Abstract
By spraying the exogenous polyamine spermidine (Spd) and the polyamine biosynthesis inhibitor d-arginine (d-Arg) in cut Paeonia lactiflora flowers, the dynamic change of superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA), soluble protein, endogenous polyamines and endogenous hormones were studied. The results showed that, the effect of exogenous polyamines and polyamine inhibitors could significantly affect the content of the vase life, which Spd decreased the vase life of flowers 1.4 days. d-Arg extended the vase life 1.8 days, improved the contents of soluble protein, activities of antioxidant enzymes, endogenous GA3, IAA, putrescine (Put), ZR and decreased the MDA content, endogenous ABA, Spd in cut herbaceous peony flowers. The effect of Spd and d-Arg might control the flower senescence through the way of affecting the hormones by affecting the endogenous polyamines or through the way of affecting the hormones and endogenous polyamines directly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paeonia lactiflora, commonly known as the herbaceous peony, is an important ornamental plant that is native to temperate Eurasia (Eason et al. 2002). It is widely distributed and cultivated in many regions, such as China, New Zealand, Turkey, Europe, North America and others (Jia et al. 2008; Walton et al. 2010). It is a famous flower-looking herbaceous plant with higher ornamental value at home and abroad owing to its beautiful colourful bouquet and abundant flower shapes. The petal colour ranges from white and cream through to a rarer lemon colour and to coral, pink, and red. Blooms may be single, semi-double, double, or bomb in form. In addition, features of strong adaptability and minimal care requirements make herbaceous peony widely used in the world.
Cut herbaceous peony flowers blossom in a short period and blossom period is concentrated. The short postharvest vase life is one of the most important problems of the cut flowers. The delay of natural postharvest senescence of cut flowers is very important to obtain high commercial benefit.
The senescence of cut flowers is complex and influenced by many internal factors, which is related to many complicated development course of physiological and biochemical reactions. Polyamines are mainly known for their essential roles in cell growth and proliferation, their functions range from a critical role in cellular translation (Michael 2016; Kumar et al. 2017). Polyamines (PAs) include spermine (Spm), putrescine (Put), spermidine (Spd) and cadaverine (Cad) which are low molecular weight cations synthesized in almost all biological systems including plants (Gupta et al. 2013; Nag and Choudhuri 2007; Altman et al. 1977; Kaur-Sawhney et al. 2003; Pandey et al. 2000; Tiburcio et al. 2014). In general, PAs levels have been reported to be high in actively growing tissues (Bachrach 1973; Galston and Kaur-Sawhney 1980; Murti 2003; Mattoo et al. 2010) and low in senescing tissues (Altman and Bachrach 1981; Fracassini et al. 1980; Nambeesan et al. 2010). Natural PAs are actively involved in events related to PCD (Kusano et al. 2008). As suppression in the levels of endogenous polyamines has been observed in senescing/aging leaves, so it is believed that they have a key role in controlling leaf senescence (Ten Chen and Kao 1991). A number of reports available show the role of PAs in delaying senescence of leaf, flower as well as fruit (Pandey et al. 2000). However the effects of polyamines in cut flowers are controversial, since Downs and Lovell (1986) reported that exgenously applied putrescine and spermidine had no effects on ethylene production or senescence of cut carnation flowers, Margrethe and Arne reported that spraying the plants with various PA concentrations (spermine, spermidine, or putrescine) or the PA synthesis inhibitor methylglyoxal guanylhydrazone did not affect postharvest life of Rosa hybrids L (Serek and Andersen 1994).
Materials and methods
Plant material, Spd and d-Arg application
Flowers of herbaceous peony ‘Qihualushuang’ were obtained from a local commercial flower center in Heze city early in the morning, cooled on the road with ice buckets and then transported by car to the laboratory within 10,800 s. The stems were selected following the industry standards for export grade flowers, i.e., buds were beginning to soften and outer petals had started to loosen and separate. Once in the laboratory, uniform and healthy cut flowers were selected and the flowers were recut underwater to a stem-length of 0.35 m to avoid air embolism, then picked up a randomized flower into 0.35 L triangle bottle containing distilled water. Every other day refreshed of the distilled water in the bottles.
