Abstract
Sugarcane has always witnessed a lower multiplication rate following conventional method of adoption for newly released variety on large scale by farmers. Tissue culture derived techniques proved as effective, reliable and alternative strategy for rapid production of quality planting materials. In vitro morphogenetic responses varies at genotype basis. Thus, an in vitro investigation was conducted to intensify the differential morphogenetic response through plant growth regulators and antibiotic supplementation in two sugarcane genotypes viz. Co 05011 and Co 0118. The cultures were initiated by explant inoculation on basal MS media and further transferred on different media formulations modified with BAP, Kn, TDZ and GA3. The best morphogenetic response was observed in media M21 [1.5 mg/L (BAP and Kn), 0.25 mg/L (TDZ and GA3)] in genotype Co 05011 whereas, genotype Co 0118 showed the best in media M22 [1.5 mg/L (BAP and Kn), 0.25 mg/L TDZ and 0.5 mg/L GA3]. The higher culture survival percent with sterile shoots was observed in 0.05% Cefotaxime fortified media. Findings of study suggests TDZ as an effective PGR for rapid and high frequency shoot regeneration and the supplementation of 0.05% Cefotaxime in media might enhance sterile culture percent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugarcane (Saccharum officinarum L.) is an economically crucial crop nurtured in the tropical and subtropical regions lying between latitudes 36.7° N to 31° S. The competency of sugarcane to carbon partitioning into sucrose upto 50% of its dry weight in its stalks (Botha and Black 2000) distinguish it among other crops for cultivation (Moore 1995; Kumar et al. 2019a). India comes at the second largest sugarcane producer country cultivating about 4.39 mha with cane production of 306.07 million tonnes (FAOSTAT 2017) that accounts 18.17% total world cane production and 27.25 million tones sugar production. In India, sugarcane is cultivated over the latitude 8° N to 33° S except cold hilly areas. Uttar Pradesh is the most sugarcane occupying state covering the area about 21.6 lakh ha with the production of about 31.52 million metric tonnes (Agricultural statistics, Govt. of India 2017). Agriculture is the most important part of Indian economy. Moreover, farmer families and large number of laborers are also involved in sugarcane cultivation.
The conventional propagation of sugarcane involve plantation of ‘setts’ in the form of seed. The limitation of these methods infers during selection and multiplication of superior developed genotypes. These methods require comparatively more time to produce sufficient quantity of seed material for newly developed varieties at commercial scale. Despite it, a large nursery space and large number of setts (40–60,000/ha) are required, so it is often difficult to ensure genetic purity and freedom from pathogens even after seed treatment (Tesfa et al. 2016). Therefore, the reduction in sugarcane productivity is mainly due to the non-availability of disease-free and true-to-type quality seed. Every year more than 35% of sugarcane crop is damaged due to the use of infected seed setts for sugarcane cultivation (Jalaja 2001). At present, the increasing demand for sugar and its products creating a thought-provoking condition for researchers and sugarcane growers. It is much important to maintain healthy cane supply to sustain sugarcane productivity. Thus, prior need is the accessibility of disease-free and quality planting materials to small-scale farmers, to fulfill the optimum production demands and rapid spread of newly released variety.
