Abstract
High-temperature stress is one of the significant abiotic stresses that reduce crop yields across the world. Heat stress is more prevalent in arid and semiarid regions of the tropics, and its occurrence has become more frequent in the subtropical areas. However, concerns related to this stress are significant due to the predicted rise in ambient temperatures due to global warming. It necessitates renewed phenotyping methods and crop breeding strategies to develop high-temperature tolerant crop cultivars. These strategies have a higher chance of success if the trait-based selection approach is implemented to achieve higher productivity under hotter environments. Hence, trait identification and phenotyping for key traits will play a crucial role in breeding programmes aiming at developing heat-tolerant crops. Although the concept has been around for decades, trait-based breeding has always been a challenge as screening large number genotypes for traits of interest is laborious and time-intensive. However, recent advances in phenomics have opened up new avenues to address this bottleneck efficiently and rapidly. It is attributed to the potential of phenomics tools to capture temporal and spatial changes in morphology and physiology and then related to the biochemistry of plants. These changes can provide clues about useful traits that can be used for selection of heat-tolerant lines in breeding programs. For this purpose, however, intensified efforts are needed to translate existing knowledge of mechanisms underlying heat tolerance into heritable traits and also into protocols for high throughput screening. In this regard, this review attempts to summarize the current status of breeding efforts to improve heat tolerance in crop plants and avenues for employing phenomic tools.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ambient air temperatures are increasing at an alarming rate as a consequence of global climate change. During the last three decades, Northern hemisphere has witnessed warmest years over the past 1400 years (Singh and Singh 2012). Plant stress due to supra-optimal ambient temperatures is highly detrimental to the normal physiological activities in plants (Wahid et al. 2007). An increase in temperature by 0.2 °C per decade, can lead to ambient temperatures exceed by 1.8–4.0 °C above the present level by 2100 (IPCC 2007). This projection is a significant concern, as high-temperature stress has several adverse effects on the growth, development, physiology and metabolism of a plant. Due to the sessile nature, plants are compelled to endure the heat stress by structural and phenological changes (Yang et al. 2020). Temperate crops are more vulnerable to high temperature than the tropical crops, which exhibit a relatively high level of stress tolerance (Govindaraj et al. 2018). To cope with extreme temperatures, crop plants exhibit adaptive traits and regulatory mechanisms, viz. reduction in leaf and canopy size, modulating essential genes, physiological and biochemical adjustments. The complexity of mechanisms and heredity of resilience to extreme temperature offers ample researchable issues to be addressed (Cossani and Reynolds 2012; Chaudhary et al. 2020). There are several reviews addressing mechanisms associated with high-temperature tolerance in plants and the methods proposed for selecting high-temperature tolerant genotypes (Asthir 2015; Janni et al. 2020). This review primarily aims at emerging opportunities to accelerate efforts through phenomics, which integrates plant science with advances in imaging tools, algorithms and automation for characterization of plant responses to various factors in its growing environment.
Impact of heat stress on crop plants and mechanisms of tolerance
Several reports (Hasanuzzaman et al. 2013; Chaudhary et al. 2020) explain many adverse effects of heat stress on plant development that impact plant phenology, reproduction, physiology, metabolism, and ultimately economic yield (Wahid et al. 2007). Phenological changes, which mark critical developmental events starting from seed emergence to grain development, respond to fluctuations in ambient temperatures.
High temperature effects on phenology and vegetative phase
The high-temperature effect on plant phenology results from the reduction in duration of crop growth phases and hence the overall duration of crop cycle (Rane et al. 2007; Hasanuzzaman et al. 2013; Chaudhary et al. 2020). This effect can be attributed to the fact that heat unit requirement for completion of each of the phenophases is met rapidly due to high temperatures. This natural escape mechanism can also help crop plants to complete their life cycle successfully, but at the cost of yield as the net duration of photosynthesis and carbon assimilation gets reduced (Akter and Rafiqul 2017). Early flowering is one of the key mechanisms at the whole plant level to escape the terminal heat during the crop cycle. It has been frequently exploited for improving grain yield of crops under high-temperature environments (Wahid et al. 2007).
The high temperature of about 45 °C severely affects embryonic cells in wheat, which reduces crop stands through impairing seed germination and emergence (Essemine et al. 2010). Heat stress mostly affects the meristems and reduces plant growth by promoting leaf senescence and abscission, and by lowering photosynthesis (Kosova et al. 2011). Plants exposed to high ambient air temperatures produce less biomass than those grown under optimum or low temperature. Day and night temperature around 30 and 25 °C, respectively, may have severe effects on leaf development and productive tiller formation in wheat (Rahman et al. 2009). There are reports that maximum tillering stage which coincides with flower primordia and spikelet/floret differentiation formation is also highly sensitive to rise in temperature. At this stage, the numbers of grains are determined (Quinones et al. 2017). High-temperature impact during the reproductive phase.
Though the reproductive phase of crop plant begins before flowering (i.e. from panicle initiation), several events that occur in sequence during anthesis, such as pollination, pollen germination and fertilization are highly sensitive to high-temperature stress (Jagadish et al. 2010). Besides, heat stress during gametogenesis impairs meiosis and the development of both male and female reproductive organs, leading to reduced reproductive organ viability (Jagadish 2020). Higher temperature coinciding with flowering can directly affect pollen and ovules viability and hence fertilization that is fundamental for grain formation (Chaudhary et al. 2020; Jagadish 2020). It can be due to impaired pollen germination, pollen tube growth, reduced ovule viability and reduction in ovule size, alteration in stigmatic and style morphology, reduction in stigma receptivity which adversely affects fertilization processes (Farooq et al. 2017; Prasad et al. 2017).
Post-fertilization events such as endosperm growth are also negatively affected due to high temperatures. Cell division, starch synthesis and starch accumulation are affected by high temperature, exceptionally high night temperature (Impa et al. 2019). These impaired processes lead to significant yield loss in essential cereal crops like rice (Jagadish et al. 2007), wheat (Jagadish et al. 2018), maize (Zaidi et al. 2016) and sorghum (Prasad et al. 2006; Nguyen et al. 2013). Increase in temperature by 1–2 °C reduces grain weight by accelerating grain growth rate and by shortening the grain-filling periods in wheat (Nahar et al. 2010). High-temperature stress degenerates mitochondria, changes the protein expression profiles, reduces ATP accumulation, and oxygen uptake in imbibing wheat embryos, resulting in an increased occurrence of loss of seed quality relating to seed mass, vigour, and germination (Balla et al. 2019).
Enzymes involved in endosperm starch biosynthesis pathway, soluble starch synthase (SSS) is very sensitive to high temperature, particularly at temperatures above 34 °C (Keeling et al. 1993). Soluble starch synthase has an optimum temperature of 20–25 °C and temperatures above 25 °C badly affect the activity of this enzyme, which results in reduced grain growth and starch accumulation (Prakash et al. 2003). This effect is found to be reversible in wheat after a short period of exposure to elevated temperature (Keeling et al. 1994). However, prolonged exposure to high temperature causes complete elimination of activity of SSS, which is much slower to reverse in wheat endosperm. Even short periods of high-temperature stress (30–40 °C) causes a decline in the rate of starch deposition due to a reduction in the activity of SSS. Keeling et al. (1994) reported that several other enzymes in starch biosynthesis pathway, including alkaline pyrophosphatase, phosphoglucomutase, UDP- glucose pyrophosphorylase, hexokinase, phosphoglucoisomerase, sucrose synthase, ADP- glucose pyrophosphorylase and bound starch synthase remained unaffected under elevated temperatures (25–45 °C).
Impact on physiological processes
Reduction in plant growth can also be attributed to adverse effects of high temperatures on various physiological processes including photosynthesis, respiration and translocation of metabolites within the plants (Hasanuzzaman et al. 2013; Akter and Rafiqul 2017; Chaudhary et al. 2020). High temperature affects both electron transport and CO2 fixation processes of photosynthesis. The sensitivity of Photo System II of different crops to high temperature has been reported in several crops. D1 proteins of the electron transport chain is highly sensitive to extreme temperature (Li et al. 2020). High temperature can drastically reduce activities of enzymes like RuBisCo and RuBisCo activase of Calvin cycle contributing to the reduction in photosynthesis and can also enhance photorespiration (HanumanthaRao et al. 2016; Bindumadhava et al. 2018). It can accelerate leaf senescence in crop plants (Kim et al. 2020). It limits the contribution of assimilates for crop growth due to curtailed green leaf area duration (Gregersen et al. 2013) in addition to the degradation of chlorophyll. Tissue degenerating elements within plant cell such as reactive oxygen species (ROS) get activated due to high temperatures (Asthir 2015; Hassan et al. 2020). This is one of the reasons for stress-induced senescence of plant parts. Hence, stay green trait has been proposed as selection criteria for many crops (Kamal et al. 2019).
Source limitation caused by high temperature can further determine the partitioning of assimilates to grains. Under such conditions, non-structural carbohydrate translocation is crucial for grain development (Li et al. 2017). Since the biochemical composition of developing grains is altered by high temperature, the quality of grains can get severely affected (Akter and Rafiqul 2017).
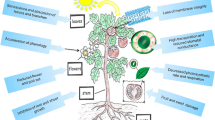
Inherent or induced changes in phenology, anatomy, morphology, metabolism and physiology of plants (Fig. 1) have been reported to contribute to high-temperature tolerance in crop plants (Hasanuzzaman et al. 2013; Asthir 2015; Hassan et al. 2020). These changes can help plants to escape, avoid or briefly tolerate high-temperature stress at the whole-plant level, organ level or at the cellular level.
