Abstract
Cyanobacteria, Anabaena cylindrica and Synechocystis PCC 6803 grown in batch culture were used to assess the effect of UV-B radiation on growth, photosynthetic pigments, photosynthetic excitation energy transfer by laser induced fluorescence, antioxidant activities, lipid peroxidation, proline and protein content. The photosynthetic apparatus, photosynthetic pigments along with excitation energy transfer are the main targets of UV-B radiation. UV-B radiation was found to inhibit the growth, photosynthetic pigments and excitation energy transfer in both cyanobacterial species. The activities of superoxide dismutase, catalase and peroxidase were significantly increased in both Anabaena cylindrica and Synechocystis PCC 6803. Malondialdehyde content was increased more than two folds in both the cyanobacterial species under prolonged incubation with UV light. In Anabaena cylindrica protein content decreased continuously with increasing duration of UV-B radiation. On the other hand in Synechocystis PCC 6803 the protein content increased up to 48 h UV-B treatment and decreased subsequently for 60 and 72 h UV-B exposure. The results indicate that UV-B radiation causes damage in cyanobacteria at several levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria are one of the most dominant group of photosynthetic organisms having cosmopolitan distribution in nature. Like all photoautotrophs, cyanobacteria also depend on solar radiation as primary source of energy. There is continuous depletion of stratospheric ozone layer, due to release of atmospheric pollutants such as chlorofluorocarbons (CFCs), chlorocarbons (CCs), and organobromide (OBs) causing an increased level of ultraviolet radiation (UVR 280–315 nm) reaching on the earth surface. UV-radiations (UVR) are potential abiotic stress, negatively affecting crop productivity and living organisms. Harmful doses of UV-B and UV-A (315–400 nm) radiation reach deep into the water level and thus may affect aquatic ecosystem (Hader et al. 2007). It is evident that a number of vital functions such as survival, growth, photosynthetic pigmentation, cell differentiation, photosynthetic oxygen production, motility, nitrogen uptake, phycobiliprotein composition, protein profiling and CO2 uptake of cyanobacteria have been reported to be affected by both UV-B and UV-A radiation (Gao et al. 2007; Lesser 2008; Sinha and Hader 2008). Since UV-A is the least energetic out of three type of radiations (UV-A, UV-B and UV-C) and acts indirectly by producing reactive oxigen species (ROS). Hence the present study is focused on UV-B radiation. The increased solar ultraviolet radiation on earth surface is supposed to be an important stress factor for all photosynthetic organisms and terrestrial ecosystem (Häder et al. 2011). Solar photo energy (400–700 nm) is required for maintaining physiology and biochemistry of cell. Cyanobacteria receive lethal dose of ultraviolet radiation (280–400 nm) with solar photo energy and directly affects cellular biomolecules, such as DNA, proteins, lipids, pigments, and indirectly by generating free radicals or ROS. The cyanobacterial photosynthetic apparatus is the main target of UV-B radiation. It is reported that out of two photosystems (PS), PSI is resistant to UV-B radiation, while PSII is more susceptible to UV-B radiation, because the D1 and D2 reaction centre protein subunits of PSII are degraded after UV-B radiation (Friso et al. 1995). All photosynthetic organisms, including cyanobacteria that are simultaneously exposed to photosynthetic active radiation (PAR; 400–700 nm) and UVR, have developed certain mechanisms to counteract the damaging effect of UV-B radiation. Besides repair of UV-induced damage of DNA by photoactivation and excision repair (Britt 1995; Kim 1995), accumulation of carotenoids and detoxifying enzymes or radical quenchers and antioxidants that provide protection from oxygen species (Middleton and Teramura 1993; Mittler and Tel-Or 1991). It has been demonstrated that UV-B radiation stimulates the formation of ROS at various site of photosynthetic electron transport and during various biochemical reactions in cellular system (He and Häder 2002). These ROS are highly deleterious for cell structure and functions (He and Häder 2002; Hidge and Vass 1996). Cell produces ROS such as superoxide radicals (O ·−2 ), while the continuous reduction of O ·−2 produces hydrogen peroxide (H2O2) and hydroxyl radicals (·HO) as final product (Halliwell and Gutteridge 2006; Lesser 2006). Antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POX) function together to inactivate O ·−2 and H2O2 molecules, resulting in the inhibition of formation of hydroxyl radicals; the most reactive of the ROS leading to cellular damage (Fridovich 1986).