In vitro propagation has established as the most reliable tool for quick multiplication of true-to-type planting material, conservation, speediness the breeding process and rejuvenation of outstanding deteriorated varieties in sugarcane (Pathak et al. 2009; Lal et al. 2015). Raising of sugarcane plantlets through tissue culture reduces the time required for seed production at commercial scale and can be used as an efficient alternative for quality seed production (Getnet 2017; Kumari et al. 2017). The explant survival and shoot establishment are the key points of in vitro propagation which depend on several factors like explant crop age, effective sterilization and especially PGRs (plant growth regulators) requirements and the genotype. The PGRs are organic molecules which play a vital role in plant growth and differentiation (Chengalrayan and Gallo-Meagher 2001). These growth regulators stimulate cell division, stem elongation and differentiate cells into various tissues such as shoots and roots. The shoot regeneration studies using MS medium have been carried by many earlier researchers (Gallo-Meagher et al. 2000; Pathak et al. 2009; Lal et al. 2015; Kumari et al. 2017), but seeking for an improved protocol is always need. In sugarcane, large scope exists in identifying response to plant growth regulators under in vitro conditions, rapid succession and validating the efficacy repeatability of the techniques under field conditions. Moreover, limited information is available pertaining to the differential morphogenetic response with number of different cytokinins and cytokinin like plant growth regulators with antibiotic supplementation. Thus, an in vitro investigation was conducted to intensify differential response of plant growth regulators and antibiotic supplementation in two high yielding sugarcane genotypes viz. Co 05011 and Co 0118.
Materials and methods
Experimental plan and protocols
The present investigation was conducted at Tissue Culture Laboratory, Department of Biotechnology, College of Agriculture, S.V.P.U.A. & T., Meerut, U.P., India. The explants were collected for present investigation from ShriRam Sugarcane Research Farm, Modipuram, Meerut, UP, India. All the chemical formulations (Hi-Media, India) and glassware (Borosil, India) used under study were procured of tissue culture grade. MS medium was prepared and pH adjusted to 6.0, autoclaved at 121 °C and 15 lbs (101 kPa) for 20 min prior to 3 days of inoculation. The autoclaved media was poured into pre-sterilized culture tubes (150 × 25 mm) under laminar air flow hood. After three days, media was screened to check any contaminants growth. The nine months old sugarcane plant tops were collected, and excised into 6–8 cm long segments containing apical meristem shoot bud and thoroughly washed under running tap water for 30 min. Initially these spindles were treated with 0.5% Calcium Hypochlorite for 10 min and rinsed properly to remove any traces of detergent. Then these spindles were dipped into 0.1% Polyvinyl pyrrolidone (PVP) solution and further surface sterilization treatments were given under laminar air flow hood (Fig. 1a, b).
Surface sterilization and explant preparation
The excised spindles were surface sterilized as per the method described by Kumar et al. (2019b). The explants were treated with a combination of 0.1% Carbendazim + Mancozeb for 10 min followed by 0.1% mercuric chloride (HgCl2) for 5 min, 6% sodium hypochlorite (NaOCl) for 10 min and 70% ethanol (EtOH) for 1 min. The explants were washed three times with autoclaved de-ionized water after each treatment. The explants for inoculation on MS medium were prepared in aseptic manner by removing upper extended outer leaf sheaths remaining only upper meristem shoot tip (1.0–1.5 cm) (Fig. 1c–e).
In vitro inoculation
The prepared explants were placed onto semisolid MS medium (Murashige and Skoog 1962) along with BAP and Kn (0.5 mg/L each), 0.01% PVP, 0.04% myo-inositol, and 3% sucrose for shoot initiation (Fig. 1f). The initiated shoots were excised aseptically and transferred on media supplemented with different concentrations of benzylamino purine (BAP) (Fig. 2a, b). The best morphogenetic responding media containing BAP was used with various Kinetin (Kn) concentrations. The best responded media combination of BAP + Kn was further used with different concentrations of thidiazuron (TDZ) and so on with gibberellic acid (GA3) to intensify the differential morphogenetic responses of cultures. The details of plant growth regulators (PGRs) concentration and combinations with their media code is given in Table 1. Each time, the media combination that performed the best morphogenetic response was further tried with various concentration of next PGR. The obtained highly intensified media combination was further supplemented with various concentrations of two antibiotics viz., streptomycin and Cefotaxime (0.01, 0.02, 0.03, 0.04, 0.05 and 0.06%). All cultures were incubated in culture room under 4000 lux white fluorescent light, 65% RH and 25 ± 1 °C temp with 16 h light and 8 h of dark photoperiod as per standard protocol (Sengar et al. 2011). All culture vessels and surgical items were sterilized prior to the inoculation.