Put these bottles within flowers divided into three groups, and spaying with 0.1 mM Spd, 0.1 mM d-Arg, or distilled water every morning and evening (8:00 a.m. and 18:00 p.m.). Spay the leaves with 0.35 L spay bottle. Throughout the experimental period, the flowers were held at 25 °C and 70–80% relative humidity (RH).
Measurements of vase life of flowers
Vase life was considered through symptoms such as crooked neck, bent neck or aging petals, full backing petals to the outside, and changing petals color and petals abscission which leads to a reduction in the attractiveness and marketability of flowers (Jowkar et al. 2012).
Measurements of the antioxidant enzymes activities in the flowers’ petals
Petals were selected from each treatment at 0, 2, 4, 6, 8 days of inserting for measurement 5 × 10−4 kg of flower tissue was suspended in 5 × 10−3 L of ice-cold HEPES buffer (25 mM, pH 7.8) containing 0.5 mM EDTA and 2% PVP. The homogenate was centrifuged at 4 °C and 100 r s−1 for 900 s and the resulting supernatants were used for the determination of SOD, CAT (Ramiro et al. 2006). The determination of SOD activity was performed at 5.6 × 10−7 m following Hwang et al. (1999). One unit of SOD activity was defined as the amount of enzyme that causes a 50% inhibition of the rate of nitroblue tetrazolium reduction. CAT activity was assayed at 2.4 × 10−7 m by measuring the conversion rate of hydrogen peroxide to water and oxygen molecules (Beers and Sizer 1952).
Measurements of (MDA) content in flowers’ petals
Petals were selected from each treatment at 0, 2, 4, 6, 8 days for MDA content measurement. MDA was extracted with 10% trichloroacetic acid and assayed at 4.5 × 10−7 m, 5.32 × 10−7 m and 6 × 10−7 m following the procedures that were described by Dhindsa et al. (1981) and modified by Xu et al. (2008).
Measurements of soluble protein in the petals of the flowers’ petals
Soluble protein was extracted with coomassie brilliant blue. Accurate 5 × 10−4 kg fresh leaves were put into mortar and ground with 5 × 10−3 L phosphate buffer solution and then transferred into centrifugal tubes. The solutions were centrifuged at 100 r s−1 for 900 s. Then the supernatant was extracted. 1 × 10−3 L supernatant was mixed with 5 × 10−3 L coomassie brilliant blue and then read at 5.95 × 10−7 m for soluble protein measurement.
Extraction and determination of endogenous hormone
3 × 10−4 kg freezing dry sample was accurately weighed, ground in ice water with 1.5 × 10−3 L 80% methanol, and then kept overnight at low temperature. The homogenate was centrifugated at 120 r s−1, 4 °C for 720 s, then the supernatant was collected. Additionally, the sediments were suspended with 0.01 L 80% methanol for 14,400 s and then centrifugated using the method mentioned above. The supernatants were mixed, decompressed, and condensed to 5 × 10−3 L at 37 °C, then the mixed solution was treated with decoloring by trichloromethane, and adjusted to pH 3.0, and extracted 3 times by adding is opyknic ethyl acetate, then decompressed and condensed the subjacent water phase dry at 50 °C, and washed out with 1 × 10−3 L. 0.1 M HCl and stored for testing; the pH value of the upper ethyl acetate phase was adjusted to 8.0 and removed polyphenolic compounds by adding insoluble polyvinyl pyrrolidone (PVPP), and then adjusted the pH value of the subjacent water phase to 3.0 and extracted it with ethyl acetate, decompressed and condensed the ethyl acetate dry at 37 °C, at last washed out with 1 × 10−3 L 50% methanol and stored for testing.
Endogenous hormones were determined by the P/ACE 5000 capillary electrophoresis (Beckman Co., USA). The testing conditions was following the method of Dong et al. (2009). The methanol used was chromatographically pure; ZR, GA3, ABA and IAA guide samples were provided by Sigma (USA); the rest chemicals were all analytical pure and home-made. Qualitative analysis was done based on the transition time of the samples and standard samples, and quantitative analysis was done using external demarcating method.
