Abstract
HIV-2, despite being less common than HIV-1, is an important cause of morbidity and mortality. As more data are published on HIV-2 infection, it is clear that despite lower viral loads and higher CD4 cell counts than those seen in HIV-1, it is important to treat these patients with antiretroviral therapy to prevent progression of disease and early mortality.
Purpose of review
To summarize the background characteristics of HIV-2, diagnostic and treatment considerations, and recent updates in treatment of HIV-2.
Recent findings
Prospective cohort studies of people with HIV-2 infection have shown that they have a significant mortality increase over HIV negative individuals and progress to AIDS and death, though at a slower rate than HIV-1. Given this progressive nature of HIV-2 infection, antiretroviral therapy is warranted, and a case can be made, as with HIV-1, that all infected people should be treated. HIV-2 RNA testing is now available in the USA and should be performed to monitor the effectiveness of treatment. Recent clinical trials have shown the efficacy of integrase inhibitors in combination with nucleoside reverse transcriptase inhibitors for HIV-2 treatment.
Summary
The natural history of HIV-2 infection includes progression to AIDS and death. Individuals with HIV-2 infection have higher mortality than the HIV negative population. Integrase inhibitors have been shown in small clinical trials to be safe and effective, and randomized clinical trials are ongoing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction/epidemiology
In the early 2000s, it was estimated that 1 to 2 million people worldwide were living with HIV-2 infection, including some who were coinfected with HIV-1 [1, 2]. Updated projections have not been made since then, and the current worldwide burden of HIV-2 infection is unknown [1, 2]. HIV-2 originated in, and is endemic to, West Africa, with the first cases documented in the mid-1980s in Senegal and Cape Verde [1, 2]. The virus is thought to have originated from a simian virus found in sooty mangabeys that live in the forests of the coasts of West Africa [1,2,3]. There is a high prevalence of this related virus in these monkeys, estimated to be over 50% [2, 4], and as opposed to humans, they have normal CD4 cell counts and lack of progression to immunodeficient states even with high viral replication [2, 4]. The two endemic groups of HIV-2 virus, groups A and B, have been tracked back to two discrete cross-species transmissions from sooty mangabeys in the Ivory Coast [2]. It is unknown exactly when the virus spread to humans, but it was thought to have occurred in the early 1900s, with one estimate predicting group A virus entered humans around 1938 [2]. Group B virus is also estimated to have entered humans in the 1930s [5]. This is later than some HIV-1 groups (such as M and O), which are thought to have entered humans in 1900s and 1920s, respectively [5, 6].
Interestingly, while group B HIV-2 is mostly concentrated in the Ivory Coast, Burkina Faso, and Ghana, group A virus is widespread throughout West Africa [2]. Infections in Guinea-Bissau are predominantly due to group A HIV-2 virus. The overall prevalence of HIV-2 in Guinea-Bissau was estimated to be around 4.7% based on one study in 1987 looking at 100 households which included over 1300 individuals and none were seropositive for HIV-1 infection [7]. The prevalence in adults (15 years and older) was 8.9% and increased to 20% in adults over the age of 40 years [7]. Other countries in West Africa have estimated prevalence > 1% [2]. The high rates in Guinea-Bissau are thought to be due to transmission from commercial sex work and iatrogenic spread (such as from reused vaccination needles and transfusions) during an internal conflict/war of independence from 1963 to 1974 [2, 8]. However, the prevalence of HIV-2 is declining in the area, particularly among younger individuals [8, 9]. In 2015, Fryer et al modeled the rate of HIV-2 infection in a rural area of Guinea-Bissau and predicted there would be no new cases by around 2048 and that the virus would go extinct by around 2068 [8].
In the USA, the first case of HIV-2 was reported in 1987. From 1988 to 2010, the CDC collected 166 cases [10], which represented 0.01% of overall HIV cases in the USA. Of the cases, 46% were reported from New York City. Of the people for whom birthplace was known, 81% were originally from West Africa. Upon closer review of these 166 cases, 72% had no definite risk factor for transmission; 23% had heterosexual contact with a partner known to have HIV; 2% were men who have sex with men (MSM), and 2% were in persons who inject drugs [10]. Although 48% of the women had a pregnancy at or after HIV-2 diagnosis, there were no known cases of HIV-2 transmission to their children (it is unclear whether these women were on antiretroviral therapy [ART]) [10].