Data collection and statistical analysis
Data was recorded for shoot generation, culture survival after two weeks and for shoot multiplication, sterile culture after four weeks of culturing. Analysis of variance was performed to determine the presence and absence of significant differences among various groups followed by Tukey’s-b multiple range test using SPSS 20.0 statistical software package. The data was represented as Mean ± SE and p ≤ 0.05 was considered significant in each case.
Results and Discussion
During in vitro propagation, shoot establishment is the dependent of explants choice. The explant should have ability to differentiate into full plantlet. The explant collection was followed by a surface sterilization step (Fig. 1a, b). The different types of explants were used by earlier researchers to establish sugarcane culture such as auxiliary buds, meristems, leaf, cotyledonary portion and root etc. The upper shoot parts of plant contain more highly divisible capacity with meristematic cells and have ability to differentiate into each type of organ as per the environment provided for its growth (Kumar et al. 2019b). Mekonnen et al. (2014), Kaur and Kapoor (2017) reported shoot tip as the best explant for in vitro direct shoot regeneration in sugarcane at commercial scale. Under study, meristem shoot from nine months mature plants were used as explant and inoculated on basal MS medium for shoot initiation (Fig. 1a–f). Shoot tip is much safer and best explant source for in vitro propagation (Kumar et al. 2019b).
Differential morphogenetic response of PGRs
Tissue culture techniques derived plantlets provides rapid development of true-to-type genetically uniform and disease-free seedling that produce its maximum production potential (Lal et al. 2015; Kumari et al. 2017; Kumar et al. 2019a). After the discovery of plant growth regulators, the protocols were standardized for in vitro clonal propagation in various crops. In current study, four PGRs were tried to intensify the morphogenetic response in sugarcane genotypes Co 05011 and Co 0118 using various concentrations. Firstly, BAP (6-benzyl aminopurine), a cytokinin hormone was used with basal MS media in various concentrations (Table1). The media responded the best in accordance to shoot regeneration, shoot per culture and shoot length was used as control for next hormone combination. A total of four hormones containing three cytokinin (BAP, Kinetin, TDZ) and one gibberellin (GA3) were used and observations were noted after four weeks (Fig. 3a, b).
Observations on media containing BAP
Under study, the significantly (p < 0.05) higher shoot regeneration percent was observed in media M5 (63%) for genotype Co 05011 and in M4 (76.67%) for genotype Co 0118. A significantly (p < 0.05) higher shoot per culture (9.2 and 9.8) were noticed in media M4 in Co 05011 and Co 0118, respectively. No markedly changes (p > 0.05) in number of shoot per culture were shown in media M4 (9.2) and M5 (9) for genotype Co 05011 whereas, media M2 and M5 for genotype Co 0118. The significantly (p < 0.05) higher shoot length was recorded in media M2 (1.84 and 2.18 cm) followed by M3 (1.58 and 2.16 cm) for both the genotype Co 05011 and Co 0118, respectively. The media M3 and M4 containing 1.5 to 2.0 mg/L of BAP, respectively was the best media for morphogenetic response and produced 52.67 to 57.33% shoot regeneration, 8 to 9 shoot per culture and 1.5 to 1.6 cm shoot length in genotype Co 05011 whereas genotype Co 0118 showed 61.33 to 74.67% shoot regeneration, 7 to 9 shoot per culture with 2 to 2.16 cm shoot length as compared to other media formulations from media M0 to M6 (Table 2). Study results are in conformity with the findings of Mamun et al. (2004). The different response of genotype for same media or PGRs combination might be the result of different nutritional requirement of genotypes. It is also supported by Pant (2004) who described that nutritional requirement of each sugarcane genotype is different. Under study, the significant increase in shoot regeneration (63–65%) and number of shoot per culture (9–10) was observed with increasing concentration of BAP however shoot length (1.71) was found to be reduced. Ali et al. (2008) also reported higher shoot initiation (80%) on MS media supplemented with 2.5 mg/L BAP in sugarcane genotype CP-77–400 after 12 days.
