Abstract
Markhamia tomentosa is used in ethno-medicine for the cure of common diseases such as malaria. There are currently few plants in their natural tropical habitats, as it is difficult to propagate. We have developed a simple protocol for in vitro regeneration of M. tomentosa using matured seeds and determined the secondary metabolites present in the in vitro-derived seedlings. Effects of two media types, i.e., Woody plant medium (WPM), and Murashige and Skoog (MS) basal medium with and without plant growth regulators, viz., 6-benzyl amino purine (BAP) and indole-3-butyric acid (IBA) @ 0, 1, 2, 3 mg l−1 were studied separately on in vitro germination and growth of seedlings. Seeds were surface disinfected, cultured in the above media and incubated at 27 °C and 16/8 light/dark photoperiod and a light intensity of 40 μmol m−2 s−1 provided by cool white fluorescent tubes prior to seeds germination. Results showed that the WPM alone produced significantly more germinated seeds (11.7) than MS (7.7) media. Significantly more germinated seedlings were produced on WPM with a combination of 1 mg l−1 BAP (10.7) or 2 mg l−1 IBA (9) than the control or other treatments. Anthraquinones were absent in the wild plants but were detected in the in vitro-derived plants (7.4 mg g−1 dry wt). This study provides the first report on a protocol for in vitro germination of this medicinally important species that could be applied to rescue it from extinction, as well as information on the phytochemical profile of the in vitro derived plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Markhamia (family Bignoniaceae) has narrow genetic diversity with only 6 species (Maroyi 2012). Among them, M. tomentosa Benth. K. Schum. ex Engl. is a tropical shrub or small tree of about 15 m height (Fig. 1A), found mostly in West Africa (Essien et al. 2013). It is used in ethno-medicine for the treatment of many diseases including diabetes (Soladoye et al. 2012), rheumatoid arthritis, oedema and as a cure of several microbial and parasitic diseases (Bouquets and Debray 1974; Arbonier 2002; Adjanohoun et al. 1996). The leaves and stem bark are also used to treat muscular pain (Burkill 1985). Several other reports showed anti-ulcer, anti-malarial, anti-cancer, anti-protozoal and anti-inflammatory activities of the phytochemical extract (Tantangmo et al. 2010; Sowemimo et al. 2013; Ibrahim et al. 2013; Shofidiya et al. 2014). Some anti-microbial, analgesic and antioxidant properties of M. tomentosa have also been reported (Aladesanmi et al. 2007; Tantangmo et al. 2010; Temdie et al. 2012). The phytochemical extracts are usually prepared as concoctions and decoctions, and administered either orally or topically on skin as remedies for various ailments (Okoli et al. 2007).
Markhamia tomentosa plant growing in the wild (A), mature fruits of M. tomentosa (B), mature seeds of M. tomentosa still in pods (C) and growth of seeds 4 weeks after initial culture on Woody Plant medium with 2 mg l−1 indole-3-butyric acid (D), and 2 mg l−1 indole-3-butyric acid and 1 mg l−1 6 benzyl amino purine (E, F)
The increasing problems of drug resistance associated with orthodox medicines and a generally increased cost of drugs in developing countries have lead to a huge dependence on ethno-medicines or plants and herbs of known medicinal value as these are relatively inexpensive. The indiscriminate harvest, habitat loss and poor propagation methods have rapidly accelerated the rate of disappearance of many important medicinal plants in the wild (Stem et al. 2005; Agyei 2010). The propagation of M. tomentosa is particularly challenging due to the low viability of the seeds. Plant tissue culture has been used for the rescue and propagation of several medicinal plant species (George and Sherrington 1984; Rout et al. 2000). However, to the best of our knowledge, there is no tissue culture protocol for M. tomentosa. An in vitro protocol would be of great importance towards both mass production and conservation. The objectives of this study were: (1) to establish a reliable protocol for the in vitro regeneration of M. tomentosa using mature seeds, specifically, the effects of two culture media types, viz., woody plant medium (WPM) (Lloyd and McCown 1980), and MS basal medium (Murashige and Skoog (1962) with and without plant growth regulators (PGR), 6-benzyl amino purine (BAP) and indole-3-butyric acid (IBA), and (2) to determine the phytochemical profile of the in vitro regenerated plants.