Extraction and quantification of endogenous polyamine
Polyamines were extracted with HClO4 and analyzed by the benzoylation method as previously reported by Serrano et al. (1991). Extracts for polyamine analysis were prepared by homogenizing 5 × 10−3 kg flowers’ petals in 5 × 10−3 L of 5% HClO4 using a mortar and pestle. The homogenate was then centrifuged for 1800 s at 330 r s−1 and 2 × 10−3 L of the supernatant was mixed with 2 × 10−3 L of 4 M NaOH and 2 × 10−2 L of benzoylchloride in an appropriate glass tube. After vortexing for 15 s, the mixture was incubated for 1,200 s at room temperature. Saturated NaCl and cold diethylether (4 × 10−3 L each) were then added. After that the tube content was vortexed for 15 s and incubated for 1800 s. Finally, 2 × 10−3 L of the ether phase that contained benzoyl-polyamines were evaporated under nitrogen and redissolved in 1 × 10−3 L of methanol (HPLC grade). Benzoyl-polyamines were analyzed by HPLC using a Waters system (Waters Associates, Milford, Mass.). The elution system consisted of methanol:water (64:36, v/v as solvent), run isocratically with a flow rate of 1.3 × 10−4 L s−1. The benzoyl-polyamines were eluted through a reverse phase column (LiChroCart 250–4, 5 µm; Merck, Darmstadt, Germany) and detected by absorbance at 2.54 × 10−7 m. A relative calibration procedure was used to determine the amounts of polyamines in samples using standard curves of putrescine, spermidine and spermine from Sigma (USA) and adding hexanediamine as the internal standard.
Statistical analysis
Data were expressed as means ± standard errors. Differences were tested with one-way ANOVA and the least significant difference (LSD) using SPSS 17.0. P values of < 0.05 were considered to be significant.
Results
Flower development
Flower development can be distinguished by two phases: flower bud slowly growing and flower decay rapidly while blooming. The plant’s life was divided into five different developmental stages, including a bud occurring colour stage (S1), a bud-soft stage (S2), an initial opening stage (S3), full opening stage (S4) and a begin wilting stage (S5) (Table 2). Visible senescence of Herbaceous peony cv. ‘Qihualushuang’ is petals falling, wilting, edge roll, and colourfading. These senescence symptoms were the same in intact plants and cut flowers placed in water (Fig. 1).
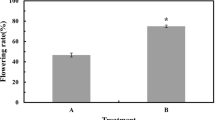
Effects of different treatments on vase life
The vase life in the cut flowers is shown in Table 1. The vase life with d-Arg treatment was 30.6% greater than that of the control, and significant difference was observed (P < 0.05). The result showed that the vase life of cut herbaceous peony flowers was extended by being sprayed with d-Arg, however, spaying Spd didn’t make effects to the vase life of flowers. Table 2 shows that the vase life was extended most in S1, S2 and S3.
Effects of different treatments on activities of SOD, CAT
As shown in Fig. 2, SOD activity increased within the first 4 days and then decreased rapidly. During the process of the flower blooming and senescence, SOD activity with the d-Arg treatment has been higher than the control group, while that with the Spd treatment was lower than the control group. The CAT activity in the flower senescence had the same change trend with SOD activity, which first rose up then decline. The results show that the SOD, CAT activity with d-Arg treatment were higher than the control group, while that with the Spd treatment was lower than the control group, which means, the d-Arg treatment was helpful for keeping up the SOD, CAT have higher ability of removing radicals.
Effects of different treatments on content of MDA
The MDA content in the vase flowers are shown in Fig. 3. The MDA content in the three treatments increased after 2 days of vase life. However, it was much lower in treatment with d-Arg comparison with that of the control and Spd treatments. The results indicate that d-Arg decreased the MDA content in the cut Herbaceous peony flowers.