HIV-2 disease/virus characteristics
HIV-2 is less sexually transmissible than HIV-1, likely due in part to lower viral loads [11,12,13]. In a prospective cohort study of female commercial sex workers in Senegal, Kanki et al showed that the annual HIV-1 incidence increased from 1985 to 1993, while the annual HIV-2 incidence did not [11]. While the relative risk of acquiring HIV-1 increased each successive year during this study period as compared to the previous year, the relative risk of HIV-2 acquisition in the same population over the same time period did not change. This finding was felt to be due to a slower, less efficient spread of HIV-2 through heterosexual contact compared to HIV-1 [11]. Gottlieb et al studied HIV RNA levels in semen of HIV-1 and HIV-2-positive men [12]. The mean RNA level in semen was significantly higher in men with HIV-1 as compared to men with HIV-2, and HIV-2 was associated with a lower RNA level in the semen when adjusted for CD4 cell count [12]. Both HIV-1 and HIV-2 seminal viral loads tracked with plasma viral loads [12]. HIV-2 infected persons also have decreased levels of viremia, as shown by Simon et al: only 10% of their cohort had plasma viremia as detected by culture [13]. Popper et al looked at HIV-2 RNA levels in a cohort of commercial sex workers in Senegal and found detectable viremia (> 100 copies) in 56% of samples [14]. Andersson et al also found lower HIV-2 RNA set points shortly after seroconversion when compared to HIV-1 positive individuals [15].
HIV-2 also has relatively low rates of vertical transmission, ranging from 1 to 4% [16,17,18]. Burgard et al evaluated the ANRS French Perinatal Cohort and found vertical transmission rates of 1.3% from women with HIV-2 versus 17.5% from women with HIV-1. Among mothers not on ART, the transmission rate was 0.7% for those with HIV-2 and 16.3% for those with HIV-1. They also found that, as compared to mothers with HIV-1, those with HIV-2 were more likely to be diagnosed later, more likely to be asymptomatic and have lower viral loads off therapy, and less likely to be on ART. When ART was started, it was usually started later. When they looked at mothers with CD4 cell counts above 350/mm3, they also noted a similar trend of lower mother-to-child transmission rates in HIV-2 [16]. In a prospective study in the Ivory Coast by Adjorlolo-Johnson et al, the rate of mother-to-child transmission was 1.2% in mothers with HIV-2 compared to 24.7% in mothers with HIV-1. In mothers with dual infection, HIV-1 was transmitted to the baby in all eleven cases whereas only one out of these eleven also acquired HIV-2 [17]. In a blinded prospective cohort in Gambia, the rate of mother-to-child transmission in HIV-2 was as high as 4%, but again significantly less than the 21% vertical transmission rate observed in mothers with HIV-1 [18]. O’Donovan et al noted that the HIV-1 infected mothers with vertical transmission had higher viral loads than their HIV-2 counterparts, and both groups had significantly higher viral loads than those who did not transmit [18]. These studies demonstrate that patients with HIV-2 infection have lower levels of viremia compared with patients with HIV-1 infection, and this likely explains the low rates of mother-to-child transmission observed for HIV-2.
HIV-2 is also considered less pathogenic than HIV-1, with persistence of higher CD4 counts and a lower risk of mortality and progression to AIDS [19,20,21]. Peterson et al looked at people with HIV-1 or HIV-2 on ART in Gambia and found a higher crude mortality rate in those with HIV-1 over those with HIV-2 (120.9/1000 person years of observations vs. 64.2 per 1000 person years of observation); of note, this analysis included everyone on ART, not just those on effective ART with undetectable viral loads. They found survival was greater among those with HIV-2, but this difference was not statistically significant [19]. In the French ANRS Cohort, in which about half the people with HIV-2 were on ART, the survival rate for all-comers was 97% 1 year after enrollment, and 93.4% at 3 years. Median viral load of the patients off ART was 3 log10 copies/mL (or around 1000 copies/mL). This study identified two variables associated with risk of progression to severe disease: age over 40 years and higher HIV-2 RNA level [20]. In a prospective matched cohort study of sex workers in Senegal from 1985 to 1993 (where they were able to estimate the time of incident infection based on seroconversion), women who had HIV-2 seroconversion were AIDS-free at 5 years (100% AIDS free time) as opposed to women who had acquired HIV-1 whose AIDS free time was 66.8% at 5 years. Women with HIV-1 were significantly more likely to develop severe disease and have declining CD4 cell counts to < 400/mm3 compared to women with HIV-2 [21]. Despite the lower pathogenicity of HIV-2, recently published data shows that people with HIV-2 have clinical progression to AIDS and death (see below).
