicrobials than those identified with nosocomial settings, toxins as Panton-Valentine leukocidinare more likely to be produced (Hultén et al. 2018). The continuing proliferation of resistant bacterial strains within the population, e.g. in health care facilities, sports teams, military recruits, and day care centers for children, produces additional difficulties in reducing pathogens [24].
Nanoparticles of trace metals, such as Ag and Au that present unique features and extensive applications, are presented in diverse fields [49]. Therefore, the characteristics of these NPs have always been of great interest to many scientists [15]. Generally, NPs display properties completely different from those of their bulk counterparts [23, 26]. Silver nanoparticles (AgNPs) show high toxicity to various types of bacteria in the human body, which is mainly related to their large specific surface area and high reactivity [14, 29,30,31]. The broad antibacterial properties of Ag-NPs have encouraged researchers to employ these materials in biomedical applications [27, 48, 50, 54]. Various investigations have demonstrated the efficacy of Ag-NPs against Methicillin-sensitive Staphylococcus aureus (MSSA) [37, 52]. Nonetheless, the attempts of combined use of Ag-NPs and antibiotics as a curative tool of antibiotic-resistant bacterial infections are scarce. Rudramurthy et al. showed the synergistic effect between antimicrobial peptides and silver nanoparticles on Gram-negative bacteria [2]. Kora and Rastogi revealed that Ag-NPs coated with polyvinylpyrrolidone (PVP) develops stronger antibacterial activity when combined with established antibiotics, compared to those capped with citrate or SDS [15]. More recently, the positive impacts of using AgNPs together with topical antibiotics to treat bacterial infections were reported by [38]. Velusamy et al. also indicated that penicillin may contribute in increasing antibiotic effectiveness in combination with silver nanoparticles [8, 12].
In late 1990, the Pulsed Laser Ablation in Liquid (PLAL) technology began to get attention. It is a top-down physical method based on the premise of splitting metal ion bulk precursors into metal atoms, It has the crucial advantage of creating extremely stable and pure nanoparticles with a surface free of reactant residue ions for biological applications, as well as very inexpensive processing setup costs. The way that materials react to light depends on the laser beam's intensity and temperature. As a result of constant laser exposure to a material, a series of reactions including heating, melting, boiling, and plasma production occur [9, 10, 18, 42]. Synthesis of Ag-NPs using PLAL is considered one of the most significant prospective strategies that was shown to be useful for many applications, especially in human health care [51].
Many efforts have been devoted to synthesising Ag- and Au-NPs [25, 28, 32, 35, 55]. Nd–YAG lasers are preferred over other laser types due to their precise processing during laser ablation in liquid. The size of AgNPs could be controlled in liquid media in the form of stable colloidal silver solutions [9, 10, 39,40,41, 43]. These colloids usually consist of Ag-NPs of different sizes with several features, such as chemical stability, non-toxicity, and easy handling. Resizing and reshaping also can be achieved via the melting and fragmentation techniques [1, 4,5,6,7, 13, 20, 36, 39, 40].
Staphylococcus aureus is a worldwide significant bacteria and it is responsible for multiple diseases. Antibiotics were used to classify resistant strains of S. aureus, including methicillin-resistant S. aureus (MRSA). Due to the presence of the mecA gene integrated in the staphylococcal cassette chromosome mecA, the MRSA strain gains resistance. This gene encodes a penicillin-binding protein of 78 kD that enhances the inhibitory effect on bacterial cell wall [29,30,31]. Antibiotic-resistant bacteria, such as MRSA, could also spread to populations outside the hospital and result in a widespread disease [27], Maribel et al. 2009).
The increasing complexity of this issue makes it necessary not only to discover new antibiotics, but also to develop alternative non-antibiotic solutions [16, 46, 50]. The size and shape of AgNps have a high impact on their antibacterial activity. Some reports had recorded a high biocidal activity of anisotropic shape of silver nanoparticles [17, 44, 47] While others reported highest antibacterial activity of isotropic silver nanoparticles due to large surface area to volume ratio of spherical shapes [3, 19, 53].
The TEM of the experimentally prepared AgNps reveal formation of spherical shaped silver nanoparticles as shown in Fig. (3).