Morphological changes include the development of trichomes, stomata distribution in upper to and suitable leaf angle to avoid heat load (Prasad et al. 2017). Anatomical changes to survive under heat stress include reduced cell size, increasing the xylem vessel diameter, expanding stomatal aperture (Chen et al. 2012, 2014). Physiological processes include transpirational cooling and delayed senescence of leaves. Transpiration is an important mechanism of heat avoidance and acts as the primary mediator of energy dissipation (Seginer 1994). In general, the transpiration rate increases with increase in ambient temperatures that affects both evaporation and vapour pressure deficit (VPD). The capacity to sustain high stomatal conductance at elevated temperatures helps transpirational heat dissipation, as noticed in high-temperature tolerant wheat cultivars (Dias et al. 2010) and also in gram genotypes (Kaushal et al. 2013). Tolerance to high-temperature stress is associated with enhanced tolerance of the photosynthetic apparatus (Hemantaranjan et al. 2014), ROS scavenging mechanisms (Das and Roychoudhury 2014; Nadeem et al. 2018; Huang et al. 2019), preservation of membrane stability (Das and Roychoudhury 2014). Biochemical adaptations have been attributed to enhanced production and recycling of antioxidative compounds and heat shock proteins, which can protect cell organelles and key enzymes (Hasanuzzaman et al. 2013). At the molecular level, heat stress leads to differential expression of genes associated with osmoprotectants, ion transporters, detoxifying of a free radicle, synthesis of LEA proteins and HSPs production. High temperatures may also activate transcriptional factors (TFs), involved in signalling cascades, which control critical processes offsetting high-temperature effects (Hasanuzzaman et al. 2013; Janni et al. 2020). All these processes are generally regulated at the molecular level by selective expression or suppression of genes (Chaudhary et al. 2020). Differential gene expression progressively results in improvement of heat tolerance in the form of acclimation, or adaptation (Hasanuzzaman et al. 2013). The details of mechanisms reported for high-temperature tolerance in plants have been extensively reviewed (Hasanuzzaman et al. 2013; Asthir 2015; Hassan et al. 2020; Chaudhary et al. 2020; Janni et al. 2020). However, much of the knowledge and insights generated so far needs to be translated into the selection traits for advancing breeding lines for high-temperature tolerance.
Traits for high temperature tolerance
Adaptation mechanisms allow crop plants to survive elevated ambient temperatures and complete their life cycle. During this process, yield and quality desired for human requirements are compromised. Hence the heat tolerance in the context of crop improvement has a dimension of economic importance in contrast to that naturally existing in resilient plant species. Therefore heat tolerance (HT) is the “capacity of the plant to grow and produce economic yield under high temperatures” (Wahid et al. 2007). While several insights exist to explain the heat tolerance mechanisms, conversion of these insights into the screening tools and methods are immensely crucial for the development of heat-tolerant cultivars of crops. Short duration crop cultivars mainly emerge from breeding strategies that focused on the adaptation of plants to high-temperature environments by escape from stress during the crop season. Opportunities for exploring diurnal escape emerge from scientific leads that have emerged from investigations on genetic variation in time of anthesis in crops such as rice. Early anthesis coinciding with cooler hours of the day is closely associated with reduced yield losses (Jagadish 2020). However, large scale screening of plants for the escape of pollination and fertilization processes reported in crops like rice is yet to be optimized for other crops. Other avenues for translation of scientific insights exist in morphological changes and short-term avoidance mechanisms viz., altered leaf orientation, transpirational cooling, modified membrane lipid compositions (Wahid et al. 2007). Leaf masking of tissues that are subtle to sunburn can be another trait with the potential to contribute to a reduction in high temperature-induced yield losses. Root branching and deep rooting can be a critical trait that can enable plants to reach more distant reserves of water (Chaudhary et al. 2020).
The heat tolerance capacity of high yielding crops such as wheat has been attributed to the conservation of photosynthesis, chlorophyll content, and maintenance of stomatal conductance under elevated temperature stress and extended grain filling duration even at increased thermal stress (Balla et al. 2019). Such observations insinuate that selection for grain yield takes into account the mechanisms of tolerance. However, the magnitude of heat stress defined by intensity and duration to which crops were exposed during the experiment under high temperature is crucial in determining the crop yield. Further, different traits and trait combinations may play a critical role in alleviating high-temperature stress depending on the type of agro-ecologies. Hence, insights gained so far on mechanisms of tolerance to high temperature in plants are crucial for trait-specific plant breeding. Some of the traits and protocols suggested for screening germplasm of crops are discussed in the following section.
Cell membrane thermo-stability
Increase in temperature can damage cellular membrane, which in turn leads to enhanced permeability and leakage of ions (Wahid et al. 2007; Govindaraj et al. 2018), which can be easily measured by the efflux of electrolytes from affected leaf tissue into an aqueous medium. This method was devised by C.Y. Sullivan in the late 1960s for assessing sorghum and maize heat tolerance (Govindaraj et al. 2018). It has been widely employed to determine cellular thermostability for heat in wheat (Bala et al. 2017; ElBasyoni et al. 2017), soybean (Srinivasan et al. 1996) maize (Naveed et al. 2016) and chickpea (Kumar et al. 2013a). A positive association between membrane injury and grain weight was observed in wheat signifying that membrane thermo-stability is a good indicator of HS tolerance (ElBasyoni et al. 2017). The membrane thermo-stability can be estimated as MTS = (1 −T1/T2) × 100, where T1 is conductivity reading after heat treatment, and T2 is conductivity reading after autoclaving (Ibrahim and Quick 2001). This trait has been tested in bajra and found effective under field condition (Govindaraj et al. 2018). However, success depends on the time of measurement and large replicates, which consumes time and labour that plant breeders cannot afford.
Photosystem-II sensitivity
Photosynthesis is sensitive to elevated ambient temperature as it severely affects photosystem II (Van der Tol et al. 2014; Kalaji et al. 2018). High temperature can also affect energy associated with CO2 fixation (Dusenge et al. 2018). Plants exhibit protective mechanisms to prevent photoinhibition when the elevated ambient temperature is coupled with high levels of solar radiation (Lu et al. 2017). Hence devices like photosynthetic meter and chlorophyll fluorescence meters have been extensively used to differentiate responses of plants to high temperatures. However, only a few plants can be screened by equipment such as IRGA, while chlorophyll fluorescence meter enables screening of a large number of plants.
Heat damage in photosynthetic tissue can be estimated by chlorophyll fluorescence (Murchie and Lawson 2013). In this method, leaf discs are subjected to short light period, and the time of dark recovery of the fluorescence parameter Fv/Fo (ratio of variable to minimum chlorophyll fluorescence) is estimated as a function of temperature. It is easy, fast, and reasonable proven approach for the quick screening in a large number of crops, e.g., barley (Rizza et al. 2011), wheat (Balouchi 2010), maize (Sinsawat et al. 2004), Olea europaea (Haworth et al. 2019) and legumes (Herzog and Chai-Arree 2012).
Membrane lipid saturation
Plant cell membranes are mainly composed of proteins and lipids, and this selectively permeable barrier is sensitive to fluctuations in ambient temperature. Heat-induced increase in the membrane fluidity is associated with a large proportion of unsaturated fatty acids in membrane lipids. To preserve the membrane fluidity, plants enhance the ratio of saturated and monounsaturated fatty acids, in response to raised temperatures. Therefore, the rise in the saturation level of fatty acids seems to be essential for maintaining membrane stability and increasing heat tolerance (Penfield 2008), Niu and Xiang 2018). High throughput mechanisms to assess crop germplasm for such traits are yet to be developed.
Canopy temperature depression (CTD)
Canopy temperature depression (CTD)—is the difference between canopy temperature (Tc) and ambient temperature (Ta)—acts as an indirect measure of transpiration (Reynolds et al. 2001) and plant water status (Araus et al. 2003). Infrared thermometer (IRT) has been extensively employed for measuring canopy temperature (CT) directly during peak sunshine hours (13:00 and 14:30 h) distantly and without interfering with the crop. Studies suggested that CT is associated with many physiological parameters such as stomatal conductance, transpiration rate, plant water status, water use, leaf area index, and crop yield (Sofi et al. 2019). Genotypes with cooler canopy temperatures maintain better hydration status. CTD has been proved to be a rapid and stable test that can be used for selection of heat-tolerant wheat (Al-Ghzawi et al. 2018). A study by Awika et al. (2017) in barley suggested that a strong link between epicuticular leaf wax QTL and CTD and that wax load influences plant canopy temperature.
Morphological traits
In many of the crop plants, reproductive organs, especially male reproductive tissues, are more sensitive to heat stress than female gametophytes (Djanaguiraman et al. 2018), and the threshold temperature for causing damage in these tissues is lower than that for vegetative tissues. Heat stress can cause damage either at pre- or post-pollination, which affects fertilization and ultimately reduce seed set (Sage et al. 2015; Prasad et al. 2017).
Pollen viability, stigma receptivity, tassel blasting, tassel sterility in maize (Zaidi et al. 2016), grain sterility in rice (Weerakoon et al. 2008), and asynchrony of male and female floral organ development in chickpea (Devasirvatham et al. 2012) have been reported as potential traits for improvement of heat tolerance. By selecting for these traits, several heat tolerance accessions have been identified in crop species like chickpea e.g., ICC1205 and ICC15614 (Devasirvatham et al. 2012).
Stem reserve mobilization for grain development
When leaves lose their functions due to high temperature, non-structural carbohydrates stored in stem are utilised for grain development (Tahir and Nakata 2005). Non-structural carbohydrates are estimated by chromatographic analysis in tree species (Raessler et al. 2010). Spectroscopic methods are employed to estimate non-structural carbohydrates in the stem, but the process is time-consuming and labour intensive (Rane and Nagarajan 2004). Dreccer et al. (2014) monitored quantitative dynamics of stem water-soluble carbohydrates in wheat in the field using hyperspectral reflectance (hyperspectral radiometer). Wang et al. (2016) employed Near-infrared spectral measurements in rice for estimation of NSC. Genetic variabilities exist for this trait in crops like wheat (Pinto et al. 2017).