Observations on media supplemented with BAP and Kn
The media M3 (1.5 mg/L BAP) and M4 (MS + 2.0 mg/L BAP) were found to the best for morphogenetic response according to the shoot regeneration percent, number of shoot per culture and shoot length, respectively (Table 2). These were selected as control media for next media formulations (MS + BAP + Kn) and coded M01 and M02, respectively. A significantly (p < 0.05) highly increased shoot regeneration percent in comparison to other media combinations was noted in media M8 (82.67%) for genotype Co 05011 and M9 (85.67%) for Co 0118. However, the media M9 also resulted in significantly (p > 0.05) similar shoot regeneration percent (77.67%) in Co 05011. A significantly (p < 0.05) higher shoots per culture were obtained in media M10 for Co 05011 (14.6) as well as Co 0118 (12.6). The media M9 was non-significantly (p > 0.05) differed with media M10 for number of shoots per culture in both genotypes. The media combinations M7, M8 and M11 did not differ significantly (p > 0.05) for both the genotypes in response to number of shoots per culture. The higher shoot length with significant (p < 0.05) difference was to be recorded in media M8 (3.11 and 3.52 cm) followed by M9 (2.72 and 3.2 cm) for both the genotype Co 05011 and Co 0118 (Table 3).
The observations recorded in media M02 to M15 were ranged from 53.33 to 85% shoot regeneration, 7 to 15.8 shoot/culture, 2.08 to 2.85 cm shoot length for genotype Co 05011 and 61.33 to 91.33% shoot regeneration, 6.4 to 13 shoot/culture and 2.22 to 4.05 cm shoot length in genotype Co 0118 (Table 4). A significantly (p < 0.05) higher increase in morphogenetic responses as compared to other media combinations was observed in media M13 for both genotypes Co 05011 and Co 0118. The media combination M12 and M14 was observed with significantly (p > 0.05) similar response in both genotypes. Results under study are in conformity with Khan and Rashid (2003). Study indicated that increase in concentration of Kn more than 1.5 mg/L while maintaining BAP 1.5 to 2.0 mg/L decreased the shoot regeneration percent, number of shoot per culture as well as shoot length in both genotype. Maintaining BAP concentration at 3.0 mg/L while stimulating the kinetin concentration from 1.5 to 2 mg/L expressively suppressed the initiated shoot percent (76.67 to 13.3%), number of shoot per explants (3.3 to 1.5) and average shoot length (4.58 to 1.22 cm), and suggested it might be the result of cell division inhibition or organogenesis (George et al. 2008).
Observations on media supplemented with BAP + Kn + TDZ
The observations noted for media combination MS + BAP + Kn were showed media M9 (MS + 1.5 mg/L each of BAP + Kn) as the best morpho-genetically responding media with comparatively higher number of shoot per culture and similar shoot length to media M13 (MS + 1.5 mg/L BAP + 2.0 Kn) (Tables 3 and 4) thus, next hormone combinations were tried by taking M9 as control media (M03) with various concentrations of TDZ. The significant changes in shoot regeneration percent, number of shoot/culture and shoot length was observed in media M03 to M20 (Table 5). The significantly (p < 0.05) higher percent shoot regeneration, shoot/culture and shoot length in comparison to other media combinations was showed in media M17 for genotype Co 05011 and media M16 for genotype Co 0118. The media M16 was also observed with significantly (p > 0.05) similar shoot regeneration percent in Co 05011. The media M17 and M18 did not differ significantly (p > 0.05) in response to number of shoots per culture for both the genotypes, similarly media M19 and M03 also showed non-significant changes in number of shoot per culture in both genotype Co 05011 and Co 0118. The difference in shoot length was found to be non-significant (p > 0.05) in media combination M19 (2.93 cm) as compared to M03 (3.11 cm) in Co 05011 whereas, genotype Co 0118 produced similar (p > 0.05) shoot length in media combinations M17 (4.3 cm), M18 (4.08 cm) and M19 (3.64 cm), M03 (3.52 cm) (Table 5).