This study aims to prepare suspensions of AgNPs by laser ablation in liquid medium, compare their antibacterial activity against S. aureus with those of common antibiotics, and evaluate the antibacterial activity of AgNPs synthesized by laser ablation against methicillin-resistant Staphylococcus aureus bacteria (MRSA) with and without irradiation with visible 405 nm blue laser light.
Experimental Procedure
Preparation and Characterization of the AgNPs
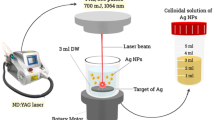
AgNPs were synthesised by pulsed laser ablation of pure Ag metal plates (ounces of 99.999%) placed at the bottom of a quartz vessel containing 1 ml of double deionised distilled water. The Q-switched Nd:YAG laser of 10 ns and various energies, in the range from 100 to1000 mj, was used. A lens with a 10 cm focal length was used to obtain a 1.2 mm diameter for laser spot by adjusting the height along the z-axis between the lens and the sample surface, as shown in Fig. (1). The proper laser energy density, 10 J/cm2, was chosen to ablate the AgNPs [29,30,31]. When the laser pulse strikes the Ag surface, a spark plume emerges, followed by a visible cloud of Ag particles, which are expanded and dispersed gradually through the liquid. This process can be recognised by the naked eye.
The synthesized AgNPs were characterized using various methods including; Transmission electron microscopy (JOEL, JEM 1400), Atomic Force Microscopy (AA2000,Angstrom, USA) and UV–Visible spectroscopy (Biotech Co., UK). Zeta potential (Brookhaven, zeta plus) tests were also carried out to explore the aggregation of silver nanoparticles in the suspension.
Examination of AgNPs Antibacterial Activity Against S. aureus
An AgNPs solution with a concentration of 60 µg/ml was prepared and its antimicrobial activity was examined based on the minimum inhibitory concentration (MIC). Different concentrations (120, 60, 30, 15 and 7.5 µg/ml) of this AgNPs solution were prepared by the two fold serial dilution method. The absorption spectrum was gained by a UV–Vis spectrophotometer. The antibacterial effects of the colloidal solutions were tested against S.aureus and compared with those of amoxicillin (25 µg), penicillin (10 µg), chloramphenicol (30 µg), and streptomycin (10 µg).
The disk diffusion method was used to examine the antimicrobial activity of each solution. Here, 50 µl of the solution was added to a sterilised filter paper and then incubated (UniMedica, China) at 37 °C until dry. An oven (Suarez, Brazil) was used for heating. Generally, S. aureus required relative humidity of 70% – 80%. The antimicrobial effects of different antibiotics were also tested by the Kirby–Bauer (disk diffusion test) method. Then, 40 µl of the solution with concentration of 60 µg/ml AgNPs was combined with the antibiotics mentioned above to investigate the synergetic effects of AgNPs and antibiotics.
The laser-enhanced antibacterial activity of AgNPs was assessed by subjecting the incubated resistant bacteria to 405 nm laser light at different exposure times (5, 10 and 15 min) and a power density of 0.1 W/cm2. MIC values were evaluated as the lowest NP concentration required to arrest the growth of bacteria in the test dish (i.e., the dish shows no turbidity) after incubation.
Results and Discussion
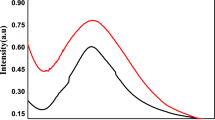
A remarkable colour change from yellow to brown (due to increases in nanoparticles density) was observed in the AgNPs colloidal solutions as the number of pulses used to prepare the solution by laser ablation increased from 100 to 1000. The absorption spectrum of AgNps colloidal suspension is given in Fig. (2). The absorption peak appears at wavelength much smaller than the incident light due to the surface plasmon resonance. The absorbance characteristics (height, width, and position) are strongly depends on the nanoparticle size. In this figure, the absorption peak which is located at 410 nm is correspond to presence of AgNps in the range in the range (20–30) nm [33, 45].
Moreover, the AgNPs colloidal solutions were chemically stable for a long time (months), as shown in Table 1. AgNPs prepared against the tested samples with high laser energy and low number of laser pulses did not have sufficient antibacterial activity. Thus, the activities of the AgNPs colloidal solutions with concentrations greater than100 µg/ml must be studied. Furthermore, AgNPs with different shapes and sizes may exhibit different antibacterial activities.
TEM investigations revealed the formation of spherical AgNPs with average size of 14 nm, as shown in Fig. (3). The findings indicate that even if prepared with a higher number of laser pulses, NPs of higher density could be obtained.