Materials and methods
Surface sterilization of explants
Mature fruits of M. tomentosa (Fig. 1B) were washed in running tap water to remove debris and then soaked for 2 min in 20 ml (v/v) fungicide solution (Fungusol Afrab-Chem Limited, Nigeria), followed by disinfection in 70% ethanol (Sigma Aldrich, Nigeria) for 2 min and 40% commercial bleach [Clorox® regular bleach (5.25% sodium hypochlorite)] for 15 min, and then rinsed 3 times in sterile distilled water (600 ml). Mature seeds (Fig. 1C) were excised from the surface-cleaned fruits before culture.
Culture medium and growth conditions
The excised seeds were planted under aseptic conditions on culture media containing either MS basal medium (Sigma Aldrich, product #M5519) @ 4.43 g l−1 or WPM (Sigma Aldrich, product #M6774) @ 2.3 g l−1 with 3% sucrose, 0.7% Agar (Sigma Aldrich, product #A1296) without PGR, and either of two PGRs, viz., BAP (0, 1, 2, 3 mg l−1) or IBA (0, 1, 2, 3 mg l−1). The most effective PGR concentrations of BAP and IBA, and culture medium (WPM or MS) were subsequently combined. Both PGRs were sourced from Sigma Aldrich (Bristol Scientific Company Limited Lagos, Nigeria). The pH of each medium was adjusted to 5.7 before adding agar. Each medium (25 ml) was dispensed into glass jars (7 × 7 cm, diameter) and autoclaved at a pressure of 15 psi and 121 °C for 15 min. The culture condition were, 16/8 h light/dark photoperiod at a temperature of 27 °C, and light intensity of approximately 40 µmol m−2 s−1 (LI-250A, LI-COR® Biosciences, USA), provided by cool white fluorescent bulbs (Eastar lighting, Zhejiang, China).
Germination data
Data on seed germination was taken at 4 weeks after initial culture of seeds. Cultured seeds were considered to have germinated with the emergence of the radical and or plumule. The number of shoots, roots, shoot length and root length per treatment were also recorded after 8 weeks of initial culture of seeds.
Extraction method
About 1 g of leaves of M. tomentosa collected from the wild and also in vitro-derived cultures (from each treatment) were air-dried at 30 ± 0.5 °C and pulverized to a coarse powder. The leaves were extracted by mixing with 4 L of distilled water and allowed to boil for 2 h. The resulting extract was left to cool and filtered. The filtrates were freeze dried, and the residues were stored at 4 °C prior to use for the phytochemical analysis.
Phytochemical profiling
Comparative tests for the screening and identification of bioactive chemical constituents of M. tomentosa plant in the wild and in vitro-derived cultures were carried out in aqueous extracts using the following standard procedures. For tannin contents, about 1 g of the extract from the plant sample was dissolved in 10 ml of distilled water and filtered. The filtrate was then treated with 10% ferric chloride solution, the appearance of a blue-black, or green coloured precipitate indicated the presence of tannins (Odebiyi and Sofowora 1978; Trease and Evans 2002).
For steroids estimation, about 0.2 g of plant extract was treated with chloroform and filtered. Few drops of acetic anhydride were added to the filtrates and then boiled. It was allowed to cool, followed by addition of sulphuric acid to form a lower layer or precipitate. Formation of a bluish green colouration indicated the presence of steroids (Sofowora 1993).
For terpenoids estimation, about 0.5 g of extract was treated with ethanol, 1 ml of acetic anhydride and concentrated sulphuric acid (H2SO4). It was vigorously shaken and allowed to settle. A yellowish colouration indicated the presence of terpenoids (Sofowora 1993).
For saponins content estimation, the plant extract of about 1 g was dissolved in distilled water in a test tube. It was shaken and filtered. A layer of foam which formed upon warming and continuous shaking for 12 min produced frothing which indicated the presence of saponins (Odebiyi and Sofowora 1978; Sofowora 1993).
For flavonoids estimation, about 1 g of plant extract was dissolved in acetone and warmed, while still hot, it was filtered and allowed to cool. One to two drops of lead acetate were added, followed by 1 ml of dilute hydrochloric acid. The resulting yellow solution indicated the presence of flavonoids (Sofowora 1993).
