Abstract
Antioxidative enzymes, non enzymic antioxidants and signaling molecules were compared in leaves and podwall of ten chickpea genotypes namely ICC 506, ICCV 10, ICC 10393, 5282, RSG 963, GL 25016, GL 26054, ICCL 86111, ICC 3137 and L 550 after Helicoverpa armigera infestation. Two chickpea genotypes (ICC 3137 and L 550) were found to be susceptible and rest of eight genotypes were found to be resistant on the basis of leaf and pod damage due to to Helicoverpa armigera infestation. The activities of defensive enzymes like, peroxidase (POD), ascorbate peroxidase(APX) and glutathione reductase (GR); content of nitric oxide (NO), 2,2-diphenyl-1-picryl hydrazyl (DPPH), ferric reducing antioxidant power(FRAP), glycine betaine (GB), total phenols (TP) and proline were higher in leaves and pod wall of resistant genotypes than susceptible genotypes. Genotype 5282 was found to be the most resistant having lower leaf and pod damage and it had higher POD, nitric oxide, DPPH, FRAP, total phenols and proline content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chickpea (Cicer arietinum L.) is the third most important pulse crop in the world after dry beans (Phaseolus vulgaris L.) and field pea (Pisum sativum L.) and had a total global production of 13.32 m tones from 13.57 m ha with an average productivity of 966.7 kg/ha (FAOSTAT 2014) and second most important legume crop after dry beans worldwide (Srivastava et al. 2015). It has an important place in human nutrition due to its high concentration of protein and carbohydrate and also used as a rotation crop, because of its N2-fixing features. Chickpea yields are quite low and remained almost stagnant for the past 2–3 decades due to different biotic and abiotic stresses. It is damaged by over 50 insect species in different parts of the world; of which the pod borer Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) remains the single most serious insect pest that causes significant yield losses of up to 80%, responsible for the major share of crop losses (US $4325 m) every year its management is very difficult due to its mobility, high polyphagy, short generation duration and high reproductive rate (Parde et al. 2012, Narayanamma et al. 2013). Therefore, there is need for alternative methods to control this pest. Host plant resistance plays an important role in pest management. These defensive mechanism can be constitutive i.e. present in the plant irrespective of any stress or inducible i.e. activated only when the plant is attacked. Insect herbivory change the metabolic machinery of the plant cell such as photosynthesis or respiration that leads to production of reactive oxygen species (ROS). The enhanced production of ROS can damage various biological molecules including nucleic acids, lipids and proteins (Gill et al. 2010). Plants have developed several enzymatic and non-enzymatic systems to protect against oxidative damage caused by these ROS that include superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), glutathione reductase (GR), glycine betaine, proline, DPPH (2,2-diphenyl-1-picryl hydrazyl), FRAP (Ferric Reducing Antioxidant Power) and total phenols (Verma et al. 2014). Chemical compounds playing an effective role in plant defense are produced and stored in tissues of the plants that are consumed by the herbivores (Hanley et al. 2007). Herbivore stressed plants produce active defense responses at the site of tissue damage and also systemically in undamaged tissues (Bostock 2005).
The potential role of defensive enzymes in the synthesis of defense compounds and in oxidative stress tolerance makes them an important weapon of plant resistance against insect herbivores. Screening of germplasm for resistance to insect pests has received considerable attention; however, there is limited progress in characterization of biochemical mechanisms conferring resistance to insects (Sharma et al. 2009). Therefore to develop cultivars with resistance to insects, it is important to understand the role of different components associated with defense mechanism. The genotypes with constitutive defense system need to be identified because they have the ability to combat oxidative stress caused due to insect infestation. The present study was designed to investigate the constitutive level of enzymatic and non enzymatic antioxidants in leaves and pod wall of different chickpea genotypes.
Materials and methods
Plant material and experimental design
Ten chickpea genotypes namely ICC 506, ICCV 10, ICC 10393, 5282, RSG 963, GL 25016, GL 26054, ICCL 86111, ICC 3137 and L 550 were sown on 1st November, 2014 at Research Farms of Pulses section, Department of Plant Breeding and Genetics, Punjab Agricultural University, Ludhiana in four rows each of 2.5 m row length with 30 × 15 cm spacing. The experiment was sown in Randomized Block Design with three replications by following the Package of Practices for chickpea crop recommended by Punjab Agricultural University, Ludhiana. The crop was kept unprotected and no spray was done for the control of insect pests.