Furthermore, the shape, size and size distribution of the laser prepared AgNPs was examined by AFM. The AFM image in Fig. (4) shows AgNPs prepared by Nd:YAG laser of 400 mj with 1000 pulses while the corresponding histogram reveals a homogeneous size distribution of AgNPs in the range 10 to 30 nm with average size of about 14 nm.
The effect of a high-concentration of colloidal AgNPs prepared with 1000 laser pulses on S. aureus was studied. It is found that higher laser energies (> 600 mj) produce larger nanoparticle sizes which exhibited no noticeable effect on this type of bacteria. Whereas, lower laser energy of 400 mj leads to prepare effective colloidal solution of AgNPs. This energy produced AgNPs with smaller sizes in comparison with those obtained using high-energy laser. It was observed that one group of Staphylococcus bacteria is sensitive and affected by silver nanoparticles while another group is not affected by AgNPs and became resistant to AgNPs. Therefore, to activate plasmonic effects on their surface, the NPs were subjected to laser light. The antimicrobial activity (diameter of inhibition zone and MIC) of these laser-enhanced AgNPs on this group of resistant bacterium was investigated. Table (2) shows the inhibition zone of Staphylococcus in agar dishes treated with various concentrations of AgNPs prepared with 400 mj laser energy. The table reveals that the MIC for this group of bacteria is 60 µg/ml. The effect of the AgNPs is attributed to their penetration of the bacterial cell wall and generation of reactive oxygen species, leading to oxidative stress in bacterial cells.
The synergistic influence of antibiotics and AgNPs leads to enhancing the antibacterial activity, particularly against strains that have been proved to be resistant [21]. In this study, the synergistic effects of AgNPs with 4 separate antibiotics against S. aureus were studied based on the method of disc-diffusion. It was found that the diameter of inhibition zone increased in response to treatment with amoxicillin (25 µg), penicillin (10 µg), chloramphenicol (30 µg), and streptomycin (10 µg) from Himedia /India in the presence of the metallic nanoparticles at different concentrations (120, 60, 30, 15, 7.5) μg/ml, as demonstrated by the results listed in Table 3. This combined impact can result either from an increase in the bio-availability of the drug after conjugation in the cell membrane of bacteria or from the assimilation of both components. Recent studies have suggested that the AgNPs will act in two ways; in the first one, they can assault the cell membrane to destabilize it; in the second way, they, under synergetic influence with antibiotics, will easily cross the barrier of the cell membrane to demonstrate their bioactivity [29, 29, 30, 30, 31, 31, 56]. Table (3) presents the diameter of inhibition zone (DIZ) for AgNPs in combination with the antibiotics. The AgNPs could improve the antibacterial activity of all antibiotics tested and increased the diameter of the inhibition zone from 4 to 28 mm when added to penicillin. Figure (5) shows the agar dish treated with the combination of various Ag-NPs concentrations and penicillin.
Finally, the effect of AgNPs on methicillin-resistant Staphylococcus aureus (MRSA) bacteria was investigated. No considerable effect was observed for all prepared concentrations of AgNPs. Therefore, a new strategy was adopted concerning irradiation AgNPs with diode laser light of 405 nm during the bacteria treatment. This strategy activates the plasmonic effect on the AgNPs surface [38]. The results showed promising antibacterial activity by irradiated AgNPs, including an increase in DIZ value to 25 mm and a decrease in MIC value from 60 µg/ml to 30 µg/ml.
The observed remarkable improvement in AgNps antibacterial activity by laser irradiation can be explained by the photo thermal effect of laser light, which leads to a rapid loss of cell membrane integrity [8, 12]. The photothermal effect is stimulated by the enhanced surface plasmon resonance which is activated by the strong absorption near 405 nm (as discussed earlier in the UV–Visible measurements) especially for smaller silver nanoparticles. Table (4) shows the antibacterial activity of AgNPs on Staphylococcus aureus before and after irradiation with 405 nm laser light.
Conclusions
Human infective S. aureus are shown here to be affected by AgNPs prepared by pulse laser ablation in liquid. The antibacterial effect of AgNPs depends on their size, shape, and concentration in its nano-suspension media. The prepared AgNPs with size distribution in the range 10 to 30 nm had a minimum inhibition concentration of 60 μg/ml and exhibit much higher antibacterial effects when applied synergistically with some antibiotics, compared with their effects alone. Notably, AgNPs synthesised in this study also showed enhanced antibacterial effects on S. aureus and reduce the inhibition zone from 25 to 12 mm when they are irradiated with a blue laser light of 405 nm for local activation against MRSA.