For glycosides content, about 5 ml of plant extract was mixed with 2 ml of glacial acetic acid with 1 drop of FeCl3. The mixture was added to 1 ml of concentrated H2SO4 so that the concentrated H2SO4 settled in the lower layer. The appearance of a brown ring colouration indicated the presence of cardiac glycoside (Trease and Evans 2002).
For alkaloids contents, about 0.2 g of plant extract was mixed with 5 ml of dilute hydrochloric acid and then filtered. About 1 ml each of this filtrate was dispensed into separate test tubes. Filtrates were treated with Mayer’s reagent (potassium mercuric iodide). The formation of a yellowish precipitate indicated the presence of alkaloids. Some filtrates were treated with Wagner’s reagent (containing potassium iodide). The appearance of a brownish red precipitate indicated the presence of alkaloids. A few drops of Dragendorff’s reagent was added resulting in red precipitate being formed, which is a positive confirmation of the presence of alkaloids (Sofowora 1993).
For cardiac glycosides (Keller-Killani), about 1 g of plant extract was diluted in water, followed by the addition of a mixture of 2 ml of glacial acetic acid and one drop of ferric chloride solution to the extract, which was then under layed with 1 ml of concentrated sulphuric acid. A brown ring formed at the interface which indicated the presence of deoxysugar, a component of cardenolides and then followed by a violet ring coloration. At the acetic acid layer, an appearance of greenish ring was formed which then spread throughout the layer (Trease and Evans 2002).
For anthraquinone, about 0.5 g of the plant extract was boiled with 10 ml of sulphuric acid and filtered while hot. The mixture was cooled and was extracted with equal volumes of benzene. The benzene layer was separated and treated with 5 ml ammonia solution. Appearance of a redish pink colouration in the ammoniacal layer indicated the presence of Anthraquinone (Sofowora 1993).
For phenolic compounds, plant extract of about 500 mg was dissolved in distilled water and then treated with 4–5 drops of ferric chloride solution. A dark green or bluish black color indicated the presence of phenolic compounds (Harborne 1973).
Quantitative phytochemicals analysis
For alkaloid determination, 5 g of plant sample was weighed and added into 200 ml of 10% acetic acid in ethanol. The mixture was kept for 4 h and then filtered. It was concentrated on a water bath. One to two drops of concentrated ammonium hydroxide were added to the concentrate until the precipitation was fully accomplished. The solution was allowed to stand and the precipitate was collected and washed with dilute ammonium hydroxide and then filtered. The residue is the alkaloid which was dried, weighed and percentage calculated (Harborne 1973).
For flavonoid estimation, about 10 g of plant sample was extracted with 100 ml of 80% methanol at room temperature, and then filtered through Whatman filter paper #42 (125 mm). The filtrate was allowed to evaporate to dryness over a water bath until a constant weight was achieved; the weight of the material and percentage quantity was calculated. All determinations were carried out in triplicates (Bohm and Kocipai-Abyazan 1994).
For saponin determination, 20 g of plant powder was added in 200 ml of 20% aqueous ethanol. The sample was continuously stirred for 4 h over a water bath at a temperature of 55 °C. The filtrate was re-extracted with another 200 ml of 20% ethanol. The resultant filtrate was allowed to evaporate to 40 ml over a water bath at 90 °C. About 20 ml of diethyl ether was added into the concentrate and shaken vigorously. The purification process was repeated on the aqueous layer. The combined n-butanol extracts was washed twice with 10 ml of 5% aqueous sodium chloride. The remaining solution was allowed to evaporate into dryness over a water bath and dry sample was kept in the oven until a constant weight was attained (Obdoni and Ochuko 2001). Saponin content was calculated as a percentage (Nahapetian and Bassiri 1975). Results were expressed in mg g−1 dry weight of plant powder.