Under study, it was observed that addition of TDZ in MS media significantly increased the number of shoots. The results under study are inconformity with the findings of Gallo-Meagher et al. (2000), Chengalrayan and Gallo-Meagher (2001) who described TDZ as the superior shoot producing cytokinin tested in sugarcane. Kumari et al. (2017) described pretreatment of explants with TDZ for higher and rapid shoot establishment. The shoot multiplication was very much higher with the increasing concentration of TDZ, but same time shoot length was decreased. Moreover, multiplied shoots were formed a clump at the media base and turned into yellowish-green color, that might be the result of limitation of nutrient availability or higher cytokinin concentration in media because shoot clump covered all the media surface in culture tube.
Observation on media supplemented with BAP + Kn + TDZ + GA3
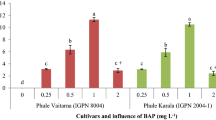
The obtained observations suggested media M16 as the best responding media in M03 to M20 thus, this media formulation was further used as control medium (M04) with GA3. The media combination M22 showed significantly higher (p < 0.05) percent of shoot regeneration in genotype Co 05011 and media combination M21 for Co 0118 (Fig. 3). The higher (p < 0.05) number of shoot per culture were recorded in media combination M21 with non-significant difference in both of the genotypes. However, shoot length (9.07 and 9.31 cm) was remarkedly higher (p < 0.05) in media combination M23 as compared to other media for both of the genotype Co 5011 and Co 0118, respectively (Table 6).
The results under study indicated, media M21 (1.5 mg/L each of BAP and Kn + 0.25 mg/L each of TDZ and GA3) as the best PGRs combination for genotype Co 05011 with 85.33% shoot regeneration, about of 14 shoot per culture and 6.33 cm shoot length. Whereas, media M22 (1.5 mg/L each of BAP and Kn + 0.25 mg/L TDZ + 0.5 mg/L GA3) was found to the best for genotype Co 0118 with 85.33% shoot regeneration, 12 shoot per culture and 8.48 cm shoot length. Study results are in conformity with Pathak et al. (2009) who had described use of various cytokinin combination for in vitro propagation in sugarcane varieties. Saini et al. (2004) also reported successful organogenesis in sugarcane in MS medium containing 1.0 mg/L GA3 and 1.0 mg/L Kn. It was noted that increased GA3 concentration resulted in lesser number of shoot per culture, however shoot length was positively influenced.
Study indicated that the genotypes response was different, even for same hormone combination. This different morphogenetic response for same PGRs combination might be the influence of diverse level of naturally occurring endogenous plant hormone and their interaction with exogenously supplied PGR. Moreover, it may be due to the different metabolic capability of genotype (George et al. 2008). The diverse concentration of receptor molecules in target tissue of genotype for PGR also determine the response potential of genotype.
Antibiotics supplementation
In tissue culture studies, mostly cultures are destroyed due to the contamination after several times of culturing that reduce the outcome. However, explants surface sterilization limits so much of contamination percent but looking for a better strategy is always required. So, media M21 and M22 which showed the best morphogenetic response were supplemented with 0.01 to 0.06% concentrations of two antibiotics (Streptomycin and Cefotaxime) to enrich culture survival and sterile culture (Table 7).