References
Abdelghany, A., Menazea, A., Abd-El-Maksoud, M., Khatab, T.: Pulsed laser ablated zeolite nanoparticles: A novel nano-catalyst for the synthesis of 1,8-dioxo-octahydroxanthene and N-aryl-1,8-dioxodecahydroacridine with molecular docking validation. ApplOrganometal. Chem. 34, e5250 (2020)
Abderrafi, K., Jimenez, E., et al.: Production of Nanometer-Size GaAs Nanocrystals by Nanosecond Laser Ablation in Liquid. J. Nanosci. Nanotechnol. 12, 6774–6778 (2012)
Agnihotri, S., Mukherji, S., Mukherji, S.: Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv 4, 3974–3983 (2014)
Ahmed, M., Meera Moydeen, A., Ismail, A., El-Naggar, M., Menazea, A., El-Newehy, M.: Wound dressing properties of functionalized environmentally biopolymer loaded with selenium nanoparticles. J. Mol. Struc. 1225, 129–138 (2021)
Ahmed, M., El-Naggar, M., Aldalbahi, A., El-Newehy, M., Menazea, A.: Methylene blue degradation under visible light of metallic nanoparticles scattered into graphene oxide using laser ablation technique in aqueous solutions. J. Molec. Liquids 315 (2020)
Ahmed, M., Menazea, A., Mansour, S., Al-Wafi, R.: Differentiation between cellulose acetate and polyvinyl alcohol nanofibrous scaffolds containing magnetite nanoparticles/graphene oxide via pulsed laser ablation technique for tissue engineering applications. J. Mater. Res. Technol. 9, 11629–11640 (2020)
Ahmed, M., Mansour, S., Al-wafi, R., Menazea, A.: Composition and design of nanofibrous scaffolds of Mg/Se- hydroxyapatite/graphene oxide @ ε-polycaprolactone for wound healing applications. J. Market. Res. 9, 7472–7485 (2020)
Akram, F., El-Tayeb, T., Abou-Aisha, K., El-Azizi, M.: A combination of silver nanoparticles and visible blue light enhances the antibacterial efficacy of ineffective antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) Comb. Ann. Clin. Microbiol. Antimicrob. 15, 48 (2016)
Alamro, F., Toghan, A., Ahmed, H., Mostafa, A., Alakhras, A., Mwafy, E.: Multifunctional leather surface embedded with zinc oxide nanoparticles by pulsed laser ablation method. Microsc. Res. Tech. 85, 1611–1617 (2021)
Alamro, F., Mostafa, A., Abu Al-Ola, K., Ahmed, H., Toghan, A.: Synthesis of ag nanoparticles-decorated cnts via laser ablation method for the enhancement the photocatalytic removal of naphthalene from water. Nanomaterials 11(8), 2142 (2021)
Ash, R., Mauck, B., Morgan, M.: Antibiotic resistance of Gram-Negative bacteria in rivers. Emerg. Infect. Dis. 7, 8 (2002)
Astuti, S., Kharisma, D., Kholimatussa, S., Zaidan, H.: An in vitro antifungal efficacy of silver nanoparticles activated by diode laser to Candida albicans. AIP Conf. Proc. 1888, 020016 (2007)
Ayman, M., Mostafa, A.: Menazea: Laser-assisted for preparation ZnO/CdO thin film prepared by pulsed laser deposition for catalytic degradation. Radiat. Phys. Chem. 176, (2020)
Bruna, T., Maldonado-Bravo, F., Jara, P., Caro, N.: Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 22, 7202 (2021)
Catalina, M.J., Eric, M., Hoek, V.: A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart, Res. 12, 1531–1551 (2010)
Czaplewski, L., Bax, R., Clokie, M., Dawson, M., Fairhead, H., Fischetti, V., et al.: Alternatives to antibiotics-a pipeline portfolio review. Lancet Infect Dis. 16, 239–251 (2016)
Dong, P., Ha, C., Binh, L., Kasbohm, J.: Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles. Int. Nano Lett 2, 1–9 (2012)
El Faham, M., Mostafa, A., Toghan, A.: Facile synthesis of Cu2O nanoparticles using pulsed laser ablation method for optoelectronic applications. Colloids Surf. A: Physicochem. Eng. Aspects 630, 127562 (2021)