For tannin determination, 50 ml of distilled water was added to 500 mg of finely ground sample which had initially been weighed into a 50 ml beaker. It was shaken for 1 h with the aid of a mechanical shaker. The extract was filtered using a double layered Whatman No. 1 filter paper into a 100 ml volumetric flask. One ml of sample extract was pipetted into 50 ml volumetric flask, and 2 ml of 0.1 M FeCl3 in 0.I N HCl and 0.008 M potassium ferrocyanide [K4Fe(CN)6] were added and mixed thoroughly. The absorbance of sample was measured at a wavelength of 120 nm within 10 min (Van-Burden and Robinson 1981). For anthraquinone determination, about 50 mg of the finely ground sample was soaked in 100 ml of distilled water for 18 h. The suspension was then heated in water bath at 70 °C for about an h. The suspension was left to cool, and 100 ml of 50% methanol was added to it and then filtered. The absorbance of clear solution was recorded by spectrophotometer at a wavelength of 450 nm. Comparison was done with a standard solution containing 1 mg per 100 ml alizarin and 1 mg per 100 ml purpurin with the absorption-maximum taken at 450 nm. For phenolic compounds determination, the extract of the sample weighing 2 g was defatted in 100 ml of diethyl ether using a soxhlet apparatus for 2 h. The fat free sample was boiled with 50 ml of ether for 20 min for the extraction of the phenolic compounds. About 10 ml of the extract was pipetted into a 50 ml flask and 15 ml distilled water, 2 ml of ammonium hydroxide solution and 5 ml of concentrated amyl alcohol were also added. The samples were made up to 50 ml in a flask and left to react for 30 min. Colour development was achieved and its absorbance was read at 505 nm.
Data collection and statistical analysis
Each experiment was a completely randomized factorial with three replicates. Each treatment including control had 20 seeds, and two seeds per culture vessel (n = 60). Data on seedling development or plant growth was subjected to student T test or the analysis of variance (ANOVA) using GENSTAT 2010 statistical package. Differences in means were grouped following DUNCAN multiple range tests at 5% level of probability. All quantitative phytochemical experiments were performed thrice, the results were averaged and reported in the form of mean ± standard error of mean.
Results and discussion
Effects of two media types (WPM and MS) without PGR on in vitro seed germination
Of the two media types (WPM and MS) without PGR, the WPM produced a significantly higher number of germinated seeds (11.7), each with a single shoot and root compared to MS (7.7) (Table 1). The shoot and root lengths were not significantly different between the media types in all the treatments.
Effects of plant growth regulators on seedling development
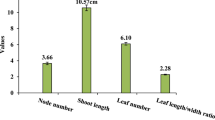
Treatment with 2 mg l−1 IBA and WPM produced a significantly higher number of germinated seeds (9) and number of roots (9) than other treatments (Table 2). The number of shoots, shoot and root lengths were not significantly different from their controls without IBA (Table 2). Seed germination was observed by 4 weeks of initial culture (Fig. 1D–E). When IBA was combined with MS, there were non-significant differences among the treatments (Table 3). The 1 mg l−1 BAP and WPM produced significantly higher germination of seeds (10.7) than other treatments (Table 4). Similarly, the number of shoots (9) and roots (10) were significantly higher at 1 mg l−1 BAP compared to other treatments (Table 4). A combination of BAP and MS produced non-significant differences in seed germination and seedling development among treatments (Table 5). The shoots appeared healthy and had good vigor under all treatments (Fig. 1F). A combination of 2 and 1 mg l−1 BAP with WPM produced plants with uniform shoot length (Fig. 2), however, the shoot lengths resulting from the above PGR combination (data not shown) were not significantly longer than when PGRs were added singly.
Many medicinally important plants are at risk of extinction. Seeds can be used as source of explant for tissue culturing, particularly for recalcitrant plants or plants with low seed viability. A simple protocol for the in vitro regeneration and rescue of M. tomentosa, a medicinally active shrub used in the treatment of malaria (Bankole et al. 2015) was developed using mature seeds. Other explants such as apical buds, leaves, shoot tips etc. could as well be used but in our preliminary tests, these alternative explants failed to establish in vitro due to high levels of contaminants (data not shown). Tissue culture techniques have earlier been used for the rescue and restoration of several other medicinal plant species (Essien et al. 2013). The success of a tissue culture procedure depends on many factors, including the physiological state of plants, source of explant material, age of explant, and the seed viability (Yildiz et al. 2012).