The present study was undertaken to elucidate the differential effects of UV-B radiation on growth, photosynthetic pigments, chlorophyll (Chl) fluorescence, excitation energy transfer, antioxidant enzymes, cellular damage measured in terms of lipid peroxidation, proline, and protein content in two cyanobacteria Anabaena cylindrica and Synechocystis PCC 6803.
Materials and methods
Organisms and culture conditions
Anabaena cylindrica, a filamentous, heterocystous cyanobacteria and Synechocystis PCC 6803, a unicellular, non-nitrogen fixing cyanobacterium, were obtained from the stock culture kept in the cyanobacterial Laboratory, Botany Department, University of Allahabad, India. Anabaena cylindrica was grown axenically in nitrogen free BG-11 medium, at 27 ± 2 °C and Synechocystis PCC 6803 was grown in nitrate containing BG-11 medium (Rippka et al. 1979) at 30 ± 2 °C, and white light supplied by fluorescent lamps of 72 μmol m−2 s−1 PAR with a 14:10 light: dark photoperiod. Exponentially growing cultures were used for the different experiments.
UV-B treatment
For UV-B exposure, well homogenized culture suspension of cyanobacteria, Anabaena cylindrica and Synechocystis PCC 6803 were taken in open petridishes (75 mm) occupying a depth of 0.5 cm. The cultures were exposed directly to UV-B radiation at influence rate of 0.4 W m−2, obtained from single Philips (TL-40W/12, RS UV-B Medical, Holland) tubes with main output at 315 nm. Exposure was done for 12–72 h in triplicate, covered with 0.14 mm cellulose acetate filter (Johnston Industrial Plastics, Canada) to remove all incidents UV-C (<280 nm) radiations.
Growth measurement
To determine the effect of UV-B radiation on growth, homogenized cyanobacterial cells exposed to UV-B for a period from 12 to 72 h were withdrawn from each test sample and growth of cyanobacteria was recorded by measuring the absorbance of cell suspension at 750 nm using UV–Vis spectrophotometer (Pharmacia Biotech Ultrospec 4000 UV/Visible Spectrophotometer, Britain).
Photosynthetic pigments estimation
For determination of photosynthetic pigments, cyanobacterial culture was centrifuged and the pellet was suspended in acetone (80 %). The cells were incubated overnight at 4 °C. The suspension was again centrifuged at 10,000 rpm for 5 min and the absorbance of supernatant for chlorophyll and carotenoids were recorded at 665 and 480 nm, respectively, with Ultrospec 4000 UV–Vis spectrophotometer (Pharmacia Biotech). The amount of Chl a was calculated according to Mackinney (1941) and total amount of carotenoids was calculated using the specific absorption coefficient described by Jensen (1978). Cyanobacterial pellet was used for the extraction of phycocyanin by repeated freezing and thawing and the absorbance of the blue supernatant formed in phosphate buffer (pH 7.5) was recorded at 620 nm, and phycocyanin content was calculated according to Bennet and Bogorad (1973).
Laser-induced pigments fluorescence spectra
Laser induced pigments fluorescence spectra of control and UV-B radiated cyanobacteria, Anabaena cylindrica and Synechocystis PCC 6803 was recorded by using 405 nm violet diode laser (Oxxus CE, Model PS-001, France), with a power output of 50 mW, as the excitation source with computer controlled Acton 0.5 M triple grating monochromator (Acton Research Corporation, USA) and Hamamatsu R928 photomultiplier (Hamamatsu Photonics, Japan) as a detector. Laser induced pigment fluorescence spectra were recorded in the region of 600–780 nm with 1800 grooves mm−1 grating blazed at 500 nm wavelength using survey mode of spectra sense software (Roper Scientific Acton Research, USA) (Pandey and Gopal 2011).
Antioxidative enzyme assay
Total super oxide dismutase (SOD) activity was determined by the inhibition of reduction of nitroblue tetrazolium (NBT) according to the method of Giannopolitis and Ries (1977) using 3 ml reaction mixture containing 50 mM potassium phosphate buffer (pH 7.8), 13 mM methionine, 75 µM NBT, 2 µM riboflavin, 0.1 mM EDTA, and 100 µl of enzyme extract. One unit of SOD activity was defined as the amount of enzyme required to cause 50 % inhibition of NBT reduction monitored at 560 nm.