El-Kheshen, A., El-Rab, S.: Effect of reducing and protecting agents on size of silver nanoparticles and their anti-bacterial activity. Pharma Chem 4, 53–65 (2012)
El-Saied, H., Mostafa, A., Hasanin, M., Mwafy, E., Mohammed, A.: Synthesis of Antimicrobial Cellulosic Derivative and its Catalytic Activity. J King Saud Univ.-Sci. 32(1), 436–442 (2020)
Fayaz, A., Balaji, K., GirilalM, Y.R., Kalaichelvan, P., Venketesan, R.: Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram negative bacteria. Nanomed.: Nanotechnol. Biol. Med. 61, 103–109 (2010)
Friedman, N.D., Temkin, E., Carmeli, Y.: The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 22, 416–422 (2016)
Georgios, A., Sotiriou, E., Pratsinis: Antibacterial activity of nanosilver ions and particles. Environ. Sci. Tech. 44, 5649 (2010)
Gurunathan, S., Han, J., Kwon, D., Kim, J.: Enhanced antibacterial and antibiofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res Lett. 9, 373 (2014)
Ilic, V., Saponjic, Z., et al.: Bactericidal efficiency of silver nanoparticles deposited onto radio frequencyplasma pretreated polyester fabrics. Ind. Eng. Chem. Res. 49, 7287 (2010)
Jeevanandam, J., Barhoum, A., Chan, Y.S., Dufresne, A., Danquah, M.K.: Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J. Nanotechnol. 9, 1050–1074 (2018)
Kim, H.S., Ryu, J.H., Jose, B., Lee, B.G., Ahn, B.S., Kang, Y.S.: Formation of silver nanoparticles induced by poly(2,6-dimethyl-1,4-phenylene oxide). Langmuir 17, 5817–5820 (2001)
Kim, J., Kuk, E., et al.: Antimicrobial effects of silver nanoparticles. Nanomedicine 3, 95 (2007)
Li, Y., Lu, W., Huang, Q., Huang, M., Li, C., Chen, W.: Copper Sulfide Nanoparticles for photothermal ablation of tumor cells. Nanomedicine 5, 1161–1171 (2010)
Li, Y., Hindi, K., Watts, K., Taylor, J., Zhan, Z., Li, Z., Hunstad, D., Cannon, C., Young, W., Wooley, K.: Shell crosslinked nanoparticles carrying silver antimicrobials as therapeutics. Chem. Commun. (Camb.) 46, 121–123 (2010)
Li, W., Xie, X., Shi, Q., Zeng, H., Ou-Yang, Y., Chen, Y.: Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 85, 1115–1122 (2010)
Liu, W., Wu, Y., Wang, C., et al.: Impact of silver nanoparticles on human cells: Effect of particle size. Nanotoxicology 4, 319–330 (2010)
Mafuné, F., Kohno, J., Takeda, Y., Kondow, T., Sawabe, T.: J. Phys. Chem. B 104(39), 9111–9117 (2000)
Mannaa, D., Mandal, A., Sen, I., et al.: Antibacterial and DNA degradation potential of silver nanoparticles synthesized via green route. Int J Biol Macromol. 80, 455–459 (2015)
Matthews, K., Roberson, J., Gillespie, B., Luther, D., Oliver, S.: Identification and differentiation of coagulase-negative Staphylococcus aureus by polymerase chain reaction. J. Food Prot. 60, 686–688 (1997)
Menazea, A., Mostafa, A.: Ag doped CuO thin film prepared via pulsed laser deposition for 4-nitrophenol degradation. J. Environ. Chem. Eng. 8, 5 (2020)
Mizajani, F., Ghassempour, A., Aliahmadi, A., Esmaeli, M.: Antibacterial activity of silver nanoparticles on Staphyloccus aureus. Res Microbiol 162, 542–549 (2011)
Mlalila, N., Shaidi, H., Hilonga, A., Kadam, D.: Antimicrobial dependence of silver nanoparticles on surface plasmon resonance bands against Escherichia coli. Nanotechnol. Sci. Appl. 10, 1–9 (2017)
Mostafa, A., Mwafy, E.: Effect of dual-beam laser radiation for synthetic SnO2/Au nanoalloy for antibacterial activity. J. Mol. Struct. 1222, 128913 (2020)
Mostafa, A., Mwafy, E.: The effect of laser fluence for enhancing the antibacterial activity of NiO nanoparticles by pulsed laser ablation in liquid media. Environ. Nanotechnol. Monitor. Manag. 14, 100382 (2020)