Effects of different treatments on content of soluble protein
The content of soluble protein was increased in the 2 days, but decreased at mid-later stage (Fig. 4). Differences in soluble protein content were found among the three treatments. Soluble protein content in the petals enhanced progressively with d-Arg over control, while the Spd treatment decrease the protein content.
Effects of different treatments on content of endogenous plant hormones
By Fig. 5A, the IAA content in peony petals was the highest in the stage S1, then decreased with the senescence the flowers, after that the content increased only slightly to 37.61 ug kg−1, and eventually declined with a low content. The GA3 content in the stage S1 was high, then slow declined while flower blooming and eventually remained at a relatively low level (Fig. 5B). ZR content in the early stage of blooming fell to the lowest, and then slightly rebounded (Fig. 5C). ABA content in peony petal in the first stage only 60.1 ug kg−1, along with the flowers blooming, ABA content was on the rise, and increased slightly in the blooming period, after that the content began to rise sharply, to almost 3 times of that in the early stage (Fig. 5D).
Differences in the hormone content were still found among the three treatments. The trends in the IAA content between control treatment and d-Arg treatment changed in the same way, and IAA content was significantly improved by d-Arg application in the first 4 days. The IAA content with d-Arg treatment much higher than the control, while the Spd treatment made the IAA content stay at low level. The GA3 content with the Spd treatment group continued to decline and lower than controls, and the trend of GA3 content with d-Arg treatment changed later than control group, which also higher than control. The change trend of ZR content with d-Arg treatment was consistent with the control, the content was significantly higher than control; the ZR content with Spd treatment did not change significantly in 1–4 days, and the content was maintained at low levels after the fourth day. The ABA content changed consistently between the d-Arg treatment and control treatment, which the only difference were the content lower than the control with d-Arg treatment, while in the process, the Spd treatment increased the ABA content.
Effects of different treatments on polyamine levels
The major polyamines in the herbaceous peony petals were putrescine and spermidine. The level of spermine was very low, just on the limit of, and in some cases below detectable limits. The spermidine level was 753.1 umol kg−1 in early stage, substantially greater than that of putrescine (66.6 umol kg−1). As senescence progressed, a clear increase in putrescine level was noted, while the opposite was observed for the spermidine level (Fig. 6). During the last stages of senescence, a decrease in the putrescine level and an increase in spermidine was observed. The endogenous Spd was lower at beginning, then increased significantly higher than control. The variation trend of Put was the same between Spd-treated and control. The values of Put with d-Arg treatment was lower than control, only higher at last stage.
Discussion
Cut flowers’ limited shelf life hinder the development of floricultural industry. Postharvest technology applied to cut flowers is need to be studied to maintain the quality of these fresh products overtime. However, the physical, biochemical and genetic mechanisms underlying some of the processes central to cut flower are still not completely delineated until now (Fanourakis et al. 2013; Tripathi and Tuteja 2007). In the experiment, exogenous d-Arg at 0.1 mM had a marked effect on promoting vase life, activity of SOD and CAT, soluble protein content of herbaceous peony flowers, decreasing MDA content. In contrary, the treatments with Spd had a negative effect compared with control group. Activities of SOD and CAT represent antioxidant defense systems that can reduce lipid peroxidation and protect plants from ROS damage. In the present study, activities of SOD and CAT increased within the first 4 days. The possible reasons for the increases are that the growth environment changed suddenly when the flowers were cut from the whole plants, inducing the production of ROS. Thus the activities of SOD and CAT were stimulated to protect the flowers from ROS damage. The decrease of the activities of SOD and CAT at late vase time (Fig. 2) might be due to accumulation of ROS which in turn inhibited the ability of the antioxidant defense system, thus the activities of SOD and CAT decreased. MDA is an important index that reflects the extent of damage in plants faced with stress or senescence. The MDA content increased within senescence accompanied with a rapid increase in membrane permeability (Gao et al. 2010). In the present study, application of exogenous d-Arg markedly lowered MDA content in the petals of herbaceous peony flowers, compared to the control, which indicated lower tissue damage and slower senescence in the d-Arg treated herbaceous peony flowers.