Under study, culture survival percent after two weeks in media containing streptomycin was ranged from 19.8 to 53.4% whereas media containing cefotaxime ranged from 35.6 to 90.8%. The media fortified with 0.05% cefotaxime showed significantly (p < 0.05) higher percent of culture survival (90.8%) after two weeks. The sterile culture percent after four weeks of sub-culturing was ranged from 34 to 79.8% in media containing streptomycin and 37.2 to 95.2% in cefotaxime fortified media. A significantly higher (p < 0.05) percent of sterile cultures (95.2%) were observed in 0.05% cefotaxime fortified media. However, media containing 0.04% of cefotaxime also showed significantly (p > 0.05) similar sterile culture (89.6%). A comparatively higher percent of sterile cultures (77.4 and 79.8%) with non-significant (p > 0.05) difference was noted in media containing 0.03% and 0.04% streptomycin. A remarked (p < 0.05) increase in culture survival percent with the increasing concentration of cefotaxime upto 0.05% suggested, supplementation of 0.05% cefotaxime positively influence culture establishment. Mittal et al. (2009) also reported a beneficial impact of cefotaxime on somatic embryogenesis and higher shoot regeneration.
Conclusion
The study findings suggested TDZ as the superior cytokinin for rapid shoot multiplication, and media M21 (1.5 mg/L each BAP and Kn + 0.25 mg/L each TDZ and GA3) as the best morpho-genetically responded PGR combination for genotype Co 05011 whereas, the media M22 (1.5 mg/L each BAP and Kn + 0.25 mg/L TDZ + 0.5 mg /L GA3) was the best for genotype Co 0118. Addition of 0.05% Cefotaxime in media can positively influence the sterile culture percent.
References
Agricultural statistics, Government of India. (2017). Ministry of Agriculture & Farmers Welfare Department of Agriculture, Cooperation & Farmers Welfare Directorate of Economics and Statistics. www.agricoop.nic.in & https://eands.dacnet.nic.in. Retrieved June 06, 2019.
Ali, A., Naz, S., Siddiqui, F. A., & Iqbal, J. (2008). An efficient protocol for largescale production of sugarcane through micropropagation. Pakistan Journal of Botany,40, 139–149.
Botha, F. C., & Black, K. G. (2000). Sucrose phosphate synthase and sucrose synthase activity during maturation of internodal tissue in sugarcane. Functional Plant Biology,27, 81–85.
Chengalrayan, K., & Gallo-Meagher, M. (2001). Effect of various growth regulators on shoot regeneration of sugarcane. Vitro Cell Developmental Biology of Plant,37, 434–439. https://doi.org/10.1007/s11627-001-0076-0.
FAOSTAT. (2017). Statistical division: production domain-Crops. Food and Agriculture Organization of the United Nations: FAO Statistical Databases. https://faostat.fao.org. Retrieved June 6, 2019.
Gallo-Meagher, M., English, R. G., & Abouzid, A. (2000). Thidiazuron stimulates shoot regeneration of sugarcane embryogenic callus. Vitro Cell Developmental Biology of Plant,36, 37–40. https://doi.org/10.1007/s11627-000-0009-3.
George, E. F., Hall, M. A., & Klerk, G. D. (2008). Plant Propagation by Tissue Culture 3rd Edition, Volume 1 (pp. 175–205). The Netherlands: Springer.
Getnet, B. (2017). Review on in-vitro propagation of sugarcane to advance the value of tissue culture. Agricultural Research and Technology: Open Access Journal,5(4), 555670.
Jalaja, N. C. (2001). Micropropagation of sugarcane varieties for quality seed production. Manual on Sugarcane Production Technology (pp. 48–52). Coimbatore: Sugarcane Breeding Institute.
Kaur, R., & Kapoor, M. (2017). In vitro direct plant regeneration using shoot tip explants in sugarcane (Saccharum officinarum L.) for rapid mass cloning. Agricultural Science Digest,37(2), 94–99. https://doi.org/10.18805/asd.v37i2.7981.
Khan, M. R., & Rashid, H. (2003). Studies on the rapid clonal propagation of Saccharum officinarum. Pakistan Journal of Biological Science,6(22), 1876–1879.