Mostafa, A., Mwafy, E., Hasanin, M.: One-pot synthesis of nanostructured CdS, CuS, and SnS by pulsed laser ablation in liquid environment and their antimicrobial activity. Opt. Laser Technol. 121, 105824 (2020)
Mostafa, A., Mwafy, E., Awwad, N., Ibrahium, H.: Synthesis of multi-walled carbon nanotubes decorated with silver metallic nanoparticles as a catalytic degradable material via pulsed laser ablation in liquid media. Colloids Surf., A 626, 126992 (2021)
Mwafy, E., Hasanin, M., Mostafa, A.: Cadmium Oxide/ TEMPO-Oxidized Cellulose Nanocomposites produced by pulsed Laser Ablation in Liquid Environment: Synthesis, Characterization, and Antimicrobial Activity. Opt. Laser Technol. 120, 105744 (2019)
Pal, S., Tak, Y., Song, J.: Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 73, 1712–1720 (2007)
Paramelle, D., Sadovoy, A., Gorelik, S., Free, P., Hobley, J., Fernig, D.: A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst, R. Soc. Chem. 139, 0003–2654 (2014)
Rudramurthy, G., Swamy, M., Sinniah, U., Ghasemzadeh, A.: Nanoparticles: alternatives against drug-resistant pathogenic microbes. Molecules 21, 836 (2016)
Sadeghi, B., Garmaroudi, F., Hashemi, M., Nezhad, H., Nasrollahi, A., Ardalan, S., Ardalan, S.: Comparison of the anti-bacterial activity on the nanosilver shapes: Nanoparticles, nanorods and nanoplates. Adv. Powder Technol 23, 22–26 (2012)
Salleh, A., Naomi, R., Utami, N.D., Mohammad, A.W., Mahmoudi, E., Mustafa, N., Fauzi, M.B.: The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomaterials (Basel, Switzerland) 10, 1566 (2020)
Sharma, V.K., Yngard, R.A., Lin, Y.: Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci. 145, 83–96 (2009)
Shuai, C., Liu, G., Yang, Y., Qi, F., Peng, S., Yang, W., He, C., Wang, G., Qian, G.: A strawberry-like Ag-decorated barium titanate enhances piezoelectric and antibacterial activities of polymer scaffold. Nano Energy 74, 104825 (2020)
Smejkal, P., Pfleger, J., Vlckova, B., Dammer, O.: Laser ablation of silver in aqueous ambient: effect of laser pulse wavelength and energy on efficiency of the process. J. Phys: Conf. Ser. 59, 185–188 (2007)
Soo-Hwan, K., Lee, H., Ryu, D., Choi, S., Lee, D.: Antibacterial activity of silver nanoparticles against Staphyloccus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 39, 77–85 (2011)
Torres, L., Gmez-Quintero, T., Padron, G., Santana, F., Hernandez, J., Castano, V.: Silver nanoprisms and nanospheres for prosthetic biomaterials. IADR/AADR/CADR General Session and Exhibition, San Francisco (2013)
Velusamy, P., Kumar, G.V., Jeyanthi, V., Das, J., Pachaiappan, R.: Bio-inspired green nanoparticles:synthesis, mechanism, and antibacterial application. Toxicol. Res. 32, 95–102 (2016)
Vogt, R., Dippold, L.: Escherichia coli O157:H7 outbreak associated with consumption of ground beef. Public Health Rep. 120, 174 (2005)
Wei, Q., Fu, J., Shen, J.: Norvancomycin-capped silver nanoparticles: synthesis and antibacterial activities against E. coli. Sci. China, Ser. B: Chem. 50, 418–424 (2007)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed Consent
Not applicable.
Conflicts of Interest / Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Ogaidi, M.A.Z., Rasheed, B.G. Enhancement of Antimicrobial Activity of Silver Nanoparticles Using Lasers. Lasers Manuf. Mater. Process. 9, 610–621 (2022). https://doi.org/10.1007/s40516-022-00192-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40516-022-00192-4