During the flower senescence, petals outward by the metabolism library into a source output nutrients outward. In the process of plant growth and development, IAA, GAs, CTKs play an important role. The IAA, GA3, ZR were at a high level in the early stage of peony flower development, and ABA was in the lowest level, which would made effects to expedite cell elongation and transfer nutrients to the petals. Then, with the development of the senescence, activity of IAA, GA3 died away, and ABA had a large rise. The levels of cytokinins within a cell gradually declined as leaf senescence progresses, and there was a general consensus that high levels of cellular cytokinins delay leaf senescence (Kim et al. 2006; Lara et al. 2004; McCabe et al. 2001). The correlation between ABA and senescence is further confirmed by the up-regulation of both ABA and leaf senescence during environmental stresses (drought, high salinity and low temperature) (Tripathi and Tuteja 2007). Auxins, referrer to as generally plant development hormones, play a significant role in leaf senescence (Kim et al. 2011; Lim and Nam 2007; Sexton and Roberts 1982). Evidence came from up-regulation of some key auxin biosynthetic genes (Eric et al. 2006), like tryptophan synthase (TSA1), IAAld oxidase (AO1), and nitrilases (NIT1-3), and consequently increased auxin concentration in the senescing leaves.
The results showed that variation trend of Spd was the same between Spd-treated and control. The values of Spd with d-Arg treatment was lower than control, only higher at last stage. That indicated that d-Arg inhibited the accumulation of endogenous Spd (Fig. 6). In the present experiment, the change trend of the Spd/Put in accordance with the change trend of ZR/ABA, showed that polyamine may adjust the balance of hormones to regulate the senescence process.
Ethylene is a gaseous hormone which promotes fruit ripening and abscission and leaf and petal senescence. It has been demonstrated that the opening and senescence of many kinds of flowers are also correlated tightly to ethylene (Woltering and Van Doorn 1988). There were significant differences in ethylene production between the different cultivars in herbaceous peony. Ethylene production also showed different patterns between vased flowers and naturally opened at plants in cultivars (Jia et al. 2006). As there is apparently no experiment values in Endogenous ethylene production, inhibitor of ethylene synthesis and exogenous ethylene, we cannot be sure that the ethylene sensitivity of herbaceous peony. We also do not know the relationship between ethylene and endogenous hormone. That requires us test further experiments to study.
Conclusion
d-Arg enhanced activities of antioxidant enzymes, reduced MDA content in the petals, and maintained a longer period of vase time in cut herbaceous peony flowers. d-Arg also enhanced contents endogenous GA3, IAA, Put, ZR and decreased endogenous ABA, Spd in cut herbaceous peony flowers. While the Spd treatment exhibited negative impact, it promoted the senescence of cut flowers.
References
Altman, A., & Bachrach, U. (1981). Involvement of polyamines in plant growth and senescence. In: C. M. Caldarea., V. Zappia., U. Bachrach. (Eds.) Advances in Polyamine Research (Vol. 3, pp. 365–375). New York: Raven Press.
Altman, A., Kaur-Sawhney, R., & Galston, A. W. (1977). Stabilization of oat leaf protoplasts through polyamine-mediated inhibition of senescence. Plant Physiology, 60, 570–574.
Bachrach, U. (1973). Function of naturally occurring polyamines[M]. New York: Academic Press. Inc.
Beers, R. F., & Sizer, I. W. (1952). A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. Journal of Biological Chemistry, 195, 133–140.
Dhindsa, R. S., Plumb-Dhindsa, P., & Thorpe, T. A. (1981). Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany, 32, 93–101.
Dong, Q., Wang, J. Z., Guo, J. M., & Heng, Z. (2009). The relation between endogenous hormones and late-germination in buds of avrolles apple. Agricultural Sciences in China, 8, 564–571.
Downs, C. G., & Lovell, P. H. (1986). The effect of spermidine and putrescine on the senescence of cut carnations. Physiologia Plantarum, 66, 679–684.
Eason, J., Pinkney, T., Heyes, J., Brash, D., & Bycroft, B. (2002). Effect of storage temperature and harvest bud maturity on bud opening and vase life of Paeonia lactiflora cultivars. New Zealand Journal of Crop & Horticultural Science, 30, 61–67.