Stay green trait
Several morpho-physiological traits have been identified for their utility in breeding for heat tolerance. Genotypes with stay-green traits constitute potential genetic resources for genetic enhancement of crop plants to overcome heat and drought stresses. Stay green genotype is characterized by delayed senescence because of prolonged chlorophyll (Chl) retention compared with a non-stay green standard genotype. Stay green trait that allows leaves to be photosynthetically active can improve the grain-filling process even under stress conditions (Kamal et al. 2019). This trait has been found desirable in developing heat-tolerant wheat variety (Kumari et al. 2012). Stay-green has been assessed in the field by using several techniques.
Visual assessment of stay-green is rapid and inexpensive and requires no specific equipment (Christopher et al. 2014. Visual inspections have been performed for the greenness of all fertile shoots (Foulkes et al. 2007) and greenness of the flag leaf and peduncle (Joshi et al. 2007). Stay green features can be measured in individual leaves by instruments such as the Minolta SPAD meter (Harris et al. 2007; Christopher et al. 2008). However, such methods time taking and not feasible for measurements of a large set of genotypes, as they require repeated measurements of multiple leaves to provide reliable information for a single trial plot. Recently, methods have been developed using normalized difference vegetative index (NDVI) measurements from devices such as the hand-held Green seeker (NTech Industries, Ukiah, CA, USA) (Kipp et al. 2014). The advantage of such methods is that they provide objective, integrated measurements of canopy greenness and only take a few seconds to measure a field plot (vs. several minutes required for SPAD measurements). NDVI-based methods can provide measurements of hundreds of field plots in an hour and thus can be used to screen large numbers of plots (e.g. breeding populations or structured populations for genetic studies). As they are non-destructive and relatively rapid, repeated measurements can be made on the same plots throughout crop development. Studies reporting NDVI-based measurements of the stay-green phenotype rely on measurements from one or a few time points late in the crop cycle (Lopes and Reynolds 2012).
Breeding for heat tolerance: a next-generation breeding approach
Plant breeding for high-temperature tolerant cultivars for present and future global climate scenario need reoriented strategy to bridge the gaps in conventional approaches that are focused on yield and yield components for selection. Such a strategy should build on experience and knowledge gained from hotspot screening and application of trait-based breeding and molecular markers approach.
Selection in hot environments
Simulating the natural heat stress environment, which is often complicated by the presence of other stresses, has been the challenge for plant scientists. However, selection based on performance in hot growing environments was found to be effective in wheat (Rane et al. 2007) and maize (Zaidi et al. 2016). Essential criteria for selection of field are high VPD, where low yield was correlated with VPD during all the growing period, the high maximum temperature during the cultivated period and low photothermal quotient corrected by VPD in the critical period of grain set before flowering (Dreccer et al. 2014).
Irrespective of the screening approach, the main objective of plant breeders is to develop a cost-effective set of thermotolerance markers which can be utilised for further implementation of breeding for heat tolerance in various crop species. Identification of tolerant germplasm/genotype is pre-requisite for significant genetic improvement through plant breeding methods. Nevertheless, identification of dependable and cost-effective heat screening methods is a major limitation in conventional breeding to facilitate identification of heat tolerance genotype (Wahid et al. 2007). Some of the preliminary screening approaches include the seedling thermo-tolerance index (STI) (Peacock et al. 1993), seed to seedling thermo-tolerance index (SSTI) in bajra (Yadav et al. 2015), and heat tolerance index (HTI) in sorghum (Setimela et al. 2005). High-temperature index (HTI) was found to be successful in identifying heat-tolerant wheat genotypes under field condition (Rane and Nagarajan 2004). High-temperature tolerance screening at germination and the early vegetative stage is useful in bajra and maize (Ashraf and Hafeez 2004). Field-based tents were used for screening of different genotypes for high night temperature stress in crops like rice (Shi et al. 2016, 2017). Recently Hein et al. (2019) developed a protocol for screening winter wheat genotypes for high night temperature stress by integrating field-based heat tents and cyber-physical system technology. These advanced field techniques can assist in screening and identification of heat-tolerant genotypes. Nevertheless, the current approaches for phenotyping high-temperature tolerance in crop plants require optimization of screening protocols for a large number of genotypes of cops for accelerating the development of heat-tolerant cultivars.
Physiological traits-based breeding
The effectiveness of direct selection for yield enhancement under stressed conditions is slowed down by low heritability and complex nature of major and minor QTLs governing them (Manavalan et al. 2009). Breeding for combined high yield and heat tolerance is hindered by the influence of the environment, complex and poor knowledge of genetic inheritance of high-temperature tolerance and a limited number of validated QTLs/cloned gene(s) (Chaudhary et al. 2020; Janni et al. 2020). Trait-based breeding would be an excellent approach for incorporating high-temperature tolerance gene(s)/QTLs. Such strategies have been implemented in wheat breeding at CIMMYT to develop heat-tolerant varieties (Reynolds et al. 2009). Traits used for selection of heat-tolerant accessions in crops breeding programs include canopy structure, delayed senescence, photosynthesis efficiency, lower transpiration rates, resilience in reproductive traits, and also the harvest index (Chaudhary et al. 2020). Genetic variability has been recorded for the above traits in several crops.
Along with, selection for morphophysiological traits conferring high-temperature adaptation, traits indirectly associated with yield, can also be exploited as demonstrated in wheat (Cossani and Reynolds 2012). Genetic variability for photosynthetic rate under heat stress has been reported in chickpea and tomato (Kumar et al. 2017; Zhou et al. 2018). Chlorophyll fluorescence, Canopy temperature depression (CTD) traits contributed positively to high-temperature tolerance in wheat and cotton, respectively (Sharma et al. 2012). The cooler canopy temperature (CT) under HS leads to higher yield in wheat (Gautam et al. 2015) The CTD, flag leaf stomatal conductance and photosynthetic rate positively associated with yield in wheat during thermal stress (Fischer et al. 1998).
Membrane thermostability was found to be a promising trait for estimating high-temperature tolerance while evaluating genetic variability in different crops (Shanahan et al. 1990; Srinivasan et al. 1996). Combination of high chlorophyll content and membrane thermostability used as selection criterion under HS in brassica and cotton (Ristic et al. 2007; Kumar et al. 2013b). Khan et al. (2008) used relative cell injury level (RCIL) index in assessing thermal tolerance in cotton. Hence, focus on the specific physiological trait in breeding is an ideal strategy to develop heat tolerance genotypes without causing yield penalty.
Contemporary plant breeding approaches for high temperature tolerance
Over the past decades’ research efforts by the conventional breeding have provided insights into several genes associated with tolerance to heat stress in addition to alleles and their mode of inheritance (Chaudhary et al. 2020; Janni et al. 2020). With the advent of molecular marker technology, QTLs and trait discovery for various morphophysiological traits have now added new dimensions for improvements of different crops (Jha et al. 2014; Chaudhary et al. 2020; Janni et al. 2020). The identification of markers associated with high-temperature tolerance QTLs has expanded opportunities to stack them in crop plants under desired agronomic background. An extensive list of QTLs, marker interval, type of mapping population has been summarized by Jha et al. (2014). Numerous major or minor QTLs and associated markers for HT have been mapped in many crops including rice (Shanmugavadivel et al. 2017; Zhu et al. 2017; Kilasi et al. 2018) wheat (Talukder et al. 2014; Sharma et al. 2016) and maize (Frey et al. 2016; McNellie et al. 2018; Inghelandt et al. 2019). Several QTLs have been mapped for component traits for HS tolerance in several crops which includes QTLs for pollen germination, pollen tube growth and cell membrane stability in maize (Ottaviano et al. 1992; Frova and Sari-Gorla 1994). Kamal et al. (2019) reviewed the significance of QTLs for stay-green traits in cereals. Stable QTLs for grain filling duration and grain number under terminal heat stress in Triticum wheat identified by Sharma et al. (2016).
An array of markers was explored for identification of high temperature tolerant and susceptible genotypes in several crops. Garg et al. (2012) identified heat tolerance and heat-sensitive cultivars in wheat by using SNPs. Microsatellite markers which are associated with grain filling rate under thermal stress were developed by Barakat et al. (2012). In cowpea RIL population, five SNPs associated with heat stress were identified (Lucas et al. 2013). A dominant gene OsHTAS was identified from the genotype HT54, which conferred thermal tolerance at 48 °C during seedling and grain-filling stages in rice (Wei et al. 2013). However, utilization of QTLs through MAS or MABB for crop improvement is hindered by large genotype x environment as well as epistasis interactions, which often lead to low breeding efficiency (Salgotra and Stewart 2020). Hence marker-assisted recurrent selection (MARS) or Genomic selection (GS) have been proposed (Godiki et al. 2016). All these selection methods aided by gene marker need assessment of a large set of breeding lines for greater power of prediction about the relation between desired trait and genes. Hence, phenomics tools assume immense significance mainly to ensure high throughput screening. It requires the identification of component traits of high breeding value for high-temperature tolerance in crops (Fig. 2).
Opportunities to employ plant phenomics
The conventional breeding methods contributed significantly in the past to achieve genetic gains in crops worldwide. However, it is possible to increase food production globally, to meet projected food demand if current breeding methods are complemented by modern tools (Araus and Cairns 2014). Hence, now the focus has been shifted towards the use of advanced genomics and other breeding strategies for fast-tracking crop improvement pipelines for cultivars resilient to abiotic stresses (Fig. 3). These advances in science are being utilized in trait discovery, genetic dissection of complex traits and discovery of associated genes and their deployment in varieties (Govindaraj et al. 2018). Despite these efforts, only a few identified QTLs/genes could be transferred to main breeding population mainly due to precision in phenotyping data of targeted traits and marginal differences in parental lines selected for mapping population (Govindaraj et al. 2018). Thus, the relationship of these QTL/genes with the phenotype in a ‘real world’ environment remains subtle as many false-positive QTL have been identified previously or these QTLs function only under specific genetic background or specific abiotic stress environments. It indicates that the phenotyping needs to be intensified to ensure location specific and genome-specific approaches. Hence high throughput phenotyping methods are essential particularly for enhancing the power of prediction about the association between genes and traits associated with high-temperature tolerance.