Diagnostic considerations for HIV-2
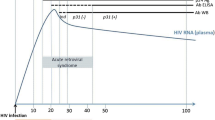
Individuals with HIV-2 may test negative on an HIV-1 only ELISA or Western blot, and HIV-1 RNA tests will usually be negative even for patients with HIV-2 viremia. The Centers for Disease Control HIV testing algorithm, updated January 2018, suggests first using the fourth-generation antibody-antigen immunoassay. If positive, then an antibody differentiation assay (which detects and distinguishes HIV-1 and HIV-2 antibodies) should be performed [22]. HIV-2 RNA testing is now available in the USA from the University of Washington and the New York State Department of Health [23]. There are important caveats to interpreting the above testing when looking for HIV-2: patients can have undetectable levels of viremia but still have progressive disease as shown in some of the studies below [24••].
Treatment considerations for HIV-2 infection
Unfortunately, there have been no completed randomized clinical trials on the timing of ART initiation or choice of first line therapy for HIV-2 infection, and most recommendations have been based on case series and cohort studies. HIV-2 is intrinsically resistant to non-nucleoside reverse transcriptase inhibitors (NNRTIs) based on the structure of its reverse transcriptase enzyme and poor binding at the active site [25,26,27] and this antiretroviral class should not be used for treatment. HIV-2 also is resistant to enfuvirtide likely due to amino acid changes in one of the helical domains of the gp41 protein in this virus compared with HIV-1 [27, 28]. Witvrouw et al looked at in vitro activity of HIV-2 and corroborated decreased activity of NNRTIs, some HIV protease inhibitors (PIs), such as amprenavir, and enfuvirtide. The nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) are usually active against HIV-2 as they are against HIV-1 [27]. However, the resistance pattern to NRTIs is different in HIV-2 than in HIV-1, leading to unique treatment considerations [29, 30]. Smith et al found that the Q151M mutation conferred resistance to zidovudine (AZT) and that HIV-2 may have a lower barrier for resistance to AZT than HIV-1 [29]. Descamps et al evaluated the French ANRS cohort and found that among patients who had received NRTIs previously with a median time of exposure of 51 months, 76% developed resistance mutations and 26% developed Q151M, often in association with K65R, making the virus resistant to many NRTI agents. They also found that although these patients had long treatment histories with thymidine analogues, they usually did not have thymidine analogue mutations, suggesting that the mechanism of acquiring NRTI resistance is different in HIV-2 compared with HIV-1 [30].
Some studies have evaluated outcomes in those on 3-NRTIs vs. 2-NRTIs plus a PI. Balestre et al evaluated the IeDEA cohort in West Africa after initiation of ART; 67.5% of the cohort was treated with a pharmacologically boosted PI-based regimen, 24.6% with an unboosted PI regimen, and 7.8% with 3-NRTIs. The people treated with boosted PI had significantly better CD4 cell count recovery at 12 months than individuals who received the other two regimens [31]. In the ACHIEV2E cohort, patients treated with boosted PI regimens (61% of whom were treated with lopinavir/ritonavir) had significantly lower viral loads and higher CD4 cell counts at 12 months than the 3-NRTIs group [32]. The boosted PI group also had significantly higher rates of meeting the endpoint of treatment success (CD4 cell recovery, viral load drop, and lack of clinical progression): 55% vs. 10% in 3-NRTIs group [32]. This finding has led to the recommendation to use boosted PI regimens and avoid 3-NRTI regimens. Certain PIs have been shown to have more activity against HIV-2 than others [33]. Desbois et al found HIV-2 to have in vitro phenotypic susceptibility to saquinavir, darunavir, and lopinavir similar to that of HIV-1. However, atazanavir, indinavir, nelfinavir, and tipranavir were found to have lower activity, and HIV-2 was resistant to amprenavir [33]. CCR5 antagonists may be a treatment option but HIV-2 also enters cells through other co-receptors.
Data on using integrase inhibitors to treat HIV-2 were initially reported in case series [34]. In five people with HIV-2 and detectable viral loads who started a raltegravir-containing regimen, the viral load dropped to either undetectable or nearly undetectable (~ 50 copies/mL); CD4 cell counts increased by a median of 238/mm3 over several years. Of note, four patients had received ART previously so were treated with a regimen of 2-NRTIs, a boosted PI, and raltegravir, while the treatment-naïve patient received 2-NRTIs and raltegravir [34]. Additional studies supporting the efficacy of integrase inhibitor-based therapy for people with HIV-2 have recently been published (below).