Eric, V. D. G., Rainer, S., Anja, S., Marcelo, D., Ulf-Ingo, F., & Reinhard, K. (2006). Transcription analysis of arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence. Plant Physiology, 141, 776–792.
Fanourakis, D., Heuvelink, E., & Carvalho, S. M. (2013). A comprehensive analysis of the physiological and anatomical components involved in higher water loss rates after leaf development at high humidity. Journal of Plant Physiology, 170, 890–898.
Fracassini, D. S., Bagni, N., Cionini, P. G., & Bennici, A. (1980). Polyamines and nucleic acids during the first cell cycle of Helianthus tuberosus tissue after the dormancy break. Planta, 148, 332–337.
Galston, A., & Kaur-Sawhney, R. (1980). Polyamines and plant cells. What’s New in Plant Physiol, 11, 5–8.
Gao, Y., Guo, Y. K., Lin, S. H., Fang, Y. Y., & Bai, J. G. (2010). Hydrogen peroxide pretreatment alters the activity of antioxidant enzymes and protects chloroplast ultrastructure in heat-stressed cucumber leaves. Scientia Horticulturae, 126, 20–26.
Gupta, K., Dey, A., & Gupta, B. (2013). Plant polyamines in abiotic stress responses. Acta Physiologiae Plantarum, 35(7), 2015–2036.
Hwang, S. Y., Lin, H. W., Chern, R. H., Lo, H. F., & Li, L. (1999). Reduced susceptibility to waterlogging together with high-light stress is related to increases in superoxide dismutase and catalase activities in sweet potato. Plant Growth Regulation, 27, 167–172.
Jia, N., Shu, Q. Y., Wang, D. H., Wang, L. S., Liu, Z. A., Ren, H. X., et al. (2008). Identification and characterization of anthocyanins by high-performance liquid chromatography–electrospray ionization–mass spectrometry in herbaceous peony species. Journal of the American Society for Horticultural Science, 133, 418–426.
Jia, P. Y., Zhou, L., Guo, W., & Dong, L. (2006). Postharvest behavior and endogenous ethylene pattern of tree-peony cut flowers. International Society for Horticultural Science. 27th international horticultural congress & exhibition, Seoul, Korea.
Jowkar, M. M., Kafi, M., Khalighi, A., & Hasanzadeh, N. (2012). Postharvest physiological and microbial impact of hydroxy quinoline citrate as ‘Cherry Brandy’rose vase solution biocide. Annals of Biological Research, 3, 2238–2247.
Kaur-Sawhney, R., Tiburcio, A. F., Altabella, T., & Galston, A. W. (2003). Polyamines in plants: An overview. Journal of Cell and Molecular Biology, 2, 1–12.
Kim, H. J., Ryu, H., Hong, S. H., Woo, H. R., Lim, P. O., Lee, I. C., et al. (2006). Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America, 103, 814–819.
Kim, J. I., Murphy, A. S., Baek, D., Lee, S. W., Yun, D. J., Bressan, R. A., et al. (2011). YUCCA6 over-expression demonstrates auxin function in delaying leaf senescence in Arabidopsis thaliana. Journal of Experimental Botany, 62, 3981–3992.
Kumar, M., Kuzhiumparambil, U., Ralph, P. J., et al. (2017). Polyamines[M]. Algal Green Chemistry
Kusano, T., Berberich, T., Tateda, C., & Takahashi, Y. (2008). Polyamines: Essential factors for growth and survival. Planta, 228, 367–381.
Lara, M. E. B., Garcia, M. C. G., Fatima, T., Ehneß, R., Lee, T. K., Proels, R., et al. (2004). Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. The Plant Cell, 16, 1276–1287.
Lim, P. O., & Nam, K. H. G. (2007). Leaf senescence. Annual Review of Plant Biology, 58, 115–136.