Though large germplasm collection is available for many crops across the countries, in many of the germplasm sets phenotypic descriptors are rather limited. It has restricted the use of genetic resources for identification of allelic variation for several traits, including those for high-temperature tolerance. The underutilization of genetic resources is also due to the absence of effective methods for precise and accurate phenotyping. Identification of key traits involved in stress tolerance requires replicated trials over multiple seasons and at multilocation. It cannot be achieved through conventional phenotyping tools that are slow, expensive and labour-intensive, often destructive and also developmental stage-specific. These impediments in phenotyping have driven extraordinary enthusiasm that has led to progress in the advancement of new high throughput phenotyping tools and techniques. These tools involve non-invasive imaging, spectroscopy, image analysis, robotics and high-performance computing and recently big data analysis with AI interventions (Yang et al. 2020). Though the initial focus of phenomics was on responses of plants under controlled environments, now the techniques have been optimized for field evaluation of plant performance in a non-destructive manner (Rahaman et al. 2015). The devoted high throughput phenomic facilities promise enhanced precision and accuracy in data recording. These facilities are equipped to facilitate structural responses of both shoot and root nondestructively. The scope for employing phenomics tools and technologies for translating ‘knowledge about mechanisms of heat tolerance’ to ‘trait identification’ and then phenotyping genotypes for high-temperature tolerance is described in this section.
Visible light (300–700 nm) based imaging
Visual examination of abiotic stress-tolerance traits and associated phenotypes has been a standard exercise in breeding plants for tolerance to stresses. But lately, the emphasis is on visual light-based imaging technologies due to the greater feasibility of monitoring plant responses to growth environment. Visible imaging techniques depends on two-dimensional (2D) digital images, which are utilised for measuring above the ground plant traits such as shoot biomass (Neilson et al. 2015), leaf morphology, shoot tip growth, panicle and seed morphology (Fahlgren et al. 2015). High temperatures influence all these traits during the growth and development of plants. The 3D imaging techniques have been improved to measure different traits, including plant height, leaf morphology, and shoot dry weight (Paproki et al. 2012) which can discriminate tolerant and intolerant genotypes grown at high temperatures under natural or controlled environments. High-resolution cameras configured for time series data have the potential to assess genetic variation in the diurnal escape of critical process such as pollination and fertilization, which are highly vulnerable to high temperature. Leaf senescence, which is one of the consequences of high-temperature stress, can also be monitored by temporal and spatial changes in red, green and blue pixels in the image of the leaf canopy. Further, stress responses of plants such as leaf folding or curling can be precisely quantified by a series of image parameters obtained from time-series images during screening for high-temperature tolerance (Singh et al. 2018).
Infrared imaging
Infrared imaging technique uses two foremost wavelength ranges, near-infrared (NIR) (0.9–1.55 μm) and far-infrared (FIR) (7.5–13.5 μm). With the development in infrared thermal technology, current thermal cameras can detect plant canopy temperature with high accuracy. Combination of NIR imaging with visible imaging, i.e., visible to short-wave infrared (VSWIR; 0.4–2.5 μm), offers a more in-depth insight into plant health under various stress situation because it provides well-defined spectral features for pigments, leaf water content, and biochemical parameters such as lignin and cellulose (Yang et al. 2013). Thermal imaging accurately estimates leaf and canopy temperature, which can indirectly provide information about leaf water status. Thermal infrared imaging can also be employed to determine relative chlorophyll content, leaf colour, and canopy temperature (Jones et al. 2009; Munns et al. 2010). Infrared thermal imaging systems have been used to measure stomatal behaviour under various stress conditions (Iseki and Olajumoke, 2020). Qiu et al. (2009) identified significant differences between leaf temperature, air temperature, and canopy temperature under drought and high-temperature stress in melons, tomatoes, and lettuce. They also suggested that the transpiration transfer coefficient can be utilized to identify different abiotic stresses in plants. Since transpiration is determined by plants capacity to extract water from the soil, it is also possible to generate preliminary information about the genetic variation in the root system of plants through canopy temperature measurements.
The NIR technique has been adopted for assessment of leaf water content. NIR equipment is exclusively used for quantification of protein and carbohydrates in seeds of different crops (Jin et al. 2017). It is essential to optimize high throughput methods for estimation of non-structural carbohydrates in the stem of crop plants at the time of grain development as conventional methods are not so efficient to screen large number of genotypes for this trait (Singh et al. 2018).
Chlorophyll fluorescence imaging
The basic principles of chlorophyll fluorescence as a tool to assess plant health have been reviewed by many researchers (Kalaji et al. 2016). Chlorophyll fluorescence imaging systems are now replacing the chlorophyll fluorescence meters for precise and rapid assessment of plant responses to stresses taking into account temporal and spatial changes. The imaging system captures fluorescence emitted by plant parts and converts into false-colour signals using computer software to interpret plant responses (Weirman 2010; Rane et al. 2019). This technique has great potential to identify genetic sources of heat tolerance as elevated temperatures have a significant effect on components of Photo System II (Weirman 2010). Further, high temperatures induced cell membrane injury can be a cause of diminishing photosynthesis and changes in chlorophyll fluorescence. Hence, it is predicted that methods for rapid assessment of cell and thylakoid membrane injury may emerge in future by engaging chlorophyll fluorescence technique. Stomatal conductance (Omasa and Takayama 2003), phloem loading and unloading (Mehdi et al. 2019), photosynthesis and growth inhibition (Pérez-Bueno et al. 2019), and plant metabolite content (Li et al. 2014) under stress can also be studied using fluorescence imaging (Osmond et al. 2004). Fluorescence imaging can also be used to resolving heterogeneity in leaf photosynthetic performance (Baker 2008). These scientific leads need to be translated into high throughput methods to facilitate crop breeding for high-temperature tolerance.
Hyperspectral imaging
Multispectral and hyperspectral cameras are engaged to capture spectral signatures of plants in a particular environment (Yang et al. 2013). Multispectral imaging systems generally limited 3–10 bands each with a descriptive title. For example, the channels may include red, green, blue, near-infrared, and short-wave infrared. In contrast, hyperspectral imaging involves hundreds and thousands of narrower (10–20 nm) bands that enhanced the capacity of the system to provide a specific spectral signature of an object like plants. Different spectral regions have been characterized as distinct for plant science, including (1) NDVI (normalized difference vegetation index) (2) CRI (carotenoid reflectance index), (3) PRI (photochemical reflectance index (Fiorani et al. 2012). The vegetation indices are associated with diverse characteristics such as water status, pigment content, and photosynthetically active biomass, which are used to compute total green biomass, leaf area, chlorophyll content, and yield in various crop species (Din et al. 2017). If optimized for high throughput, these techniques can help to identify genetic markers associated with critical plant processes such as leaf senescence with greater precision. Also, the higher resolution of hyperspectral data can predict leaf growth (Cheng et al. 2017) and panicle emergence (Liu et al. 2010) in rice. These methods promise the possibility of screening crop genotypes for a diurnal escape from high-temperature tolerance. Further, hyperspectral techniques need to be optimized for plant metabolic changes that impart tolerance to temperature.
Limitations in phenotyping tools
Though the image-based phenomics is being increasingly focused for high throughput phenotyping, there are some limitations in employing them in plant breeding programs. The successful implementation of high throughput phenotyping tools needs huge funds, experienced personnel in data handling. Plant phenomics in fields is very complicated as they are the outcome of considerable G x E x management interaction. The effective use of UAVs for field phenotyping relies on stability, safety, control, reliability, positioning, autonomy, sensor mount, and controller. Other factors are such as specific spectral wavelengths, resolution, weight, calibration, and field of view. The UAV operation is often limited by sensor pay-load (size/weight), operating altitude (regulatory issues), and flight time (Deery et al. 2014). Measurements of plant phenotypes are complicated as it includes measurement morphological, biochemical, physiological traits to identify critical traits responsible for stress tolerance. The challenge is to separate the developmental changes from high temperature-induced changes.
Conclusion and way forward
Novel strategies are essential to developing heat-tolerant cultivars to make them climate-smart as conventional approaches are not sufficient to meet the expectations. Traits based plant breeding is the key to accomplish this task. Attempts have been made to use traits identified so far as selection criteria in plant breeding programme for high-temperature tolerance. However, the utility of these traits often highly restricted due to lack of feasibility to screen a large number of genotypes. Phenomics tools equipped by different sensors and imaging system are being extensively engaged to generate phenome data and promise to overcome existing bottleneck in screening germplasm in large scale. However, many of these techniques have to be optimized for different crops. The imaging systems that have been designed to capture different wavelengths of the electromagnetic spectrum can be engaged in complex measure traits. For example, excised leaf water, cell membrane stability, change in lipid composition, pollen and ovule response can be assessed by engaging imaging systems. These traits, which have emerged from our insights into the mechanisms of high-temperature tolerance, can help in detecting the promising genetic sources from germplasm collections. While investigations on the mechanisms will continue to explore new information, some of the current scientific leads can be translated into the selection-traits and also can be used for developing screening protocol. There is a need to optimize image-based methods to assess phenological changes, diurnal escape from stress, changes in spike morphology, as well as intra-spike, assimilate distribution. Such efforts can facilitate the application of phenomics tools for supporting OMICS’ approaches along with system biology approach to develop high-temperature tolerant cultivars. Known sets of heat-tolerant and intolerant cultivars of different crops available in germplasm bank can be of immense use in optimizing the phenomics methods and then extending them to a large set of germplasm.