The United States Department of Health and Human Services guidelines on HIV-2 have recently been updated based on the emerging data discussed below. Where they previously suggested starting ART before clinical progression, they now recommend that ART should be started at the time of diagnosis or soon thereafter. They recommend using a regimen of 2-NRTIs plus an integrase inhibitor as initial therapy based on the trials discussed below. An HIV-2-active boosted protease inhibitor (darunavir or lopinavir) plus 2-NRTIs is an alternative regimen. They also recommend continuing periodic CD4 cell count monitoring even with undetectable viral loads as HIV-2 disease can still progress despite undetectable viremia. Importantly, this is a different approach than in HIV-1, where CD4 count monitoring is stopped if there is CD4 cell count recovery and the viral load remains suppressed [23]. The British HIV Association guidelines from 2010 recommend treatment initiation at a HIV-2 RNA > 1000 copies/mL since this is reported to be predictive of progression. They recommend using 2-NRTIs +1 boosted PI [35], but these guidelines have not been updated since 2010 and do not reflect the newer evidence showing efficacy and safety of integrase inhibitor-based therapy as discussed below and incorporated into the US guidelines.
New and emerging research in HIV-2
A prospective cohort study published early in 2019 by Esbjörnsson et al provided important information about the clinical outcomes and progression of HIV-2 infection and highlighted the importance of starting ART for all patients with HIV-2, regardless of CD4 count [36••]. The cohort they studied, from 1990 to 2009, was unique because they documented a large number of HIV-2 seroconversions during the study period, so they could more accurately estimate time of acquisition than in prior cohorts. Median survival for those who seroconverted was significantly higher for HIV-2 patients compared to HIV-1 infected individuals by nearly twofold (15.6 years in HIV-2 patients vs. 8.2 years in HIV-1 patients). Median time to development of AIDS for those who seroconverted was also significantly longer in people who acquired HIV-2 than in those who acquired HIV-1: 14.3 years vs. 6.2 years. When they looked at the subset of 560 HIV-1 and HIV-2 patients who were already positive at time of enrollment, the survival again was significantly higher in those with HIV-2 (15.7 years in the HIV-2 sub-cohort vs. 6.6 years in the HIV-1 sub-cohort), and time to AIDS was demonstrated to be longer in those with HIV-2 (11.2 years in those with HIV-2 vs. 5.2 years in people with HIV-1) [36••].
They also compared mortality age among HIV-1, HIV-2, and HIV negative patients and found a significant 10-year difference in mortality age between each group (51 years in HIV-1, 62.9 years in HIV-2, 73.7 years in the HIV negative cohort). It had been previously thought that HIV-2 untreated patients had similar mortality as HIV negative patients, but the findings from this cohort refute this long-held view. The mean rate of decline in CD4 cell percentage in people with HIV-2 was about half that of people with HIV-1. The authors also looked at rate of progression to AIDS in HIV-2 patients, using a clinical definition of AIDS based on WHO criteria, independent of CD4 cell counts, and found that HIV-2 patients developed AIDS at higher CD4 cell counts than their HIV-1 counterparts (median CD4 8.2%/136.8 cells/mm3 in HIV-1 patients vs. 18.2%/236.7 cells/mm3 in HIV-2 patients). They also modeled the disease course of HIV-1 and HIV-2: HIV-2 had a similar disease course as HIV-1 when participants were not on ART, although HIV-2 did progress at a slower rate and had a lower overall mortality. This study challenged long-standing views that HIV-2 is benign and suggested ART should be started in people with HIV-2 regardless of CD4 cell counts to prevent progression of disease and premature mortality [36••].
In correspondence between a French group and the authors of the above study, the question of long-term non-progressors in this cohort was considered [37, 38]. When using a similar definition as the French ANRS cohort, they identified 7% of individuals with HIV-2 in their cohort as long-term non-progressors. With a more stringent denominator, their estimates were closer to 11–12%. They also noted that a significant number of these non-progressors eventually went on to have drops in their CD4 cell counts [38], again demonstrating that HIV-2 may be more pathogenic than once thought.
In terms of diagnostic tests for HIV-2, Chang et al examined the Bio-Rad Geenius HIV 1/2 supplemental assay in known HIV-2-positive samples from the USA/Canada [39]. They found that only 41.5% of the samples tested HIV-2 positive by the assay, and 48% tested HIV-2 positive with HIV-1 cross-reactivity [39]. This finding highlights the need for improved diagnostics for HIV-2.