Mattoo, A. K., Minocha, S. C., Minocha, R., & Handa, A. K. (2010). Polyamines and cellular metabolism in plants: Transgenic approaches reveal different responses to diamine putrescine versus higher polyamines spermidine and spermine. Amino Acids, 38, 405–413.
McCabe, M. S., Garratt, L. C., Schepers, F., Jordi, W. J., Stoopen, G. M., Davelaar, E., et al. (2001). Effects of PSAG12-IPT gene expression on development and senescence in transgenic lettuce. Plant Physiology, 127, 505–516.
Michael, A. J. (2016). Polyamines in eukaryotes, bacteria and archaea. Journal of Biological Chemistry, 291(29), 14896.
Murti, G. S. R. (2003). Changes in the levels of common endogenous polyamines in the pericarp and seeds of mango fruits during development. Indian Journal of Plant Physiology, 8(2), 111–114.
Nag, S., & Choudhuri, M. A. (2007). Role of endogenous auxin and polyamines in adventitious root formation in mungbean cuttings. Indian Journal of Plant Physiology, 12(3), 228–233.
Nambeesan, S., Datsenka, T., Ferruzzi, M. G., Malladi, A., Mattoo, A. K., & Handa, A. K. (2010). Overexpression of yeast spermidine synthase impacts ripening, senescence and decay symptoms in tomato. The Plant Journal, 63, 836–847.
Pandey, S., Ranade, S. A., Nagar, P. K., & Kumar, N. (2000). Role of polyamines and ethylene as modulators of plant senescence. Journal of Biosciences, 25, 291–299.
Ramiro, D. A., Guerreiro-Filho, O., & Mazzafera, P. (2006). Phenol contents, oxidase activities, and the resistance of coffee to the leaf miner Leucoptera coffeella. Journal of Chemical Ecology, 32, 1977–1988.
Serek, M., & Andersen, A. S. (1994). Polyamine uptake does not affect floral longevity in miniature potted roses. HortScience, 29, 196–198.
Serrano, M., Romojaro, F., Casas, J., & Acosta, M. (1991). Ethylene and polyamine metabolism in climacteric and nonclimacteric carnation flowers. HortScience, 26, 894–896.
Sexton, R., & Roberts, J. A. (1982). Cell biology of abscission. Annual Review of Plant Physiology, 33, 133–162.
Ten Chen, C., & Kao, C. H. (1991). Senescence of rice leaves XXIX. Ethylene production, polyamine level and polyamine biosynthetic enzyme activity during senescence. Plant Science, 78, 193–198.
Tiburcio, A. F., Altabella, T., & Bitrián, M. (2014). The roles of polyamines during the lifespan of plants: From development to stress. Planta, 240(1), 1–18.
Tripathi, S. K., & Tuteja, N. (2007). Integrated signaling in flower senescence: An overview. Plant Signaling & Behavior, 2, 437–445.
Walton, E., Boldingh, H., McLaren, G., Williams, M., & Jackman, R. (2010). The dynamics of starch and sugar utilisation in cut peony (Paeonia lactiflora Pall.) stems during storage and vase life. Postharvest Biology and Technology, 58, 142–146.
Woltering, E. J., & Van Doorn, W. G. (1988). Role of ethylene in senescence of petals—Morphological and taxonomical relationships. Journal of Experimental Botany, 39, 1605–1616.
Xu, P. L., Guo, Y. K., Bai, J. G., Shang, L., & Wang, X. J. (2008). Effects of long-term chilling on ultrastructure and antioxidant activity in leaves of two cucumber cultivars under low light. Physiologia Plantarum, 132, 467–478.
Acknowledgements
This work was supported by China Agriculture Research System (Grant CARS-25), Nature Science Foundation of China (Grant No. 31070589) and Shandong Agricultural Engineering Forest Tree Breeding Project Corpus for High-grade Garden Flower Breeding (Grant No. 2130106).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, L., Sun, X., Xu, Jg. et al. The influence of polyamine and polyamine inhibitors in herbaceous peony postharvest physiology. Ind J Plant Physiol. 23, 499–506 (2018). https://doi.org/10.1007/s40502-018-0398-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-018-0398-0