Catalase activity was determined by measuring the consumption of H2O2 (extinction coefficient 39.4 mM−1 cm−1) at 240 nm for 3 min as per Aebi (1984). The 3 ml reaction mixture contained 50 mM potassium phosphate buffer (pH 7), 10 mM H2O2 and 200 µl of enzyme extract. One unit of CAT activity was defined as the amount of enzyme utilized to decompose 1.0 µmol of H2O2. Peroxidase activity in a 3 ml reaction mixture containing 16 mM H2O2, 10 mM pyrogallol and enzyme extract (650 µg protein ml−1) was determined spectrophotometrically according to the method of Gahagen et al. (1968) and the activity was measured as increase in optical density at 430 nm.
Lipid peroxidation
Lipid peroxidation was measured according to method of Heath and Packer (1968). Harvested cyanobacterial cells were homogenized in 1 % Trichloroacetic acid (TCA) and then centrifuged at 10,000 rpm for 15 min at room temperature. The same volume of supernatant and 0.5 % Thiobarbituric acid (TBA) in 20 % TCA solutions (freshly prepared) were added into a new test tube and heated at 95 °C for 30 min in water bath. The heated supernatant was re-centrifuged at 10,000 rpm for 5 min and the absorbance of the supernatant was measured at 532 and 600 nm. 5 % TBA in 20 % TCA was used as the blank. MDA content was determined using the extinction coefficient 155 mm−1cm−1.
Proline estimation
Proline estimation was carried out by using cyanobacterial cells (treated and untreated), suspended in 3 % sulphosalicylic acid and centrifuged at 10,000 rpm for 10 min to remove cell debris. The reaction mixture contained 2 ml of supernatant to which 2 ml of ninhydrin was added, followed by addition of 2 ml glacial acetic acid and incubated at boiling temperature 1 h. The mixture was extracted with toluene. Proline was measured spectrophotometrically at 520 nm from toluene phase (Bates et al. 1973).
Protein content estimation
The protein content was estimated at 700 nm spectrophotometrically following the method of Lowry et al. (1951).
Statistical analysis
Percentage reductions in activities were calculated by comparing with control samples using the formula,
All the data were analyzed by 2-way ANOVA and multivariate analysis in SPSS 16.0 for windows. All the experiments were carried out in three replicates.
Results and discussion
Growth
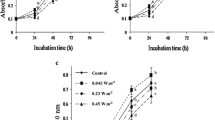
The growth responses of two tested cyanobacteria Anabaena cylindrica and Synechocystis PCC 6803 to UV-B exposure were inhibitory and varied with duration of UV-B exposure. In Anabaena cylindrica and Synechocystis PCC 6803 after 72 h of UV-B exposure growth decreased by 43 and 36 %, respectively compared to control (Table 1). The decreasing trend in growth of cyanobacteria after UV-B radiation was due to the different degree of damage caused by UV-B directly or indirectly on various structural components, physiological factors and genetic material (Melis et al. 1992; Friso et al. 1994).
Photosynthetic pigments
The concentration of chlorophyll a (Chl a) and phycocyanin decreased considerably with UV-B exposure in tested cyanobacteria. As a result of increased UV-B radiation duration from 12 to 72 h, Chl a content decreased by 38 and 48 % in Anabaena cylindrica and Synechocystis PCC 6803, respectively (Table 1). It was found that effect of UV-B radiation was greater in case of phycocyanin than Chl a. The UV-B induced reduction in phycocyanin content was 69 and 92 % in Anabaena cylindrica and Synechocystis PCC 6803, respectively over control after 72 h of UV-B exposure (Table 1). Carotenoids content showed varied response to varying duration of UV-B exposure. In Anabaena cylindrica, carotenoids showed an increase up to 36 h and then reduced to 32 % of control at 72 h, while in Synechocystis PCC 6803 it increased up to 36 h and thereafter decreased by 22 % of control (Table 1). It has been reported that the damaging effect of UV-B radiation on photosynthetic pigments was due to photobleaching or through ROS mediated peroxidation (Nultsch and Agel 1986). The loss of phycocyanin was due to the direct interaction of UV-B radiation with phycocyanin. Phycocyanin directly absorbs radiation in UV-B region (280 nm and above), because it is proteinaceous in nature and localized on the outer surface of thylakoid membrane. Increase in carotenoids content due to UV-B radiation may be attributed to their protective role in scavenging of singlet oxygen. The production of singlet oxygen increased under stress and UV-B radiation (Xue et al. 2005). UV-B radiation induced damaging effect on photosynthetic pigments is also reported in brown and blue green strains of Nostoc spongiforme, where blue green strain of cyanobacteria showed high damaging effect (Tyagi et al. 1992). UV-B exposure results in a high level of ROS formation though photosynthetic pigments and also by redox components.