Leaf and Pod damage
Leaf damage score was calculated by observing the leaf area damaged by Helicoverpa armigera on particular chickpea genotypes by using 1–9 scale; where 1 ≤ 10% leaf area damaged/eaten; 2 = 10–20% leaf area damaged/eaten; 3 = 21–30% leaf area damaged/eaten and 9 ≥ 80% leaf area damaged/eaten. After harvest of crop, the per cent pod damage was recorded by counting the total number of pods and pods damaged by the H. armigera by examining 200 pods from 10 randomly selected plants per replication per genotype. The damage caused by Helicoverpa armigera was calculated and converted into percent damage by using the following equation:
Per cent pod damage = (Number of damaged pods/Total number of pods) × 100.
The percent pod damage was converted into pest susceptibility/resistance (%) by using the formula derived from Abbott (1925):
Pest Susceptibility or Resistance (%) = (P.D. of check–P.D of test genotype)/P.D. check × 100
Where P.D. = Mean of per cent pod damage
Pest Susceptibility or Resistance percentage was converted to pest susceptibility/resistance rating (PSRR) scale (1–9) (Kooner and Cheema 2006).
The antioxidative defense enzymes (SOD, CAT, POD, APX, GR); non enzymatic antioxidants and signaling molecules were estimated in leaves at the time of flowering at 120 days after sowing (DAS), when 1st instar larvae of Helicoverpa armigera was feeding on the leaves. The uniform young pods were tagged and all the mentioned parameters were estimated in tagged pods at 140 DAS when the third instar larvae were feeding on them. The samples were collected early in the morning and brought to the laboratory in an ice bucket.
Enzyme extraction
The sample (100 mg) was extracted in prechilled pestle and mortar with 2 ml of ice cold 0.1 M potassium phosphate buffer (pH 7.5) containing 1 mM EDTA, 1% PVP, 10 mM β-mercaptoethanol. Homogenate was centrifuged at 10,000 × g at 4 °C for 25 min and clear supernatant was used for enzyme assay. All the enzymes were extracted with relevant extraction buffers at 4 °C to minimize denaturation and assayed at 30 °C.
Determination of Enzymatic antioxidants
SOD activity was assayed by using 1.4 ml of 0.1 mM Tris HCl buffer (pH 8.2), 0.5 ml of 6 mM EDTA, 1 ml of 6 mM pyrogallol solution and 0.1 ml of enzyme extract and absorbance was recorded at 420 nm using spectrophotometer after an interval of 30 s up to 3 min (Marklund and Marklund 1974). Catalase activity was measured using Chance and Maehly (1955) method and POD activity was assayed by using 3 ml of 0.05 M guaiacol, 20 µl of enzyme extract and 0.1 ml of 0.8 M H2O2. The reaction was initiated by adding H2O2 and rate of change in absorbance was recorded at 470 nm (Shannon et al. 1966). APX activity was measured by using the Nakano and Asada (1987) method and decrease in absorbance was recorded at 290 nm. GR activity was measured by using the method of Esterbaur and Grill (1978) and expressed as ηmoles of NADP+ formed/min/g protein.
Determination of non-enzymatic antioxidants and signaling molecules
Four hundred mg of sample was refluxed with 5 ml of 80% aqueous methanol for 1 h. The refluxed material was filtered and the volume was made to 10 ml by washing with hot 80% methanol. This extract was used for estimation of nitric oxide (NO), 2,2-diphenyl-1-picryl hydrazyl (DPPH), ferric reducing antioxidant power (FRAP), glycine betaine and total phenols. Nitric oxide was generated from sodium nitroprusside and the nitrite formed was measured by the Griess reaction (Marcocci and Packer 1994). DPPH free radical scavenging activity was measured by Blois (1958) method. FRAP activity is based on the ability of the sample to reduce Fe+3 to Fe+2 ions in the presence of 2,4,6-Tri-(2-pyridal)5-triazine (TPTZ) was measured (Benzie and Strain (1996). Glycine betaine was estimated by Grieve and Grattan (1983) and the concentration of glycine betaine was determined from betaine hydrochloride standards (50–100 μg) run simultaneously. Total phenols were estimated by using folin-phenol reagent (Swain and Hills 1959). Proline was extracted with 3% sulphosalicylic acid estimated by using ninhydrin reagent (Bates 1973). The procedure of Lowry et al. (1951) was used for determination of protein content.