Further, extensive efforts through multi-institutional and multidisciplinary approaches are essential to identify genetic resources for each of the traits associated with temperature tolerance based on the superior trait values relative to a popular cultivar of the targeted agro-ecologies. Image-based parameters can serve as surrogate traits to amplify such difference and for high throughput plant phenotyping. The enhanced power of prediction about trait-gene association generated through the large screen can help in precise identification of genes associated with each of component traits relevant to heat tolerance. Such genes can be stacked to develop novel cultivars possessing temperature resilience which is essential for the sustainability of agriculture in arid and Semi-arid region, particularly in the context of climate change.
References
Akter, N., & Rafiqul, I. M. (2017). Heat stress effects and management in wheat. A review. Agronomy for Sustainable Development, 37(5), 37. https://doi.org/10.1007/s13593-017-0443-9.
Al-Ghzawi, A., Khalaf, Y., Al-Ajlouni, Z., AL-Quraan, N., Musallam, I., & Hani, N. (2018). The effect of supplemental irrigation on canopy temperature depression, chlorophyll content, and water use efficiency in three wheat (Triticum aestivum L. and T. durum Desf.) varieties grown in dry regions of Jordan. Agriculture, 8(5), 67.
Araus, J. L., Bort, J., Steduto, P., Villegas, D., & Royo, C. (2003). Breeding cereals for Mediterranean conditions: Eco-physiological clues for biotechnology application. Annals of Applied Biology, 142, 129–141. https://doi.org/10.1111/j.1744-7348.2003.tb00238.x.
Araus, J. L., & Cairns, J. E. (2014). Field high-throughput phenotyping: The new crop breeding frontier. Trends in Plant Science, 19(1), 52–61. https://doi.org/10.1016/j.tplants.2013.09.008.
Ashraf, M., & Hafeez, M. (2004). Thermotolerance of pearl millet and maize at early growth stages: Growth and nutrient relations. Biologia Plantarum, 48, 81–86.
Asthir, B. (2015). Protective mechanisms of heat tolerance in crop plants. Journal of Plant Interactions, 10(1), 202–210. https://doi.org/10.1080/17429145.2015.1067726.
Awika, H. O., Hays, D. B., Mullet, J. E., Rooney, W. L., & Weers, B. D. (2017). QTL mapping and loci dissection for leaf epicuticular wax load and canopy temperature depression and their association with QTL for stay green in Sorghum bicolor under stress. Euphytica, 213, 1–22. https://doi.org/10.1007/s10681-017-1990-5.
Baker, N. R. (2008). Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annual Review of Plant Biology, 59, 89–113.
Bala, P., Lalmia, H., & Sikde, S. (2017). Evaluation of heat tolerance of wheat genotypes through membrane thermostability test. MAYFEB Journal of Agricultural sciences, 2, 1–6.
Balla, K., Karsai, I., Bónis, P., Kiss, T., Berki, Z., & Horvath, A. (2019). Heat stress responses in a large set of winter wheat cultivars (Triticum aestivum L.) depend on the timing and duration of stress. PLoS ONE, 14(9), e0222639.
Balouchi, H. R. (2010). Screening wheat parents of mapping population for heat and drought tolerance, detection of wheat genetic variation. International Journal of Nuclear and Quantum Engineering, 4, 63–73.
Barakat, M. N., Al-Doss, A. A., Elshafeim, A. A., & Moustafa, K. A. (2012). Bulked segregant analysis to detect quantitative trait loci (QTL) related to heat tolerance at grain filling rate in wheat using simple sequence repeat (SSR) markers. African Journal of Biotechnology, 11, 12436–12442.
Bindumadhava, H., Sharma, L., Nair, R. M., Nayyar, H., Riley, J. J., & Easdown, W. (2018). High temperature-tolerant mungbean (Vigna radiata L.) lines produce better yields when exposed to higher CO2 levels. Journal of Crop Improvement. https://doi.org/10.1080/15427528.2018.1439132.
Chaudhary, S., Devi, P., Bhardwaj, A., Jha, U. C., Sharma, K. D., Prasad, P. V. V., et al. (2020). Identification and characterization of contrasting genotypes/cultivars for developing heat tolerance in agricultural crops: Current status and prospects. Frontiers in Plant Sciences, 11, 587264. https://doi.org/10.3389/fpls.2020.587264.
Chen, W. L., Yang, W. J., Lo, H. F., & Yeh, D. M. (2014). Physiology, anatomy, and cell membrane thermostability selection of leafy radish (Raphanussativus var. Oleiformis Pers.) with different tolerance under heat stress. Scientia Horticulturae, 179, 367–375.
Chen, W. R., Zheng, J. S., Li, Y. Q., & Guo, W. D. (2012). Effects of high temperature on photosynthesis, chlorophyll fluorescence, chloroplast ultrastructure, and antioxidant activities in fingered citron. Russian Journal of Plant Physiology, 59, 732–740.
Cheng, T., Rivard, B., Sánchez-Azofeifa, A. G., Féret, J., Jacquemoud, S., & Ustin, S. L. (2017). Deriving leaf mass per area LMA from foliar reflectance across a variety of plant species using continuous wavelet analysis. ISPRS Journal of Photogrammetry and Remote Sensing, 87, 28–38.
Christopher, J. T., Manschadi, A. M., Hammer, G. L., & Borrell, A. K. (2008). Developmental and physiological traits associated with high yield and stay-green phenotype in wheat. Australian Journal of Agricultural Research, 59, 354–364. https://doi.org/10.1071/AR07193.
Christopher, J. T., Veyradier, M., Borrell, A. K., Harvey, G., Fletcher, S., & Chenu, K. (2014). Phenotyping novel stay-green traits to capture genetic variation in senescence dynamics. Functional Plant Biology, 41(11), 1035. https://doi.org/10.1071/fp14052.
Cossani, C. M., & Reynolds, M. P. (2012). Physiological traits for improving heat tolerance in wheat. Plant Physiology, 160, 1710–1718.
Das, K., & Roychoudhury, A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Frontiers in Environmental Science. https://doi.org/10.3389/fenvs.2014.00053.
Deery, D., Jimenez-Berni, J., Jones, H., Sirault, X., & Furbank, R. (2014). Proximal remote sensing buggies and potential applications for field-based phenotyping. Agronomy, 4, 349–379. https://doi.org/10.3390/agronomy4030349.
Devasirvatham, V., Gaur, P., Mallikarjuna, N., Raju, T. N., Trethowan, R. M., & Tan, D. K. Y. (2012). Effect of high temperature on the reproductive development of chickpea genotypes under controlled environments. Functional Plant Biology, 39, 1009–1018.
Dias, A. S., Barreiro, M. G., Campos, P. S., Ramalho, J. C., & Lidon, F. C. (2010). Wheat cellular membrane thermo tolerance under heat stress. Journal of Agronomy and Crop Science, 196, 100–108.
Din, M., Zheng, W., Rashid, M., Wang, S., & Shi, Z. (2017). Evaluating hyperspectral vegetation indices for leaf area index estimation of Oryza sativa L. at diverse phenological stages. Frontiers in Plant Sciences, 8, 820.
Djanaguiraman, M., Perumal, R., Ciampitti, I. A., Gupta, S. K., & Prasad, P. V. V. (2018). Quantifying pearl millet response to high temperature stress: Thresholds, sensitive stages, genetic variability and relative sensitivity of pollen and pistil. Plant Cell Environment, 41, 993–1007. https://doi.org/10.1111/pce.12931.
Dreccer, M. F., Wockner, K. B., Palta, J. A., McIntyre, C. L., Borgognone, M. G., Bourgault, M., et al. (2014). More fertile florets and grains per spike can be achieved at higher temperature in wheat lines with high spike biomass and sugar content at booting. Functional Plant Biology, 41, 482–495.
Dusenge, M. E., Duarte, A. G., & Way, D. A. (2018). Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytologist. https://doi.org/10.1111/nph.15283.
ElBasyoni, I., Saadalla, M., Baenziger, S., Bockelman, H., & Morsy, S. (2017). Cell membrane stability and association mapping for drought and heat tolerance in a worldwide wheat collection. Sustainability, 9(9), 1606.
Essemine, J., Ammar, S., & Bouzid, S. (2010). Impact of heat stress on germination and growth in higher plants: Physiological, biochemical and molecular repercussions and mechanisms of defense. Journal of Biological Science, 10, 565–572. https://doi.org/10.3923/jbs.2010.565.572.
Fahlgren, N., Gehan, M. A., & Baxter, I. (2015). Lights, camera, action: High-throughput plant phenotyping is ready for a close-up. Current Opinion in Plant Biology, 24, 93–99.
Farooq, M., Nadeem, F., Gogoi, N., Ullah, A., Alghamdi, S. S., Nayyar, H., et al. (2017). Heat stress in grain legumes during reproductive and grain-filling phases. Crop Pasture Science, 68, 985–1005. https://doi.org/10.1071/CP17012.
Fiorani, F., Rascher, U., Jahnke, S., & Schurr, U. (2012). Imaging plants dynamics in heterogenic environments. Current Opinion in Plant Biology, 23, 227–235.
Fischer, R. A., Rees, D., Sayre, K. D., Lu, Z. M., Condon, A. G., & Larque Saavedra, A. (1998). Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Science, 38, 1467–1475. https://doi.org/10.2135/cropsci1998.0011183X003800060011x.
Foulkes, M. J., Sylvester-Bradley, R., Weightman, R., & Snape, J. (2007). Identifying physiological traits associated with improved drought resistance in winter wheat. Field Crops Research, 103, 11–24. https://doi.org/10.1016/j.fcr.2007.04.007.
Frey, F. P., Presterl, T., Lecoq, P., Orlik, A., & Stich, B. (2016). First steps to understand heat tolerance of temperate maize at adult stage: Identification of QTL across multiple environments with connected segregating populations. Theoretical and Applied Genetics, 129(5), 945–961. https://doi.org/10.1007/s00122-016-2674-6.