Blando et al. (2013) reported successful culturing of seeds of Eugenia myrtifolia Sims, a commercially important medicinal plant on MS medium with 2.5 M thidiazuron (TDZ). In our study treatments with BAP generally produced more germinated seeds than those with only IBA. Cytokinins are known to induce a break in seed dormancy, resulting in the formation of micro-shoots (Devi et al. 1994; Sujatha and Reddy 1998). Our studies also showed that WPM was better for the in vitro germination of seeds of M. tomentosa compared to MS (Table 1). This is possibly due to the differences in the media compositions of WPM and MS which may have impacted plant nutrient requirement. The MS basal medium has higher nitrate contents than WPM. The seeds of M. tomentosa normally take several months (12–24) to germinate ex vitro or under tropical field conditions (oral communication, Forestry Research Institute of Nigeria). However, under in vitro culture condition, germination of seeds was observed on the 4th week after initial culture (Fig. 1D–F) suggesting that the in vitro culture method is quicker for seedling production than traditional methods. This is likely due to inhibitors present in the seed pod that inhibit germination, which was circumvented by the use of PGR.
Phytochemical analysis
The phytochemical analysis indicated the presence of anthraquinones in the samples obtained from tissue culture-derived plants of M. tomentosa, while it was absent in the parent plant collected from the wild (Table 6). Most of the secondary metabolites assayed were present in both plant types (parent and in vitro-derived plants), except for cardiac glycosides, phlobatannins and steroids. The quantity of tannin in the wild plant (parent) was significantly higher (29 mg g−1 dry wt.) than the in vitro-derived plants (19 mg g−1 dry wt.). However, the quantities of anthraquinones was significantly higher in the in vitro-derived plants (7 mg g−1 dry wt.) compared to wild plants, which have none (Table 7). There were similar amounts of other secondary metabolites in both the plant types.
The phytochemical profiling of tissue culture-derived plants of M. tomentosa for the presence or absence of specific phytochemicals indicated the presence of anthraquinones, however, this was absent in plant samples collected from the wild (Table 6). Tantangmo et al. (2010) reported isolation of 2- acetylnaphtho [2,3-β] furan-4,9-dione and 2- acetyl-6-methoxynaphtho[2,3-β] furan-4,9-dione, a derivative of anthraquinones from the stem bark of M. tomentosa. It is not clear why anthraquinones was not detected in M. tomentosa samples collected from the wild. Probably the physiological status of the parent plants affected the bioavailability of anthraquinones. The results of this study indicated that in vitro manipulations with hormones could potentially improve the availability and quantity of phytochemicals in M. tomentosa. Similar findings have been reported by Luczkiewicz and Głod (2003), which suggested that secondary metabolites could change during tissue culturing. The types of PGRs in the culture medium could result in morphological, metabolic and physiological modifications in the regenerated plantlets (Van Staden et al. 2006) which in turn could change or modify the plant’s secondary metabolites (Patnaik et al. 1999). Plants that contain anthraquinones are useful for the preparation of special dyes and laxatives that are known to improve digestion (Müller-Lissner 1993). Anthraquinones may also help to reduce inflammation in arthritic patients and also has been shown to inhibit the growth of cancerous cells (Radha and Laxmipriya 2015; Sahu et al. 2013).
In conclusion, this study provided the first report on in vitro germination protocol for M. tomentosa for the purpose of regeneration and rescue of M. tomentosa. This protocol is directly applicable towards the large scale multiplication of clean plant materials to meet the increasing demand for quality planting materials and sources of tissues for phytochemical extraction by herbal drug industries, pharmaceutical companies and plant improvement programs.
References
Adjanohoun, J. E., Aboubakar, N., Dramane, K., Ebot, M. E., Ekpere, J. A., Enoworock, E. G., Focho, D., Gbile,. Z. O., Kamanyi, A., Kamsu, K. J., Keita, A., Mbenkum, T., Mbi, C. N., Mbiele, A. C., Mbome, J. C., Muberu, N. K., Nancy, W. L., Kongmeneck, B., Satabie, B., Sowora, A., Tamze, V., & Wirmum, C. K. (1996). Organization of African Unity. In Scientific, Technical and Research Commission (Ed). Traditional medicine and pharmacopoeia: Contribution to ethno botanical and floristic studies in Cameroon (p. 641). OAU/STRC.
Agyei, A. O. (2010). Bushfires and management policies in Ghana. Environment, 8, 221–228.
Aladesanmi, A. J., Iwalewa, E. O., Adebajo, A. C., Akinkunmi, E. O., Taiwo, B. J., Olorunmola, F. O., et al. (2007). Antimicrobial and antioxidant activities of some Nigerian medicinal plants. African Journal of Traditional and Complementary Alternative, 4, 173–184.