Statistical analysis
Mean and standard deviation were calculated. The results of leaves and pod wall data were analyzed by Duncan’s multiple range tests to determine the significant differences (Software SPSS 20.0, P ≤ 0.05) and correlation analysis was carried out using MS Excel 2007.
Results and discussion
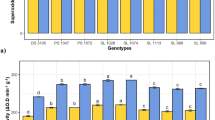
The leaf damage data was calculated for ten chickpea genotypes namely ICC 506, ICCV 10, ICC 10393, 5282, RSG 963, GL 25016, GL 26054, ICCL 86111, ICC 3137 and L 550. The pod damage data was available for all the genotypes except ICC 10393 and ICC 3137. Two chickpea genotypes namely ICC 3137 and L 550 were found to be susceptible due to higher leaf and pod damage in them by H. armigera as compared to other genotypes which were found to be resistant (Tables 1, 2). Leaf damage by H. armigera in ICC 3137 and L 550 was found to be 58–65% while in other genotypes it was varied from 16 to 37% (Table 1). Pod damage due to Helicoverpa armigera infestation in genotypes ICC 506, 5282 and GL 25016 was 9.67, 9.59 and 10.33% respectively, in RSG 963 and ICCL 86111 was 13.33 and 12.50% respectively, in ICCV 10 and GL 26054 was 17.43 and 17% respectively and in L 550 pod damage was found to be 35% (Table 2). During active period of H. armigera invasion in field antioxidative defense enzymes; non-enzymatic antioxidants and signaling molecules were estimated in leaves and pod wall of these genotypes. Reactive oxygen species (ROS) play an important role in plant defense against various pathogens (Mittler et al. 2004). Superoxide anion (O2•−), hydrogen peroxide (H2O2) and hydroxyl radical (OH•) are the three major forms of ROS. These molecules are highly reactive and toxic and can lead to the oxidative destruction of cells. Several mechanisms have been proposed for ROS generation in plants of which the NADPH-dependent oxidase system has received the most attention (Apel and Hirt 2004). NADPH-dependent oxidases catalyze the one-electron reduction of molecular oxygen to form O2•−, which then undergoes dismutation to form H2O2 either spontaneously or catalyzed by superoxide dismutases. NADPH-dependent oxidases are also linked with O2•− production in response to pathogen attack and wounding in plants (Razem and Bernards 2003). Superoxide dismutase plays an important role in plant stress tolerance and provides the first line of defense against the toxic effects of elevated levels of ROS (Gharari et al. 2014). It removes O2 •− by catalyzing its dismutation, one O2•− being reduced to H2O2 and another oxidized to O2. It removes O2•− and decreases the risk of OH• formation and hence avoid damage to the cell (Labudda and Azam 2014). The activity and specific activity of SOD in leaves of resistant chickpea genotypes varied from 289.72 to 580.24 units/min/g and 4.82–9.59 units/min/mg respectively and in susceptible genotypes varied from 432.22 to 509.63 units/min/g and 5.53–7.59 units/min/mg respectively and maximum SOD activity was observed in ICCL 86111 and minimum in ICC 10393 (Table 3). The activity and specific activity of SOD in pod wall of resistant chickpea genotypes varied from 35.41 to 64.95 units/min/g and 1.36–3.35 units/min/mg respectively and in susceptible genotype (L 550) SOD activity was 60.83 units/min/g and specific activity was 2.38 units/min/mg respectively (Table 4). Higher SOD activity was observed in leaves and pod wall of ICCL 86111. The activity and specific activity of CAT in leaves of resistant chickpea genotypes was 1.79 fold and 1.69 fold respectively higher than the susceptible genotypes (Table 3). The activity and specific activity of CAT in pod wall of resistant chickpea genotypes varied from 383.39 to 524.49 nmoles of H2O2 decomposed/min/g and 19.85–29.84 nmoles of H2O2 decomposed/min/mg respectively and in susceptible genotype (L 550) the activity was 466.04 nmoles of H2O2 decomposed/min/g and specific activity was 25.49 nmoles of