Frova, C., & Sari-Gorla, M. (1994). Quantitative trait loci (QTLs) for pollen thermo tolerance detected in maize. Molecular and General Genetics, 1294954, 424–430.
Garg, D., Sareen, S., Dalal, S., Tiwari, R., & Singh, R. (2012). Heat shock protein based SNP marker for terminal heat stress in wheat (Triticum aestivum L.). Australian Journal of Crop Science, 6, 1516–1521.
Gautam, A., Sai Prasad, S. V., Jajoo, A., & Ambati, D. (2015). Canopy temperature as a selection parameter for grain yield and its components in durum wheat under terminal heat stress in late sown conditions. Agricultural Research, 4(3), 238–244. https://doi.org/10.1007/s40003-015-0174-6.
Godiki, Y., Bhanu, A. N., & Singh, M. N. (2016). Marker assisted recurrent selection: An overview. Advances in Life Sciences, 5(17), 6493–6499.
Govindaraj, M., Pattanashetti, S. K., Patne, N., & Kanatti, A. A. (2018). Breeding Cultivars for Heat Stress Tolerance in Staple Food Crops. Next Generation Plant Breeding. https://doi.org/10.5772/intechopen.76480.
Gregersen, P. L., Culetic, A., Boschian, L., & Krupinska, K. (2013). Plant senescence and crop productivity. Plant Molecular Biology, 82(6), 603–622. https://doi.org/10.1007/s11103-013-0013-8.
HanumanthaRao, B., Nair, R. M., & Nayyar, H. (2016). Salinity and high temperature tolerance in mungbean [Vigna radiata (L.) Wilczek] from a physiological perspective. Frontiers in Plant Sciences., 7, 957. https://doi.org/10.3389/fpls.2016.00957.
Harris, K., Subudhi, P., Borrell, A., Jordan, D., Rosenow, D., Nguyen, H., et al. (2007). Sorghum stay-green QTL individually reduce post flowering drought-induced leaf senescence. Journal of Experimental Botany, 58, 327–338. https://doi.org/10.1093/jxb/erl225.
Hasanuzzaman, M., Nahar, K., Alam, M., Roychowdhury, R., & Fujita, M. (2013). Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. International Journal of Molecular Sciences, 14(5), 9643–9684.
Hassan, M. U., Chattha, M. U., Khan, I., Chattha, M. B., Barbanti, L., Aamer, M., & Aslam, M. T. (2020). Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies—A review. Plant Biosystems—An International Journal Dealing with All Aspects of Plant Biology, 1–56. https://doi.org/10.1080/11263504.2020.1727987.
Haworth, M., Marino, G., Brunetti, C., Killi, D., De Carlo, A., & Centritto, M. (2019). The impact of heat stress and water deficit on the photosynthetic and stomatal physiology of olive (Oleaeuropaea L.)—A case study of the 2017 heat wave. Plants, 7(4), 76.
Hein, N. T., Wagner, D., Bheemanahalli, R., Šebela, D., Bustamante, C., Chiluwal, A., et al. (2019). Integrating field-based heat tents and cyber-physical system technology to phenotype high night-time temperature impact on winter wheat. Plant Methods. https://doi.org/10.1186/s13007-019-0424-x.
Hemantaranjan, A., NishantBhanu, A., & Singh, M. N. (2014). Heat stress responses and thermo- tolerance. Advances in Plants Agriculture Research, 1(3), 62–70.
Herzog, H., & Chai-Arree, W. (2012). Gas exchange of five warm-season grain legumes and their susceptibility to heat stress. Journal of Agronomy and Crop Science, 198, 466–474. https://doi.org/10.1111/j.1439-037X.2012.00517.x.
Huang, H., Ullah, F., Zhou, D.-X., Yi, M., & Zhao, Y. (2019). Mechanisms of ROS regulation of plant development and stress responses. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2019.00800.
Ibrahim, A. M. H., & Quick, J. S. (2001). Genetic control of high temperature tolerance in wheat as measured by membrane thermal stability. Crop Science, 41(5), 1405–1407.
Impa, S. M., Vennapusa, A. R., Bheemanahalli, R., Sabela, D., Boyle, D., Walia, H., et al. (2019). High night temperature induced changes in grain starch metabolism alters starch, protein and lipid accumulation in winter wheat. Plant, Cell and Environment. https://doi.org/10.1111/pce.13671.
Inghelandt, D. V., Frey, F. P., Ries, D., & Stich, B. (2019). QTL mapping and genome-wide prediction of heat tolerance in multiple connected populations of temperate maize. Scientific Reports. https://doi.org/10.1038/s41598-019-50853-2.
Intergovernmental Panel on Climate Change (IPCC). Climate change (2007)—The physical science basis. In Contribution of working group I to the fourth assessment report of the intergovernmental. Panel on climate change. Cambridge: Cambridge University Press, 2007.
Iseki, K., & Olajumoke, O. (2020). A new indicator of leaf stomatal conductance based on thermal imaging for field grown cowpea. Plant Production Science, 23(1), 36–147.
Jagadish, S. V. K. (2020). Heat stress during flowering in cereals—Effects and adaptation strategies. New Phytologist. https://doi.org/10.1111/nph.16429.
Jagadish, S. V. K., Bahuguna, R. N., Djanaguiraman, M., Gamuyao, R., Prasad, P. V. V., & Craufurd, P. Q. (2018). Implications of high temperature and elevated CO2 on flowering time in plants. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2016.00913.
Jagadish, S. V. K., Craufurd, P. Q., & Wheeler, T. R. (2007). High temperature stress and spikelet fertility in rice (Oryza sativa L.). Journal of Experimental Botany, 58, 1627–1635.
Jagadish, S. V. K., Muthurajan, R., Oane, R., Wheeler, T. R., Heuer, S., Bennett, J., et al. (2010). Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). Journal of Experimental Botany, 61(1), 143–156. https://doi.org/10.1093/jxb/erp289.
Janni, M., Gullì, M., Maestri, E., Marmiroli, M., Valliyodan, B., Nguyen, H. T., et al. (2020). Molecular and genetic bases of heat stress responses in crop plants and breeding for increased resilience and productivity. Journal of Experimental Botany. https://doi.org/10.1093/jxb/eraa034.
Jha, U. C., Chaturvedi, S. K., Bohra, A., Basu, P. S., Khan, M. S., & Barh, D. (2014). Abiotic stresses, constraints and improvement strategies in chickpea. Plant Breeding, 133, 163–178.
Jin, X., Shi, C., Yu, C. Y., Yamada, T., & Sacks, E. J. (2017). Determination of leaf water content by visible and near-infrared spectrometry and multivariate calibration in Miscanthus. Frontiers in Plant Science, 8, 721.
Jones, H. G., Serraj, R., Loveys, B. R., Xiong, L., Wheaton, A., & Price, A. H. (2009). Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Functional Plant Biology, 36, 978–989.
Joshi, A. K., Kumari, M., Singh, V. P., Reddy, C. M., Kumar, S., Rane, J., et al. (2007). Stay green trait: Variation, inheritance and its association with spot blotch resistance in spring wheat (Triticum aestivum L.). Euphytica, 153, 59–71. https://doi.org/10.1007/s10681-006-9235-z.
Kalaji, H. M., Jajoo, A., Oukarroum, A., Brestic, M., Zivcak, M., Samborska, I. A., et al. (2016). Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiologiae Plantarum, 38(4), 102.
Kalaji, H. M., Rastogi, A., Živèák, M., Brestic, M., Daszkowska-Golec, A., & Sitko, K. (2018). Prompt chlorophyll fluorescence as a tool for crop phenotyping: An example of barley landraces exposed to various abiotic stress factors. Photosynthetica, 56, 953–961. https://doi.org/10.1007/s11099-018-0766-z.
Kamal, Alnor Gorafi, Abdelrahman, Abdellatef, & Tsujimoto, (2019). Stay-green trait: A prospective approach for yield potential, and drought and heat stress adaptation in globally important cereals. International Journal of Molecular Sciences, 20(23), 5837. https://doi.org/10.3390/ijms2023583.
Kaushal, N., Awasthi, R., Gupta, K., Gaur, P. M., Siddique, K. H. M., & Nayyar, H. (2013). Heat-stress-induced reproductive failures in chickpea (Cicer arietinum) are associated with impaired sucrose metabolism in leaves and anthers. Functional Plant Biology, 40, 1334–1349.
Keeling, P. L., Bacon, P. J., & Holt, D. C. (1993). Elevated temperature reduces starch deposition in wheat endosperm by reducing the activity of soluble starch synthase. Planta, 191(3), 342–348.
Keeling, P. L., Banisadr, R., & Barone, L. (1994). Effect of temperature on enzymes in the pathway of starch biosynthesis in developing wheat and maize grain. Australian Journal of Plant Physiology, 21(6), 807–827.
Khan, A. I., Khan, I. A., & Sadaqat, H. A. (2008). Heat tolerance is visible in cotton (Gossypium hirsutum L.) and can be exploited for breeding of better yielding cultivars under high temperature. Pakistan Journal of Botany, 40, 2053–2058.
Kilasi, N. L., Singh, J., Vallejos, C. E., Ye, C., Jagadish, S. V. K., Kusolwa, P., et al. (2018). Heat stress tolerance in rice (Oryza sativa L.): Identification of quantitative trait loci and candidate genes for seedling growth under heat stress. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2018.01578.
Kim, C., Kim, S. J., Jeong, J., Park, E., Oh, E., Park, Y., et al. (2020). High ambient temperature accelerates leaf senescence via PHYTOCHROME-INTERACTING FACTOR 4 and 5 in Arabidopsis. Molecules and Cells, 43(7), 645–661. https://doi.org/10.14348/molcells.2020.0117.