Arbonier, M. (2002). Trees, shrubs and lianas of West African dry zones (pp. 196–426). Utrecht: Cirad Margraf Publishers.
Bankole, A. E., Adekunle, A. A., Sowemimo, A. A., Umebese, C. E., Abiodun, O., & Gbotosho, G. O. (2015). Phytochemical screening and in vivo antimalarial activity of extracts from three medicinal plants used in malaria treatment in Nigeria. Parasitology Research, 115, 299–305.
Blando, F., Onlu, S., Colella, G., & Konczak, I. (2013). Plant regeneration from immature seeds of Eugenia myrtifolia Sims. In Vitro Cell and Development Biology-Plant, 49, 388–395.
Bohm, B. A., & Kocipai-Abyazan, R. (1994). Flavonoid and condensed tannins from the leaves of Vaccinum raticulation and Vaccinum calcyimium. Pacific Science, 48, 458–463.
Bouquets, A., & Debray, M. (1974). Medicinal plants of the Ivory Coast. Travaux et Doc de L’orstom, 32, 1–18.
Burkill, H.M. (1985) Entry for Markhamia tomentosa (Benth.) K. Schum. [family BIGNONIACEAE]. In: Sprague TA (ed) The useful plants of west tropical Africa (Vol. 1, pp. 258–259). Royal Botanic Gardens, Kew.
Devi, Y. S., Mukherjee, B. B., & Gupta, S. (1994). Rapid cloning of elite teak (Tectona grandis Linn) by in vitro multiple shoot production. Indian Journal of Experimental Biology, 32, 668–671.
Essien, B. A., Essien, J. B., Nwite, J. C., Ogbu, J. U., Okereke, S., & Agunannah, M. U. (2013). Contribution of plant species in homestead farms to food security and sustainability in Ebonyi state, South eastern Nigeria. African Journal of Plant Science, 7, 317–324.
George, E. F., & Sherrington, P. D. (1984). Plant propagation by tissue culture handbook and dictionary of commercial laboratories (p. 71). Eversley: Exegetics Publisher Ltd.
Harborne, J. B. (1973). Phytochemical methods: a guide to modern techniques of plant analysis (2nd ed., p. 309). London: Chapman and Hall Publishers.
Ibrahim, B., Sowemimo, A., Spies, L., Koekomoer, T., Van de Venter, M., & Odukoya, O. (2013). Antiproliferative and apoptosis inducing activity of Markhamia tomentosa leaf extract on HeLa cells. Journal of Ethnopharmacology, 149, 745–749.
Lloyd, G., & McCown, B. (1980). Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Combined Proceedings Int Plant Prop Soc, 30, 421–427.
Łuczkiewicz, M., & Głód, D. (2003). Callus cultures of Genista plants: in vitro material producing high amounts of isoflavones of phytoestrogenic activity. Plant Science, 165, 1101–1108.
Maroyi, A. (2012) Markhamia lutea (Benth.) K. Schum. [Internet] Record from PROTA4U. In Lemmens, R. H. M. J., Louppe, D., & Oteng-Amoako, A. A. (Eds). PROTA (Plant Resources of Tropical Africa/Resources végétales de l’Afrique tropicale), Wageningen, Netherlands. http://www.prota4u.org/search.asp. Accessed 9 Oct 2015.
Müller-Lissner, S. A. (1993). Adverse effects of laxatives, fact and fiction. Pharmacology, 47, 138–145.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiology, 15, 473–497.
Nahapetian, A., & Bassiri, A. (1975). Changes in concentrations and interrelationships of phytate, phosphorus, magnesium, calcium and zinc in wheat during maturation. Journal of Agricultural Food and Chemistry, 23, 1179–1182.
Obdoni, B. O., & Ochuko, P. O. (2001). Phytochemical studies and comparative efficacy of the crude extracts of some Homostatic plants in Edo and Delta States of Nigeria. Global Journal of Pure and Applied Science, 8, 203–208.
Odebiyi, A., & Sofowora, E. A. (1978). Phytochemical screening of Nigeria medicinal plants Part II. Lioydia, 403, 234–246.
Okoli, R. I., Aigbe, O., Ohaju-Obodo, J. O., & Mensah, J. K. (2007). Medicinal herbs used for managing some common ailments among Esan people of Edo State, Nigeria. Pakistan Journal of Nutrition, 6, 490–496.