H2O2 decomposed/min/g (Table 4). The activity and specific activity of POD in leaves of resistant chickpea genotypes was 41.02 and 44.02% respectively higher than susceptible genotypes (Table 3). But in genotype ICC 506, POD activity was lower as compared to susceptible genotype and maximum POD activity was observed in leaves of ICCL 86111. The activity and specific activity of POD in pod wall of resistant chickpea genotypes was found to be 60.00% higher than susceptible genotype (L 550) (Table 4). Peroxidase apart from detoxification of H2O2 also performs other diverse functions like production of semiquinone free radicals and quinones that acts as direct toxicants against insects (Zhu-Salzman et al. 2008). It also mediates the oxidation of hydroxylcinnamyl alcohols into free radical intermediates, oxidation of phenols, cross-linking of polysaccharides and monomers, lignification and suberization that lead to the production of antinutritive compounds (He et al. 2011). Thus the enhanced POD in leaves and pod wall of resistant chickpea genotypes might be enhancing the physical barrier of these genotypes to insect attack thereby minimizing the insect damage. Sharma et al. (2016) reported that enhanced activity of peroxidase and total phenols also contribute to resistance to Helicoverpa armigera infestation under increased levels of CO2.
The activity of APX in leaves of resistant chickpea genotypes varied from 15852.1 to 16849.6 nmoles of monodehydroascorbate formed/min/g and specific activity varied from 208.89 to 268.23 nmoles of monodehydroascorbate formed/min/mg respectively and in susceptible genotypes the activity and specific activity varied from 16228.9 to 16239.8 nmoles of monodehydroascorbate formed/min/g and 213.15–243.77 nmoles of monodehydroascorbate formed/min/mg respectively (Table 3). But in genotypes ICCV 10 and RSG 963, APX activity was lower than susceptible genotypes and no significant difference was observed in genotypes, 5282, GL 26054 and ICCL 86111. Average mean of APX activity and specific activity in pod wall of resistant chickpea genotypes was 10642.4 nmoles of monodehydroascorbate formed/min/g and 570.14 nmoles of monodehydroascorbate formed/min/mg respectively and in susceptible genotype (L 550) APX activity was 10292.3 nmoles of monodehydroascorbate formed/min/g and specific activity was 403.73 nmoles of monodehydroascorbate formed/min/mg respectively (Table 4). It was observed that higher APX activity in leaves and pod wall of resistant chickpea genotypes as compared to susceptible genotypes make them resistant towards Helicoverpa armigera. Ascorbate peroxidase reduces excessive H2O2 to water by utilizing ascorbic acid as the electron donor and also oxidizes phenolic compounds to quinones, which inhibit insect feeding (Gill and Tuteja 2010).
The activity and specific activity of GR in leaves of resistant chickpea genotypes was 2.23 fold and 2.01 fold respectively higher than the susceptible genotypes except in ICCV 10 and maximum GR activity was observed in GL 26054 and minimum in ICC 3137 (Table 3). The activity and specific activity of GR in pod wall of resistant chickpea genotypes varied from 115.41 to 405.19 nmoles of NADP+ formed/min/g and 5.11–17.14 nmoles of NADP+ formed/min/mg respectively and in susceptible genotype (L 550) GR activity was 355.27 nmoles of NADP+ formed/min/g and specific activity was 7.48 nmoles of NADP+ formed/min/g respectively (Table 4). GR activity was lower in genotype ICCV 10 as compared to susceptible genotypes and in genotypes RSG 963 and GL 25016 no significant difference was observed with susceptible genotypes. GR activity was greater in leaves and pod wall of resistant chickpea genotypes as compared to susceptible genotypes. Glutathione reductase is a potential enzyme of the ASH-GSH cycle and plays an essential role in defense system against ROS by sustaining the reduced status of GSH (Romero-Puertas et al. 2006).