Kumar, D., Malik, N., & Sengar, R. S. (2019a). Physio-biochemical insights into sugarcane genotypes under water stress. Biological Rhythm Research. https://doi.org/10.1080/09291016.2019.1587838.
Kumar, D., Sengar, R. S., Yadav, M. K., Chand, P., Singh, G., & Gupta, S. (2019b). Evaluation of sterilant effect on in-vitro culture establishment in sugarcane variety Co 0118. International Journal of Current Microbiology and Applied Sciences,8(7), 1226–1233. https://doi.org/10.20546/ijcmas.2019.807.146.
Kumari, K., Lal, M., & Saxena, S. (2017). Enhanced micropropagation and tiller formation in sugarcane through pretreatment of explants with thidiazuron (TDZ). 3Biotech,7, 282. https://doi.org/10.1007/s13205-017-0910-7.
Lal, M., Tiwari, A. K., Gupta, G. N. N., & Kavita, (2015). Commercial scale micropropagation of sugarcane: Constraints and remedies. Sugar Tech,17(4), 339–347.
Mamun, M. A., Skidar, M. B. H., Paul, D. K., Rehman, M. M., & Islam, M. (2004). In-vitro micropropagation of some important sugarcane varieties of Bangladesh. Asian Journal of Plant Sciences,3(6), 666–669.
Mekonnen, T., Diro, M., Sharma, M., & Tadesse, N. (2014). Protocol optimization for in-vitro mass propagation of two sugarcane (Saccharum officinarum L.) clones grown in Ethiopia. African Journal of Biotechnology,13(12), 1358–1368.
Mittal, P., Gosal, S. S., Senger, A., & Kumar, P. (2009). Impact of cefotaxime on somatic embryogenesis and shoot regeneration in sugarcane. Physiology and Molecular Biology of Plants,15(3), 257–265.
Moore, P. H. (1995). Temporal and spatial regulation of sucrose metabolism in the sugarcane stem. Australian Journal of Plant Physiology,22, 661–679.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum,15, 473–497.
Pant, N. M. (2004). Sugarcane micropropagation (pp. 58–59). Pune: VSI.
Pathak, S., Lal, M., Tiwari, A. K., & Sharma, M. L. (2009). Effect of growth regulators on in vitro multiplication and rooting of Sugarcane. Sugar Tech,11(1), 80–82.
Saini, N., Saini, M. L., & Jain, R. K. (2004). Large scale production, field performance and RAPD analysis of micropropagated sugarcane plants. Indian Journal of Genetics and Plant Breeding,64, 102–107.
Sengar, K., Sengar, R. S., & Garg, S. K. (2011). The effect of in-vitro environmental conditions on some sugarcane varieties for micropropagation. African Journal of Biotechnology,10(75), 17122–17126.
Tesfa, M., Admassu, B., & Bantte, K. (2016). In-vitro rooting and acclimatization of micropropagated elite sugarcane (Saccharum officinarum L.) Genotype- N52 and N53. Journal of Tissue Science and Engineering,7, 164. https://doi.org/10.4172/2157-7552.1000164.
Acknowledgements
The authors extend their hearty gratitude to VC, SVPUA&T, Meerut for providing necessary facility to conduct this research work. Author is also grateful to his advisor Prof. R.S. Sengar, Head, Department of Agricultural Biotechnology for his advice and constant motivation to undertake all research activities.
Author information
Authors and Affiliations
Contributions
DK and NM: performed the experiment, RSS: designed and supervised the study. MKY, SG, PC, GS and PK: Associated and supervised study. DK and RSS: Analyzed the data and Wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, D., Sengar, R.S., Malik, N. et al. In vitro evaluation to intensify the differential morphogenetic response through plant growth regulators and antibiotic supplementation in sugarcane. Plant Physiol. Rep. 25, 335–346 (2020). https://doi.org/10.1007/s40502-020-00516-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-020-00516-6