Kipp, S., Mistele, B., & Schmidhalter, U. (2014). Identification of stay-green and early senescence phenotypes in high-yielding winter wheat, and their relationship to grain yield and grain protein concentration using high throughput phenotyping techniques. Functional Plant Biology, 41, 227–235. https://doi.org/10.1071/FP1322.1.
Kosova, K., Vitamvas, P., Prasil, I. T., & Renaut, J. (2011). Plant proteome changes under abiotic stress-contribution of proteomics studies to understanding plant stress response. Journal of Proteome, 74, 1301–1322. https://doi.org/10.1016/j.jprot.2011.02.006.
Kumar, N., Nandwal, A. S., Waldia, R. S., Kumar, S., Devi, S., Singh, S., et al. (2013a). High temperature tolerance in chickpea (Cicer arietinum) genotypes as evaluated by membrane integrity, heat susceptibility index and chlorophyll fluorescence techniques. Indian Journal of Agricultural Sciences, 83(4), 467–471.
Kumar, S., Sairam, R. K., & Prabhu, K. V. (2013b). Physiological traits for high temperature stress tolerance in Brassica juncea. Indian Journal of Plant Physiology, 18, 89–93.
Kumar, P., Shah, D., & Singh, M. P. (2017). Evaluation of chickpea (Cicer arietinum L.) genotypes for heat tolerance: A physiological assessment. Indian Journal of Plant Physiology, 22, 164–177. https://doi.org/10.1007/s40502-017-0301-4.
Kumari, M., Pudake, R. N., Singh, V. P., & Joshi, A. (2012). Association of stay green trait with canopy temperature depression and yield traits under terminal heat stress in wheat (Triticum aestivum L.). Euphytica, 190, 87–97.
Li, G., Pan, J., Cui, K., Yuan, M., Hu, Q., Wang, W., et al. (2017). Limitation of unloading in the developing grains is a possible cause responsible for low stem non-structural carbohydrate translocation and poor grain yield formation in rice through verification of recombinant inbred lines. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2017.01369.
Li, Y.-T., Xu, W.-W., Ren, B.-Z., Zhao, B., Zhang, J., Liu, P., et al. (2020). High temperature reduces photosynthesis in maize leaves by damaging chloroplast ultrastructure and photosystem II. Journal of Agronomy and Crop Science. https://doi.org/10.1111/jac.1240.
Li, L., Zhang, Q., & Huang, D. (2014). A review of imaging techniques for plant phenotyping. Sensors, 14, 20078–20111.
Liu, Z., Shi, J., Zhang, L., & Huang, J. (2010). Discrimination of rice panicles by hyperspectral reflectance data based on principal component analysis and support vector classification. Journal of Zhejiang University science B, 11(1), 71–78.
Lopes, M. S., & Reynolds, M. P. (2012). Stay-green in spring wheat can be determined by spectral reflectance measurements (normalized difference vegetation index) independently from phenology. Journal of Experimental Botany, 63, 3789–3798. https://doi.org/10.1093/jxb/ers071.
Lu, T., Meng, Z., Zhang, G., Qi, M., Sun, Z., Liu, Y., et al. (2017). Sub-high temperature and high light intensity induced irreversible inhibition on photosynthesis system of tomato plant (Solanum lycopersicum L.). Frontiers in Plant Science, 08, 365.
Lucas, M. R., Ehlers, J. D., Huynh, B. L., Diop, N. N., Roberts, P. A., & Close, T. J. (2013). Markers for breeding heat-tolerant cowpea. Molecular Breeding, 31, 529–536.
Manavalan, L. P., Guttikonda, S. K., Tran, L. S., & Nguyen, H. T. (2009). Physiological and molecular approaches to improve drought resistance in soybean. Plant and Cell Physiology, 50, 1260–1276.
McNellie, J. P., Chen, J., Li, X., & Yu, J. (2018). Genetic mapping of foliar and tassel heat stress tolerance in maize. Crop Science, 58(6), 2484. https://doi.org/10.2135/cropsci2018.05.0291.
Mehdi, R., Lamm, C. E., Anjanappa, R. B., Müdsam, C., Saeed, M., Klima, J., et al. (2019). Symplasmic phloem unloading and radial post-phloem transport via vascular rays in tuberous roots of Manihot esculenta. Journal of Experimental Botany, 70(10), 5559–5573.
Munns, R., James, R. A., Sirault, X. R., Furbank, R. T., & Jones, H. G. (2010). New phenotyping methods for screening wheat and barley for beneficial responses to water deficit. Journal of Experimental Botany, 61(13), 3499–3507.
Murchie, E. H., & Lawson, T. (2013). Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. Journal of Experimental Botany, 64(13), 3983–3998.
Nadeem, M., Li, J., Wang, M., Shah, L., Lu, S., Wang, X., et al. (2018). Unraveling field crops sensitivity to heat stress mechanisms, approaches, and future prospects. Agronomy, 8(7), 128.
Nahar, K., Ahamed, K. U., & Fujita, M. (2010). Phenological variation and its relation with yield in several wheat (Triticum aestivum L.) cultivars under normal and late sowing mediated heat stress condition. Notulae Scientia Biologicae, 2, 51–56.
Naveed, M., Ahsan, M., Akram, H. M., Aslam, M., & Ahmed, N. (2016). Measurement of cell membrane thermo-stability and leaf temperature for heat tolerance in maize (Zea mays L.): Genotypic variability and inheritance pattern. Maydica, 61(2), 7.
Neilson, E. H., Edwards, A. M., Blomstedt, C. K., Berger, B., Moller, B. L., & Gleadow, R. M. (2015). Utilization of a high-throughput shoot imaging system to examine the dynamic phenotypic responses of a C-4 cereal crop plant to nitrogen and water deficiency over time. Journal of Experimental Botany, 66, 1817–1832.
Nguyen, C. T., Singh, V., van Oosterom, E. J., Chapman, S. C., Jordan, D. R., & Hammer, G. L. (2013). Genetic variability in high temperature effects on seed-set in sorghum. Functional Plant Biology, 40(5), 439. https://doi.org/10.1071/fp12264.
Niu, Y., & Xiang, Y. (2018). An overview of Biomembrane functions in plant responses to high-temperature stress. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2018.00915.
Omasa, K., & Takayama, K. (2003). Simultaneous measurement of stomatal conductance, non-photochemical quenching, and photochemical yield of photosystem II in intact leaves by thermal and chlorophyll fluorescence imaging. Plant and Cell Physiology, 44(12), 1290–1300.
Osmond, B., Ananyev, G., Berry, J., Langdon, C., Kolber, Z., Lin, G., et al. (2004). Changing the way we think about global change research: Scaling up in experimental ecosystem science. Global Change Biology, 10, 393–407.
Ottaviano, E., Mulcahy, D. L., Sari Gorla, M., & Bergamini Mulcahy, G. (1992). Angiosperm pollen and ovules (pp. 355–358). New York: Springer. 10.1007/978-1-4612-2958-2.
Paproki, A., Sirault, X., Berry, S., Furbank, R., & Fripp, J. (2012). A novel mesh processing based technique for3D plant analysis. BMC Plant Biology, 12, 63–71.
Peacock, J. M., Soman, P., Jayachandran, R., Rani, A. V., Howarth, C. J., & Thomas, A. (1993). Effect of high soil surface temperature on seedling survival in pearl millet. Experimental Agriculture, 29, 215–225.
Penfield, S. (2008). Temperature perception and signal transduction in plants. New Phytologist, 179, 615–628. https://doi.org/10.1111/j.1469-8137.2008.02478.x.
Pérez-Bueno, M. L., Pineda, M., & Baron, M. (2019). Phenotyping plant responses to biotic stress by chlorophyll fluorescence imaging. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2019.01135.
Pinto, R. S., Molero, G., & Reynolds, M. P. (2017). Identification of heat tolerant wheat lines showing genetic variation in leaf respiration and other physiological traits. Euphytica. https://doi.org/10.1007/s10681-017-1858-8.
Prakash, P., Sharma-Natu, P., & Ghildiyal, M. C. (2003). High temperature effect on starch synthase activity in relation to grain growth in wheat cultivars. Indian Journal of Plant Physiology, 8, 390–398.
Prasad, P. V. V., Bheemanahalli, R., & Jagadish, S. K. (2017). Field crops and the fear of heat stress—Opportunities, challenges and future directions. Field Crops Research, 200, 114–121. https://doi.org/10.1016/j.fcr.2016.09.024.
Prasad, P. V., Boote, K. J., & Allen, L. H., Jr. (2006). Adverse high temperature effects on pollen viability, seed-set, seed yield and harvest index of grain sorghum [Sorghum bicolor (L.) Moench] are more severe at elevated carbon dioxide due to higher tissue temperatures. Agricultural and Forest Meteorology, 139, 237–251. https://doi.org/10.1016/j.agrformet.2006.07.003.
Qiu, G. Y., Omasa, K., & Sase, S. (2009). An infrared-based coefficient to screen plant environmental stress: Concept, test and applications. Functional Plant Biology, 36, 990–997.
Quinones, C., Mattes, N., Faronilo, J., & Jagadish, S. V. K. (2017). Drought stress reduces grain yield by altering the floral meristem development and sink size under dry-seeded rice cultivation. Crop Science, 57(4), 1–11.
Raessler, M., Wissuwa, B., Breul, A., Unger, W., & Grimm, T. (2010). Chromatographic analysis of major non-structural carbohydrates in several wood species—An analytical approach for higher accuracy of data. Analytical Methods, 2(5), 532. https://doi.org/10.1039/b9ay00193j.
Rahaman, M. M., Chen, D., Gillani, Z., Klukas, C., & Chen, M. (2015). Advanced phenotyping and phenotype data analysis for the study of plant growth and development. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2015.00619.