Nitric oxide (NO) is an essential signal for mediating the defense against the insect attacks and act as a secondary messengers for the activation of defense-related genes and it can react with the free radical superoxide (O .−2 ) to form the reactive molecule peroxynitrite (ONOO−). NO and ROS are essential and closely connected signaling components in plant herbivory interactions (Scheler et al. 2013). Nitric oxide radical scavenging activity in leaves of resistant chickpea genotypes was 1.98 fold higher than susceptible genotypes (Table 5). Nitric oxide radical scavenging activity in pod wall of resistant chickpea genotypes was 32.53% higher than in susceptible genotype (Table 6). DPPH scavenging activity in leaves of resistant chickpea genotypes was 22.69% higher than susceptible genotypes (Table 5). Average mean of DPPH scavenging activity in pod wall of resistant chickpea genotypes was 48.59% and in susceptible genotype L 550 was 44.21% (Table 6). FRAP content in leaves of resistant chickpea genotypes varied from 9.61 to 14.82 mg/g and in susceptible genotypes ICC 3137 and L 550 were found to be 8.74 and 7.76 mg/g respectively (Table 5). FRAP content in pod wall of resistant chickpea genotypes varied from 3.39 to 5.80 mg/g and in susceptible genotype L 550 was 4.99 mg/g (Table 6). FRAP content was lower in genotypes ICC 506, ICCV 10, RSG 963 and GL 25016 as compared to susceptible genotypes. Antioxidants like DPPH and FRAP donate hydrogen to free radicals, leading to non toxic species and therefore to inhibition of the propagation phase of lipid peroxidation (Li et al. 2008). Kaur et al. (2015) reported increased DPPH activity in leaves, pod wall and seeds of pigeonpea indicated the upregulation of antioxidants upon Helicoverpa armigera feding. Average mean of glycine betaine in leaves of resistant chickpea genotypes was 2149.34 µg/g and in susceptible genotypes was found to be 1760.5 µg/g (Table 5). The content of glycine betaine was lower in ICCV 10, 5282 and RSG 963 as compared to susceptible genotypes. Glycine betaine content in pod wall of resistant chickpea genotypes was 27.07% higher than susceptible genotype (Table 6). Glycine betaine (GB) is a polyol which occurs in small families of higher plants, particularly in species adapted to dry and saline environments (Sarwar et al. 2006). Glycine betaine act as a signaling molecule in abiotic stress tolerance as it balances the osmotic pressure between outside and inside of cells to cope with osmotic stress and hence maintains turgor. However its role in biotic stress tolerance was not known. It was observed that pod wall of resistant chickpea genotypes has greater content of GB than susceptible genotypes.
Quinones formed by oxidation of phenols bind covalently to leaf proteins and inhibit the protein digestion in herbivores. Quinones also exhibit direct toxicity to insects. Alkylation of amino acids reduces the nutritional value of plant proteins for insects, which in turn negatively affects the insect growth and development (Bhonwong et al. 2009). The content of total phenols in leaves of resistant chickpea genotypes varied from 2.93 to 3.85 mg/g and in susceptible genotypes ICC 3137 and L 550 were found to be 3.13 and 3.01 mg/g respectively (Table 5). The content of total phenols in 5282, RSG 963, GL 25016 and GL 26054 was not significantly different than the susceptible genotypes. Average mean of total phenols in pod wall of resistant chickpea genotypes was 1.91 mg/g and in susceptible genotype L 550 was 1.21 mg/g (Table 6). Total phenols content was greater in resistant chickpea genotypes than susceptible genotypes.
Free proline has been proposed to act as an osmoprotectant, a protein stabilizer, a metal chelator, an inhibitor of lipid peroxidation and OH and 1O2 scavenger (Ashraf and Foolad 2007). The ability of proline to scavenge ROS and ability to inhibit ROS-mediated apoptosis can be an important function in response to cellular stress. Proline content in leaves of resistant chickpea genotypes was 1.63 fold higher than susceptible genotypes (Table 5). Proline content in pod wall of resistant chickpea genotypes was 2.57 fold higher than susceptible genotype (Table 6). Proline content was greater in leaves and pod wall of resistant chickpea genotypes.