Laser-induced pigments fluorescence and effect on excitation energy transfer
The laser-induced pigment fluorescence spectra of control (untreated) Anabaena cylindrica showed mainly four fluorescence maxima, one near 645 nm, which was due to the fluorescence of phycocyanin (Kana et al. 2009), a very small peak near 673 nm due to the fluorescence of allophycocyanin (Kana et al. 2009) and other two fluorescence maxima near 684 and 723 nm were due to the Chl a fluorescence (Pandey et al. 2014) (Fig. 1). In case of control set of Synechocystis PCC 6803 three fluorescence maxima were observed on excitation with 405 laser (Fig. 3). The fluorescence maxima near 660 nm was due to the allophycocyanin (Kana et al. 2009; Govindjee and Shevela 2011), whereas fluorescence maxima near 684 and 715 nm were due to the Chl a fluorescence. Other than these three maxima, a tiny hump was present at 645 nm, which was the region of fluorescence emission of phycocyanin (Kana et al. 2009). As the absorbance of major accessory photosynthetic pigments of cyanobacteria (phycocyanin and allophycocyanin) in violet region is very less (Govindjee 2004), that is why the Chl fluorescence maxima are dominant in these fluorescence emission spectra (Figs. 1, 3). In cyanobacteria, phycobilins transfer energy very efficiently to Chl a (Govindjee and Shevela 2011), but in the present study the emission maxima for phycocyanin and allophycocyanin showed that this excitation energy transfer was not 100 %.
Exposure of both the cyanobacterial strains with UV-B radiation for various time duration showed drastic change in pigment fluorescence spectra, which can be used as an indicator of effect of UV-B treatment on structural change and excitation energy transfer in cyanobacterial photosynthetic apparatus. Chl a fluorescence intensity maxima near 685 nm showed a direct correlation with the duration of UV-B treatment. The fluorescence intensity of Chl a at 685 nm decreased continuously with increase in the duration of the UV-B treatment in both the strains. This decrease in the fluorescence intensity was due to the decrease in the Chl a content (Table 1) due to the photobleaching or ROS mediated peroxidation, which is caused due to the prolonged irradiation of UV-B light (Nultsch and Agel 1986). Photosynthetic pigments content was lowest at 72 h UV-B treatment. The minimal Chl a fluorescence in Synechocystis PCC 6803 was recorded for 72 h UV-B light as compared to Anabaena cylindrica, where minimum fluorescence was observed at 36 h UV-B light treatment. For both the cyanobacterial strains 48 and 72 h UV-B light treatments were very detrimental. The pigment fluorescence of Anabaena cylindrica showed more variation than Synechocystis PCC 6803, due to the filamentous nature of the former. The shoulder of the Chl a fluorescence maxima near 715–725 nm showed continuous decrease in Synechocystis PCC 6803 with increase in the duration of UV-B treatment (Fig. 3). The decrease was greater than the decrease at 685 nm fluorescence emission maxima, which is seen in normalized spectra (Fig. 4). Due to this the ratio of F685/F715 increased continuously (data not shown) with increase in duration of UV-B treatment. In case of Anabaena cylindrica, the intensity of fluorescence maxima near 725 nm was greater for control (Fig. 1) but beyond 685 nm it was variable. Cyanobacteria treated with UV-B light for 72 h showed a blue shift (fluorescence maxima near 718 nm) for the second Chl a fluorescence maxima. For 12 and 24 h UV-B treated set a great decrease was observed at the 725 nm maxima than at 685 nm maxima (Fig. 2). Though the ratio of F685/F715 increased over the control, but for 36, 48 and 72 h UV-B treatments decrease for 725 nm fluorescence maxima was less in comparision to decrease for 685 nm maxima (Fig. 2), and the ratio of F685/F715 (data not shown) decreased over the control, which was clearly visible in Fig. 2. UV radiation significantly modifies the structure and function of the photosynthetic apparatus. The most important target of UV light is Mn cluster (Mn4Ca) and oxygen evolving/H2O splitting complex. The higher valence state of Mn absorbs the UV quanta, leading to breakup of the bridging ligand between two Mn ions (Tyystjärvi 2008). Other most important target is PS II complex of thylakoids. UV-B causes degradation of D1 and D2 proteins. It also affects primary (QA) and