Patnaik, J., Sahoo, S., & Debata, B. K. (1999). Somaclonal variation in cell suspension culture derived regenerants of Cymbopogon martinii (Roxb.) Wats var. motia. Plant Breeding, 118, 351–354.
Radha, M. H., & Laxmipriya, N. P. (2015). Evaluation of biological properties and clinical effectiveness of Aloe vera. A systemic review. Journal of Traditional and Complementary Medicine, 5, 21–26.
Rout, G. R., Samantaray, S., & Das, P. (2000). In vitro manipulation and propagation of medicinal plants. Biotechnology Advances, 18, 109–120.
Sahu, P. K., Giri, D. D., Singh, R., Pandeyi, P., Gupta, S., Shrivastava, A. K., et al. (2013). Therapeutic and medicinal uses of Aloe vera: A Review. Pharmacology and Pharmacy, 4, 599–610.
Shofidiya, M. O., Agunbiade, F. O., Koorbanally, N. A., Sowemimo, A. A., Soesan, D., & Familusi, T. (2014). Antiulcer activity of the ethanolic extract and ethyl acetate fractions of the leaves of Markhamia tomentosa in rats. Journal of Ethnopharmacology, 157, 1–6.
Sofowora, A. (1993). Screening plants for bioactive agents. Medicinal plants and traditional medicinal in Africa. Ibadan: Spectrum Books.
Soladoye, M. O., Chukwuma, E. C., & Owa, F. P. (2012). An ‘avalanche’ of plant species for the traditional cure of Diabetes mellitus in South-Western Nigeria. Journal of Natural Product and Plant Resources, 2, 60–72.
Sowemimo, A., Samuel, F., & Fageyinbo, M. (2013). Anti-inflammatory activity of Markhamia tomentosa (Benth.) K.Schum. Ex Engl. ethanolic leaf extract. Journal of Ethnopharmacology, 149, 191–194.
Stem, C., Margoluis, R., Salfasky, N., & Brown, M. (2005). Monitoring and evaluation in conservation: A review of trends and approaches. Conservation Biology, 19, 295–309.
Sujatha, M., & Reddy, T. P. (1998). Differential cytokinin effects on the stimulation of in vitro shoot proliferation from meristematic explants of castor (Ricinus communis L.). Plant Cell Reproduction, 17, 561–566.
Tantangmo, F., Lenta, B. N., Boyom, F. F., Ngouela, S., Kaiser, M., Tsamo, E., et al. (2010). Antiprotozoal activities of some constituents of Markhamia tomentosa (Bignoniaceae). Annual Tropical and Medical Parasitology, 104, 391–398.
Temdie, G. R., Fotio, A. L., Dimo, T., Beppe, J., & Tsague, M. (2012). Analgesic and anti-inflammatory effects of extracts from the leaves of Markhamia tomentosa (Benth.) K. Schum. (Bignoniaceae). Pharmacology, 3, 565–573.
Trease, G. E., & Evans, W. C. (2002). Pharmacognosy (p. 393). London: Saunders Publishers.
Van Staden, J., Fennell, C. W., & Taylor, N. J. (2006). Plant stress in vitro: The role of phytohormones. Acta Horticulture, 725, 55–61.
Van-Burden, T., & Robinson, W. (1981). Formation of complexes between protein and Tannin acid. Journal of Agricultural and Food Chemistry, 1, 77.
Yıldız, M., Özcan, S. F., Telci, K. C., & Tuna, E. (2012). The effect of sodium hypochlorite solutions on the viability and in vitro regeneration capacity of the tissue. The Natural Products Journal, 2, 328–331.
Acknowledgement
This study was possible through the funds provided by the Alliance for a Green Revolution in Africa (AGRA), Nairobi, Kenya and the National Biotechnology Development Agency (NABDA), Abuja, Nigeria for the upgrade of the Biotechnology laboratory of the Department of Agronomy, University of Ibadan. Part of this work was carried out at the University of Lagos, Nigeria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bankole, A.E., Uchendu, E.E. & Adekunle, A.A. In vitro germination of Markhamia tomentosa Benth K. Schum ex. Engl. and preliminary phytochemical screening for medicinal compounds. Ind J Plant Physiol. 22, 85–93 (2017). https://doi.org/10.1007/s40502-016-0279-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-016-0279-3