Correlation coefficient (r) between enzymatic antioxidants, non enzymatic antioxidants and signaling molecules in leaves and pod wall with leaf and pod damage by H. armigera has been depicted in Table 6. Leaf damage by H. armigera is negatively correlated with DPPH, FRAP and proline (r = −0.86, r = −0.69, r = −0.72 respectively). As DPPH, FRAP and proline content was higher in leaves of resistant chickpea genotypes which mean these genotypes has higher scavenging activities than susceptible genotypes. Pod damage by H. armigera is negatively correlated with APX, glycine betaine and total phenols (r = −0.73, r = −0.73, r = −0.67 respectively). The higher content of antioxidative enzymes and non-enzymatic antioxidants in resistant chickpea genotypes than the susceptible genotypes makes them resistant towards Helicoverpa armigera feeding. The pod wall of resistant genotype namely 5282 has higher APX, nitric oxide, DPPH, FRAP, total phenols and proline content in it.
References
Abbott, W. S. (1925). A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, 18, 265–267.
Apel, K., & Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annual Review of Plant Biology, 55, 373–399.
Ashraf, M., & Foolad, M. R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and Experimental Botany, 59, 206–216.
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39, 205–207.
Benzie, I. F. F., & Strain, J. J. (1996). The ferric Reducing ability of plasma (FRAP) as a measure of antioxidant power. The FRAP assay. Analytical Biochemistry, 239, 70–76.
Bhonwong, A., Stout, M. J., Attajarusit, J., & Tantasawat, P. (2009). Defensive role of tomato polyphenol oxidase against cotton bollworm (Helicoverpa armigera) and beet armyworm (Spodoptera exigua). Journal of Chemical Ecology, 35, 28–38.
Blois, M. S. (1958). Antioxidant determinations by the use of a stable free radical. Nature, 181, 1199–1200.
Bostock, R. M . (2005). Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annu Rev Phytopathol, 43, 545–580.
Chance, B., & Maehly, A. C. (1955). Assay of catalase and peroxidases. Methods in Enzymology, 2, 764–775.
Esterbaur, H., & Grill, D. (1978). Seasonal variation of glutathione and glutathione reductase in needles of Picea abies. Plant Physiology, 61, 119–121.
FAOSTAT (2014) Food and Agriculture Organisation of the United Nations (FAO) Statistical Databases, http://faostat.fao.org.
Gharari, Z., Nejad, R. K., Band, R. S., Najafi, F., Nabiuni, M., & Irian, S. (2014). The role of Mn-SOD and Fe SOD genes in the response to low temperature in chs mutants of Arabidopsis. Turkish Journal of Botany, 38, 80–88.
Gill, R. S., Gupta, A. K., Taggar, G. K., & Taggar, M. S. (2010). Role of oxidative enzymes in plant defenses against insect herbivory. Acta Phytopathologica et Entomologica Hungarica, 45, 277–290.
Grieve, C. M., & Grattan, S. R. (1983). Rapid assay for the determination of water soluble quaternary ammonium compounds. Plant and Soil, 70, 303–307.
Hanley, M. E., Lamont, B. B., Fairbanks, M. M., & Rafferty, C. M. (2007). Plant structural traits and their role in antiherbivore defense. Perspect Plant Ecol, 8, 157–178.
He, J., Chen, F., Chen, S., Lv, G., Deng, Y., & Fang, W. (2011). Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation. Journal of Plant Physiology, 168, 687–693.
Kaur, R., Gupta, A. K., & Taggar, G. K. (2015). Induced resistance by oxidative shifts in pigeonpea (Cajanus cajan L.) following Helicoverpa armigera (Hubner) herbivory. Pest Management Science, 71, 770–782.
Kooner, B. S., & Cheema, H. K. (2006). Evaluation of pigeon pea genotypes for resistance to pod borer complex. Indian Journal of Crop Science, 1, 194–196.
Labudda, M., & Azam, F. M. S. (2014). Glutathione-dependent responses of plants to drought: a review. Acta Societatis Botanicorum Poloniae, 83, 3–12.
Li, Y., Jiang, B., Zhang, T., Mu, Z., & Liu, J. (2008). Antioxidant and free radical scanenging activities of chickpea protein hydrolysate (CPH). Food Chemistry, 106, 444–450.
Lowry, O. H., Rosebrough, N. I., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Marcocci, L., & Packer, L. (1994). Antioxidant action of Ginkgo biloba extract EGB 761. Methods in Enzymology, 234, 462–475.