Two clinical trials published in the latter half of 2018 demonstrated the efficacy of integrase inhibitors for treatment of HIV-2 [24••, 40••]. In a non-comparative phase 2 multicenter trial conducted in France between 2012 and 2015, treatment-naïve adults with HIV-2 infection received raltegravir plus tenofovir disoproxil fumarate (TDF) and emtricitabine; these participants also needed to meet certain criteria of immunologic compromise (such as CD4 cell count < 500/mm3 or CD4 decrease of > 50 cells/mm3 per year over 3 years) or HIV-2 RNA of greater than or equal to 100 copies/mL. They used a composite primary endpoint (“therapeutic success”) consisting of surviving at week 48 without any of the following events: (1) CD4 gain of < 100 cells/mm3 from baseline, (2) HIV-2 RNA greater than or equal to 40 copies/mL starting from week 24, (3) discontinuing raltegravir altogether, and (4) a new CDC HIV class B or C event. They analyzed 30 participants, and their composite endpoint for therapeutic success was met in 40% of these patients. The main cause for failure was not achieving a CD4 cell count gain of > 100 cells/mm3. At week 48, the median CD4 cell gain was 87 cells/mm3 and 96% of participants had a VL < 40 copies/mL. They observed a trend for improved success in patients with higher CD4 cell count > 500/mm3 (50% met endpoint) versus patients with CD4 cell count < 500/mm3 (36% met endpoint). This was a pilot trial intended to launch further randomized trials, and a larger, follow-up randomized trial is underway currently. The authors note the difficulty in conducting randomized trials in HIV-2 given the small number of infected patients and that HIV-2 RNA is difficult to use as both an inclusion criterion or outcome as the viral loads tend to be lower than in HIV-1 and patients can have progression of disease even at lower viral loads [40••].
The first clinical trial (not randomized) assessing the fixed-dose single-tablet regimen of elvitegravir/cobicistat/emtricitabine/TDF for treatment of HIV-2 was published in late 2018 [24••]. This trial recruited ART-naïve adults and evaluated HIV-2 RNA and CD4 cell count at 48 weeks. They found a median CD4 cell count increase of 161/mm3; there was a median increase of over 100 CD4 cells/mm3 in all CD4 cell strata. Participants had similar increases in CD4 cell counts regardless of whether they had detectable or undetectable HIV-2 RNA levels prior to starting ART, suggesting an immunologic benefit even in those who do not have detectable viremia [24••]. Smith et al evaluated the in vitro activity of bictegravir [41], demonstrating activity against both HIV-1 and HIV-2. Bictegravir was also found to be as potent in vitro as dolutegravir and cabotegravir for HIV-2 [41]. These data, although only in vitro, are promising that bictegravir may be effective in people with HIV-2; clinical trials, however, are needed to be certain that bictegravir-based ART is an option for people with HIV-2.
A case report published in 2019 by Ceia et al described HIV-2 acquisition in a person with HIV-1 who had been on ART [42] which may have implications for pre-exposure prophylaxis. The person was diagnosed with HIV-1 in 2002 (also tested negative for HIV-2 at the time) and started on zidovudine, lamivudine, and efavirenz. Over the ensuing 9 years, he was on a variety of different antiretroviral regimens; his CD4 cell count increased from 123/mm3 to over 1000/mm3 and he had continued viral suppression. Starting in 2013, his CD4 cell count started to drop, eventually declining to 89/mm3 despite having an undetectable HIV-1 RNA. He was re-tested in 2016 and was found to be both HIV-1 and HIV-2 positive by antibody testing with a detectable HIV-2 RNA. The hypothesis was that he acquired HIV-2 through an unprotected sexual encounter in Brazil and then developed HIV-2 viremia and CD4 cell decline while on an NNRTI regimen. He was eventually controlled on a regimen of two NRTIs, one boosted protease inhibitor, and one integrase inhibitor. This case report also raises questions about pre-exposure prophylaxis for HIV-2, as the patient was on a regimen of TDF/emtricitabine at the time of HIV-2 acquisition [42] but this remains an important area yet to be studied.
Conclusion
While HIV-2 has been shown to be less virulent and less efficiently transmitted than HIV-1, it retains the ability to clinically progress to AIDS leading to increased morbidity and premature death. A recent cohort study has shown that people with HIV-2 have higher mortality as compared to the general HIV negative population and can progress to immunocompromised states despite higher CD4 counts. This finding has implications for initiation of therapy in people with HIV-2: ART should be started before clinical progression and even with intact CD4 counts or undetectable viremia, as these patients have been shown to progress when off antiretroviral treatment. Diagnosing HIV-2 still remains a challenge especially in resource-limited settings. There are important treatment differences between HIV-1 and HIV-2 as NNRTIs are not effective against HIV-2, and some mechanisms of resistance appear to be different between the two viruses. Recent clinical trials have shown that integrase inhibitor-based regimens are effective in decreasing viral load and increasing CD4 cell counts as well as improving clinical outcomes. Randomized clinical trials are currently underway looking at integrase inhibitors and will hopefully inform future treatment guidelines. Knowledge gaps still remain regarding optimal treatment strategies and pre-exposure prophylaxis for prevention of HIV-2 acquisition. More research, particularly randomized clinical trials, is needed to determine the optimal time of treatment initiation and what is the most effective first-line therapy.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Gottlieb GS, Raugi DN, Smith RA. 90-90-90 for HIV-2? Ending the HIV-2 epidemic by enhancing care and clinical management of patients infected with HIV-2. Lancet HIV. 2018;5(7):e390–e9. https://doi.org/10.1016/S2352-3018(18)30094-8.