Rahman, M. A., Chikushi, J., Yoshida, S., & Karim, A. J. M. S. (2009). Growth and yield components of wheat genotypes exposed to high temperature stress under control environment. Bangladesh Journal of Agriculture Research, 34, 360–372. https://doi.org/10.3329/bjar.v34i3.3961.
Rane, J., & Nagarajan, S. (2004). High temperature index—For field evaluation of heat tolerance in wheat varieties. Agricultural Systems, 79, 243–255.
Rane, J., Pannu, R., Sohu, V. S., Saini, R. S., Mishra, B., Crossa, J., et al. (2007). Performance of yield and stability of advanced wheat genotypes under heat stress environments of the indo-gangetic plains. Crop Science. https://doi.org/10.2135/cropsci2006.07.0479.
Rane, J., Sharma, D., Ekatpure, S., Aher, L., Kumar, M., Prasad, S. V. S., et al. (2019). Relative tolerance of photosystem II in spike, leaf, and stem of bread and durum wheat under desiccation. Photosynthetica, 57(4), 1100–1108.
Reynolds, M., Manes, Y., Izanloo, A., & Langridge, P. (2009). Phenotyping approaches for physiological breeding and gene discovery in wheat. The Annals of Applied Biology, 155, 309–320.
Reynolds, M. P., Nagarajan, S., Razzaque, M. A., and Ageeb, O. A. A. (2001). Heat tolerance, in Application of Physiology in Wheat Breeding, eds M. P. Reynolds, and A. McNab (Mexico: CIMMYT), 124–135.
Ristic, Z., Bukovnik, U., & Prasad, P. V. V. (2007). Correlation between heat stability of thylakoid membranes and loss of chlorophyll in winter wheat under heat stress. Crop Science, 47, 2067–2073.
Rizza, F., Pagani, D., Gut, M., Prasil, I. T., Lago, C., & Tondelli, A. (2011). Diversity in the response to low temperature in representative barley genotypes cultivated in Europe. Crop Science, 51, 2759–2779. https://doi.org/10.2135/cropsci2011.01.0005.
Sage, T. L., Bagha, S., Lundsgaard-Nielsen, V., Branch, H. A., Sultmanis, S., & Sage, R. F. (2015). The effect of high temperature stress on male and female reproduction in plants. Field Crop Research, 182, 30–42. https://doi.org/10.1016/j.fcr.2015.06.011.
Salgotra, R. K., & Stewart, C. N. (2020). Functional markers for precision plant breeding. International Journal of Molecular Sciences, 21(13), 4792. https://doi.org/10.3390/ijms21134792.
Seginer, I. (1994). Transpirational cooling of a greenhouse crop with partial ground cover. Agricultural and Forest Meteorology, 71(3–4), 265–281.
Setimela, P. S., Andrews, D. J., Partridge, J., & Eskridge, K. M. (2005). Screening sorghum seedlings for heat tolerance using a laboratory method. European Journal of Agronomy, 23(2), 103–107.
Shanahan, J. F., Edwards, I. B., Quick, J. S., & Fenwick, J. R. (1990). Membrane thermostability and heat tolerance of spring wheat. Crop Science, 30, 247–251.
Shanmugavadivel, P. S., Amitha Mithra, S. V., Prakash, C., Ramkumar, M. K., Mohapatra, T., & Singh, N. K. (2017). High resolution mapping of QTLs for heat tolerance in rice using a 5 k SNP array. Rice. https://doi.org/10.1186/s12284-017-0167-0.
Sharma, D. K., Andersen, S. B., Ottosen, C. O., & Rosenqvist, E. (2012). Phenotyping of wheat cultivars for heat tolerance using chlorophyll a fluorescence. Functional Plant Biology, 39, 936–947.
Sharma, D., Rane, J., Gupta, V., Mamrutha, H., & Tiwari, R. (2016). Mapping quantitative trait loci associated with grain filling duration and grain number under terminal heat stress in bread wheat (Triticum aestivum L.). Plant Breeding, 135, 538–545.
Shi, W., Yin, X., Struik, P. C., Solis, C., Xie, F., & Schmidt, R. C. (2017). High day- and night-time temperatures affect grain growth dynamics in contrasting rice genotypes. Experimental Botany, 68, 5233–5245. https://doi.org/10.1093/jxb/erx344.
Shi, W., Yin, X., Struik, P., Xie, F., Schmidt, R., & Jagadish, S. V. (2016). Grain yield and quality responses of tropical hybrid rice to high night-time temperature. Field Crops Research, 190, 18–25. https://doi.org/10.1016/j.fcr.2015.10.006.
Singh, B., Mishra, S., Bohra, A., Joshi, R., & Siddique, K. H. M. (2018). Crop phenomics for abiotic stress tolerance in crop plants In. Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress Tolerance in Plants (pp. 277–296).
Singh, B. R., & Singh, O. (2012). Study of impacts of global warming on climate change: Rise in sea level and disaster frequency. Global Warming—Impacts and Future Perspective, Bharat Raj Singh, IntechOpen,. https://doi.org/10.5772/50464.
Sinsawat, V., Leipner, J., Stamp, P., & Fracheboud, Y. (2004). Effect of heat stress on the photosynthetic apparatus in maize (Zea mays L.) grown at control or high temperature. Environmental and Experimental Botany, 52, 129. https://doi.org/10.1016/j.envexpbot.2004.01.010.
Sofi, P., Ara, A., Gull, M., & Rehman, K. (2019). Canopy temperature depression as an effective physiological trait for drought screening, drought. Detection and Solutions. https://doi.org/10.5772/intechopen.85966.
Srinivasan, A., Takeda, H., & Senboku, T. (1996). Heat tolerance in food legumes as evaluated by cell membrane thermostability and chlorophyll fluorescence techniques. Euphytica, 88, 35–45.
Tahir, I. S. A., & Nakata, N. (2005). Remobilization of nitrogen and carbohydrate from stems of bread wheat in response to heat stress during grain filling. Journal of Agronomy and Crop Science, 191, 106–115. https://doi.org/10.1111/j.1439-037X.2004.00127.x.
Talukder, S. K., Babar, M. A., Vijayalakshmi, K., Poland, J., Prasad, P. V. V., Bowden, R., et al. (2014). Mapping QTL for the traits associated with heat tolerance in wheat (Triticum aestivum L.). BMC Genetics. https://doi.org/10.1186/s12863-014-0097-4.
Van der Tol, C., Berry, J. A., Campbell, P. K. E., & Rascher, U. (2014). Models of fluorescence and photosynthesis for interpreting measurements of solar induced chlorophyll fluorescence. Journal of Geophysical Research: Biogeosciences, 119, 2312–2327. https://doi.org/10.1002/2014JG002713.
Wahid, A., Gelani, S., Ashraf, M., & Foolad, M. R. (2007). Heat tolerance in plants: An overview. Environmental and Experimental Botany, 61, 199–223.
Wang, D. R., Wolfrum, E. J., Virk, P., Ismail, A., Greenberg, A. J., & McCouch, S. R. (2016). Robust phenotyping strategies for evaluation of stem non-structural carbohydrates (NSC) in rice. Journal of Experimental Botany, 67(21), 6125–6138. https://doi.org/10.1093/jxb/erw375.
Weerakoon, W. M. W., Maruyama, A., & Ohba, K. (2008). Impact of humidity on temperature induced grain sterility in rice (Oryza sativa L.). Journal of Agronomy and Crop Science, 194, 135–140.
Wei, H., Liu, J., Wang, Y., Huang, N., Zhang, X., Wang, L., et al. (2013). Dominant major locus in chromosome 9 of rice (Oryza sativa L.) confers tolerance to 48 °C high temperature at seedling stage. The Journal of Heredity, 104, 287–294.
Weirman, A. (2010). Plant phenomics teacher resource. www.plantphenomics.org.au/files/teacher/FinalPhenomicsforwordwith_image.doc.
Yadav, A. K., Narwal, M. S., & Arya, R. K. (2015). Genetic dissection of temperature tolerance in pearl millet (Pennisetumglaucum). Indian Journal of Agricultural Sciences, 81, 203–213.
Yang, W., Duan, L., Chen, G., Xiong, L., & Liu, Q. (2013). Plant phenomics and high-throughput phenotyping: Accelerating rice functional genomics using multidisciplinary technologies. Current Opinion in Plant Biology, 16, 180–187.
Yang, W., Feng, H., Zhang, X., Zhang, J., Doonan, J. H., Batchelor, W. D., et al. (2020). Crop phenomics and high-throughput phenotyping: Past decades, current challenges and future perspectives. Molecular Plant. https://doi.org/10.1016/j.molp.2020.01.008.
Zaidi, P. H., Zaman-Allah, M., Trachsel, S., Seetharam, K., Cairns, J. E., & Vinayan, M. T. (2016). Phenotyping for abiotic stress tolerance in maize—Heat stress. A field manual. Hyderabad: CIMMYT.
Zhou, R., Wu, Z., Wang, X., Rosenqvist, E., Wang, Y., & Zhao, T. (2018). Evaluation of temperature stress tolerance in cultivated and wild tomatoes using photosynthesis and chlorophyll fluorescence. Horticulture, Environment, and Biotechnology, 59, 499–509. https://doi.org/10.1007/s13580-018-0050-y.
Zhu, S., Huang, R., Wai, H. P., Xiong, H., Shen, X., He, H., et al. (2017). Mapping quantitative trait loci for heat tolerance at the booting stage using chromosomal segment substitution lines in rice. Physiology and Molecular Biology of Plants, 23(4), 817–825. https://doi.org/10.1007/s12298-017-0465-4.
Funding
Funding was provided by NICRA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Basavaraj, P.S., Rane, J. Avenues to realize potential of phenomics to accelerate crop breeding for heat tolerance. Plant Physiol. Rep. 25, 594–610 (2020). https://doi.org/10.1007/s40502-020-00552-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-020-00552-2