Marklund, S., & Marklund, G. (1974). Involvement of superoxide anion radical in the autoxidation of pyragallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry, 47, 169–174.
Mittler, R., Vanderauwera, S., Gollery, M., & Breusegem, F. V. (2004). Reactive oxygen gene network of plants. Trends in Plant Sciences, 9, 490–498.
Nakano, Y., & Asada, K. (1987). Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell and Physiology, 28, 131–140.
Narayanamma, L. V., Sharma, H. C., Vijay, P. M., Gowda, C. L. L., & Sriramulu, M. (2013). Expression of resistance to the pod borer Helicoverpa armigera (Lepidoptera: noctuidae) in relation to high performance liquid chromatography fingerprints of leaf exudates of chickpea. International Journal of Tropical Insect Science, 33, 276–282.
Parde, V. D., Sharma, H. C., & Kachole, M. S. (2012). Protease inhibitors in wild relatives of pigeonpea against the cotton bollworm/legume pod borer Helicoverpa armigera. American Journal of Plant Sciences, 3, 627–635.
Razem, F. A., & Bernards, M. A. (2003). Reactive oxygen species production in association with suberization: evidence foe an NADPH-dependent oxidase. Journal of Experimental Botany, 54, 935–941.
Romero-Puertas, M. C., Corpas, F. J., Sandalio, L. M., Leterrier, M. Rodriguez-, Serrano, M., Rio, L. A., et al. (2006). Glutathione reductase from pea leaves: response to abiotic stress and characterization of the peroxisomal isozyme. New Phytology, 170, 43–52.
Sarwar, M. K. S., Ullh, I., Rahman, M. U., Ashraf, M. Y., & Zafar, Y. (2006). Glycine betaine accumulation and its relation to yield and yield components in cotton genotypes grown under water deficient condition. Pakistan Journal of Botany, 38, 1449–1456.
Scheler, C., Duner, J., & Astier, J. (2013). Nitric oxide and reactive oxygen species in plant biotic interactions. Current Opinion in Plant Biology, 16, 534–539.
Shannon, L. M., Kay, E., & Lew, J. Y. (1966). Peroxidase isozymes from horseradish roots I. Isolation and physical properties. Journal of Biological Chemistry, 241, 2166–2172.
Sharma, H. C., Sujana, G., & Rao, D. M. (2009). Morphological and chemical components of resistance to pod borer Helicoverpa armigera in wild relatives of pigeonpea. Arthropod Plant Interactions, 3, 151–161.
Sharma, H.C., War, A.R., Pathania, M., Sharma, S.S., Akbar, S.M.D., & Munghate, R.S. (2016). Elavated CO2 influences host plant defense response in chickpea against Helicoverpa armigera. Arthopod Plant Interactions, 10, 171–181.
Srivastava, S., Zheng, Y., Kudapa, H., Jagadeeswaran, G., Hivrale, V., Varshney, R. K., et al. (2015). High throughput sequencing of small RNA component of leaves and inflorescence revealed conserved and novel miRNAs as well as phasiRNA loci in chickpea. Plant Science, 235, 46–57.
Swain, T., & Hillis, W. E. (1959). Phenolic constituents of Prunus domestica. The qualitative analysis of phenolic constituents. Journal of Science Food and Agriculture, 10, 63–68.
Verma, K. K., Singh, M., Gupta, R. K., & Verma, C. L. (2014). Photosynthetic gas exchange, chlorophyll fluorescence, antioxidant enzymes and growth responses of Jatropha curcas during soil flooding. Turkish Journal of Botany, 38, 130–140.
Zhu-Salzman, K., Luthe, D. S., & Felton, G. W. (2008). Arthropod-induced proteins: broad spectrum defenses against multiple herbivores. Plant Physiology, 146, 852–858.
Acknowledgements
The authors are thankful to Pulses section, Department of Plant Breeding and Genetics, Punjab Agricultural University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, A., Grewal, S.K., Singh, R. et al. Defense system in chickpea genotypes differing in tolerance to Helicoverpa armigera infestation. Ind J Plant Physiol. 22, 324–331 (2017). https://doi.org/10.1007/s40502-017-0310-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-017-0310-3