Visseaux B, Damond F, Matheron S, Descamps D, Charpentier C. Hiv-2 molecular epidemiology. Infect Genet Evol. 2016;46:233–40. https://doi.org/10.1016/j.meegid.2016.08.010.
McCutchan FE. Global epidemiology of HIV. J Med Virol. 2006;78(Suppl 1):S7–S12. https://doi.org/10.1002/jmv.20599.
Santiago ML, Range F, Keele BF, Li Y, Bailes E, Bibollet-Ruche F, et al. Simian immunodeficiency virus infection in free-ranging sooty mangabeys (Cercocebus atys atys) from the Tai Forest, Cote d’Ivoire: implications for the origin of epidemic human immunodeficiency virus type 2. J Virol. 2005;79(19):12515–27. https://doi.org/10.1128/JVI.79.19.12515-12527.2005.
Wertheim JO, Worobey M. Dating the age of the SIV lineages that gave rise to HIV-1 and HIV-2. PLoS Comput Biol. 2009;5(5):e1000377. https://doi.org/10.1371/journal.pcbi.1000377.
Worobey M, Gemmel M, Teuwen DE, Haselkorn T, Kunstman K, Bunce M, et al. Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960. Nature. 2008;455(7213):661–4. https://doi.org/10.1038/nature07390.
Poulsen AG, Kvinesdal B, Aaby P, Molbak K, Frederiksen K, Dias F, et al. Prevalence of and mortality from human immunodeficiency virus type 2 in Bissau, West Africa. Lancet. 1989;1(8642):827–31.
Fryer HR, Van Tienen C, Van Der Loeff MS, Aaby P, Da Silva ZJ, Whittle H, et al. Predicting the extinction of HIV-2 in rural Guinea-Bissau. AIDS. 2015;29(18):2479–86. https://doi.org/10.1097/QAD.0000000000000844.
De Cock KM, Adjorlolo G, Ekpini E, Sibailly T, Kouadio J, Maran M, et al. Epidemiology and transmission of HIV-2. Why there is no HIV-2 pandemic. JAMA. 1993;270(17):2083–6.
Centers for Disease Control and Prevention. HIV-2 Infection Surveillance--United States, 1987-2009. MMWR Morb Mortal Wkly Rep. 2011;60(29):985–8.
Kanki PJ, Travers KU, S MB, Hsieh CC, Marlink RG, Gueye NA, et al. Slower heterosexual spread of HIV-2 than HIV-1. Lancet. 1994;343(8903):943–6.
Gottlieb GS, Hawes SE, Agne HD, Stern JE, Critchlow CW, Kiviat NB, et al. Lower levels of HIV RNA in semen in HIV-2 compared with HIV-1 infection: implications for differences in transmission. AIDS. 2006;20(6):895–900. https://doi.org/10.1097/01.aids.0000218554.59531.80.
Simon F, Matheron S, Tamalet C, Loussert-Ajaka I, Bartczak S, Pepin JM, et al. Cellular and plasma viral load in patients infected with HIV-2. AIDS. 1993;7(11):1411–7.
Popper SJ, Sarr AD, Travers KU, Gueye-Ndiaye A, Mboup S, Essex ME, et al. Lower human immunodeficiency virus (HIV) type 2 viral load reflects the difference in pathogenicity of HIV-1 and HIV-2. J Infect Dis. 1999;180(4):1116–21. https://doi.org/10.1086/315010.
Andersson S, Norrgren H, da Silva Z, Biague A, Bamba S, Kwok S, et al. Plasma viral load in HIV-1 and HIV-2 singly and dually infected individuals in Guinea-Bissau, West Africa: significantly lower plasma virus set point in HIV-2 infection than in HIV-1 infection. Arch Intern Med. 2000;160(21):3286–93.
Burgard M, Jasseron C, Matheron S, Damond F, Hamrene K, Blanche S, et al. Mother-to-child transmission of HIV-2 infection from 1986 to 2007 in the ANRS French Perinatal Cohort EPF-CO1. Clin Infect Dis. 2010;51(7):833–43. https://doi.org/10.1086/656284.
Adjorlolo-Johnson G, De Cock KM, Ekpini E, Vetter KM, Sibailly T, Brattegaard K, et al. Prospective comparison of mother-to-child transmission of HIV-1 and HIV-2 in Abidjan, Ivory Coast. JAMA. 1994;272(6):462–6.
O’Donovan D, Ariyoshi K, Milligan P, Ota M, Yamuah L, Sarge-Njie R, et al. Maternal plasma viral RNA levels determine marked differences in mother-to-child transmission rates of HIV-1 and HIV-2 in The Gambia. MRC/Gambia Government/University College London Medical School working group on mother-child transmission of. HIV AIDS. 2000;14(4):441–8.
Peterson I, Togun O, de Silva T, Oko F, Rowland-Jones S, Jaye A, et al. Mortality and immunovirological outcomes on antiretroviral therapy in HIV-1 and HIV-2-infected individuals in the Gambia. AIDS. 2011;25(17):2167–75. https://doi.org/10.1097/QAD.0b013e32834c4adb.
Matheron S, Pueyo S, Damond F, Simon F, Lepretre A, Campa P, et al. Factors associated with clinical progression in HIV-2 infected-patients: the French ANRS cohort. AIDS. 2003;17(18):2593–601. https://doi.org/10.1097/01.aids.0000096907.73209.b9.
Marlink R, Kanki P, Thior I, Travers K, Eisen G, Siby T, et al. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science. 1994;265(5178):1587–90.
Centers for Disease Control and Prevention (U.S.); National Center for HIV/AIDS VH, and TB Prevention (U.S.). Division of HIV/AIDS Prevention; Association of Public Health Laboratories. 2018 Quick reference guide: Recommended laboratory HIV testing algorithm for serum or plasma specimens. 2018. https://stacks.cdc.gov/view/cdc/50872. Accessed March 3, 2019 2019.
Department of Health and Human Services (U.S.). Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. 2019. https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/24/hiv-2-infection. Accessed July 12 2019.
•• Ba S, Raugi DN, Smith RA, Sall F, Faye K, Hawes SE, et al. A trial of a single-tablet regimen of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate for the initial treatment of human immunodeficiency virus type 2 infection in a resource-limited setting: 48-week results from Senegal, West Africa. Clin Infect Dis. 2018;67(10):1588–94. https://doi.org/10.1093/cid/ciy324. This clinical trial demonstrated clinical efficacy of integrase inhibitors in HIV-2 infection and suggested immunologic benefit even in the absence of detectable viremia.
Tuaillon E, Gueudin M, Lemee V, Gueit I, Roques P, Corrigan GE, et al. Phenotypic susceptibility to nonnucleoside inhibitors of virion-associated reverse transcriptase from different HIV types and groups. J Acquir Immune Defic Syndr. 2004;37(5):1543–9.
Rodes B, Holguin A, Soriano V, Dourana M, Mansinho K, Antunes F, et al. Emergence of drug resistance mutations in human immunodeficiency virus type 2-infected subjects undergoing antiretroviral therapy. J Clin Microbiol. 2000;38(4):1370–4.
Witvrouw M, Pannecouque C, Switzer WM, Folks TM, De Clercq E, Heneine W. Susceptibility of HIV-2, SIV and SHIV to various anti-HIV-1 compounds: implications for treatment and postexposure prophylaxis. Antivir Ther. 2004;9(1):57–65.
Poveda E, Rodes B, Toro C, Soriano V. Are fusion inhibitors active against all HIV variants? AIDS Res Hum Retroviruses. 2004;20(3):347–8. https://doi.org/10.1089/088922204322996590.
Smith RA, Anderson DJ, Pyrak CL, Preston BD, Gottlieb GS. Antiretroviral drug resistance in HIV-2: three amino acid changes are sufficient for classwide nucleoside analogue resistance. J Infect Dis. 2009;199(9):1323–6. https://doi.org/10.1086/597802.
Descamps D, Damond F, Matheron S, Collin G, Campa P, Delarue S, et al. High frequency of selection of K65R and Q151M mutations in HIV-2 infected patients receiving nucleoside reverse transcriptase inhibitors containing regimen. J Med Virol. 2004;74(2):197–201. https://doi.org/10.1002/jmv.20174.
Balestre E, Ekouevi DK, Tchounga B, Eholie SP, Messou E, Sawadogo A, et al. Immunologic response in treatment-naive HIV-2-infected patients: the IeDEA West Africa cohort. J Int AIDS Soc. 2016;19(1):20044. https://doi.org/10.7448/IAS.19.1.20044.
Benard A, van Sighem A, Taieb A, Valadas E, Ruelle J, Soriano V, et al. Immunovirological response to triple nucleotide reverse-transcriptase inhibitors and ritonavir-boosted protease inhibitors in treatment-naive HIV-2-infected patients: The ACHIEV2E Collaboration Study Group. Clin Infect Dis. 2011;52(10):1257–66. https://doi.org/10.1093/cid/cir123.
Desbois D, Roquebert B, Peytavin G, Damond F, Collin G, Benard A, et al. In vitro phenotypic susceptibility of human immunodeficiency virus type 2 clinical isolates to protease inhibitors. Antimicrob Agents Chemother. 2008;52(4):1545–8. https://doi.org/10.1128/AAC.01284-07.
Peterson K, Ruelle J, Vekemans M, Siegal FP, Deayton JR, Colebunders R. The role of raltegravir in the treatment of HIV-2 infections: evidence from a case series. Antivir Ther. 2012;17(6):1097–100. https://doi.org/10.3851/IMP2303.
Gilleece Y, Chadwick DR, Breuer J, Hawkins D, Smit E, McCrae LX, et al. British HIV Association guidelines for antiretroviral treatment of HIV-2-positive individuals 2010. HIV Med. 2010;11(10):611–9. https://doi.org/10.1111/j.1468-1293.2010.00889.x.
Esbjornsson J, Mansson F, Kvist A, da Silva ZJ, Andersson S, Fenyo EM, et al. Long-term follow-up of HIV-2-related AIDS and mortality in Guinea-Bissau: a prospective open cohort study. Lancet HIV. 2018;6:e25–31. https://doi.org/10.1016/S2352-3018(18)30254-6. This prospective cohort study included a cohort where time of acquisition was more accurately estimated. It provides information about longitudinal clinical outcomes of HIV-2 infection.
Hingrat QL, Charpentier C, Ghosn J, Thiebaut R, Peytavin G, Descamps D, et al. New insights are game-changers in HIV-2 disease management. Lancet HIV. 2019;6(4):e214. https://doi.org/10.1016/S2352-3018(19)30088-8.
Esbjornsson J, Mansson F, Lindman J, Rowland-Jones SL, Jansson M, Medstrand P, et al. New insights are game-changers in HIV-2 disease management – authors’ reply. Lancet HIV. 2019;6(4):e214–e5. https://doi.org/10.1016/S2352-3018(19)30089-X.
Chang M, Deng E, Ortega J, Raugi DN, Faye B, Simal F et al. Differentiation Capability of the Geenius Assay for HIV-2 and HIV-1/2 Dual Infections. Conference on Retroviruses and Opportunistic Infections; Seattle, WA 2019.
•• Matheron S, Descamps D, Gallien S, Besseghir A, Sellier P, Blum L, et al. First-line raltegravir/emtricitabine/tenofovir combination in human immunodeficiency virus type 2 (HIV-2) infection: a phase 2, noncomparative trial (ANRS 159 HIV-2). Clin Infect Dis. 2018;67(8):1161–7. https://doi.org/10.1093/cid/ciy245. This pilot phase-2 non-comparative trial demonstrated safety and clinical efficacy of integrase inhibitors in HIV-2 infection.
Smith R, Raugi D, Wu V, Zavala CG, Song J, Seydi M et al. Activity of bictegravir against HIV-2 isolates and INI-resistant HIV-2 Mutants Conference on Retroviruses and Opportunistic Infections; Seattle, WA 2019.
Ceia F, Silva-Pinto A, Carvalho AC, Pineiro C, Soares J, Serrao R, et al. Human immunodeficiency virus (HIV) 2 superinfection in a patient receiving antiretroviral therapy With longstanding HIV-1 viral load suppression. Open Forum Infect Dis. 2019;6(4):ofz063. https://doi.org/10.1093/ofid/ofz063.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Shauna Gunaratne declares that she has no conflict of interest.
Rajesh Gandhi has received grants and personal fees from Gilead, grants and personal fees from Merck, grants and personal fees from Theratechnologies, grants from ViiV, and grants from Janssen.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on HIV Medicine
Rights and permissions
About this article
Cite this article
Gunaratne, S.H., Gandhi, R.T. HIV-2 Infection: Latest Advances. Curr Treat Options Infect Dis 11, 233–242 (2019). https://doi.org/10.1007/s40506-019-00201-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40506-019-00201-9