secondary (QB) quinone electron acceptor and the Try-D and Tyr-Z electron donors (Wua et al. 2011; Zlatko et al. 2012). Thus, UV-B severely affects the PSII to PSI excitation energy transfer. At room temperature, PSII is mainly responsible for the Chl a fluorescence maxima (Govindjee 2004) and there is little contribution of PSI for the second Chl a maxima near 725 nm (Kana et al. 2009). As in cyanobacterial PSI, Chl a is present in large quantity (Govindjee and Shevela 2011) and UV-B interrupts the electron transfer from PSII to PSI, there is possibility of increase in Chl a fluorescence from PSI. Most probably due to this reason UV-B treatment for 36 h or more increased the fluorescence maxima at 725 nm in comparison to fluorescence maxima near 685 nm in case of Anabaena cylindrica. The major accessory photosynthetic pigments; phycocyanin and allophycocyanin were more adversely affected by UV-B radiation than Chl and carotenoids (Table 1), therefore the pigment fluorescence and excitation energy transfer were most affected for these accessory pigment. In Anabaena cylindrica 12 and 24 h UV-B treatments the fluorescence maxima for phycocyanin near 645 nm increased over the control set, where as for allophycocyanin near 673 nm forms a hump (Figs. 1, 2). It shows that in Anabaena cylindrica the treatment of UV-B light up to 24 h adversely affected the excitation energy transfer between phycocyanin and allophycocyanin, whereas it remained more or less smooth between allophycocyanin and Chl a. This effect was maximally visible for 12 h UV-B treatment. For longer duration UV-B treatments, the fluorescence emission near 645 nm decreased drastically over the control (Figs. 1, 2). At 36 h UV-B treatment a small peak was observed near 645 nm, whereas for 48 and 72 h, the fluorescence emission maxima near 645 nm was totally absent. This decrease in fluorescence maxima near 645 nm was due to the considerable decrease in phycocyanin over the Chl a (Table 1). In Synechocystis PCC 6803, the UV-B treatment for 12 h showed considerable increase in fluorescence maxima for phycocyanin and allophycocyanin over the control (Figs. 3, 4). The maxima due to phycocyanin (near 645), which was not clearly visible in control set was clearly seen for 12 and 24 h UV-B treatment. These variations showed the interruption of excitation energy transfer to phycocyanin and allophycocyanin and also between allophycocyanin and Chl a. For longer duration of UV-B treatment (more than 36 h) the fluorescence in these regions decreased considerably and there was no fluorescence in this region for 72 h UV-B treatment (Figs. 3, 4). This decrease was most probably due to the considerable decrease in these pigments over the Chl and carotenoids.
Antioxidative enzyme assay
The activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and peroxidase (POX) increased with exposure of UV-B irradiation. The activity of SOD, CAT and POX increased by 40, 42 and 40 %, respectively, in Anabaena cylindrica, while in Synechocystis PCC 6803 the increase in activities of SOD, CAT and POX was 70, 73 and 48 %, respectively (Table 2). The increase in activities of SOD, CAT and POX in Anabaena cylindrica and Synechocystis PCC 6803 after UV-B radiation may be a consequence of production of O ·−2 in the cyanobacterial cell. SOD is a prominent biomarker of defense against oxidative stress. Similar results of increased antioxidant enzyme activity has also been reported in UV-B exposed cucumber plant (Kondo and Kawashima 2000). The greater increase in CAT activity in Synechocystis PCC 6803 than Anabaena cylindrica under increasing UV-B radiation suggested that Synechocystis PCC 6803 synthesized high amount of this enzyme to scavenge the excess of O ·−2 and H2O2. The excessive ROS level in cyanobacteria Synechocystis PCC 6803 caused imbalance in redox potential, which exhibited an elevated oxidative capacity. This was due to changes in biomolecular metabolism (Stork et al. 2005). The greater increase in the activity of SOD, CAT and POX in Synechocystis PCC 6803 than Anabaena cylindrica under UV-B irradiation indicates the high efficiency of stress tolerance in Synechocystis PCC 6803 (Table 2).
Lipid peroxidation
The level of lipid membrane damage measured as malondialdehyde (MDA) content showed enhancement in lipid peroxidation in UV-B exposed cyanobacteria. MDA content increased two fold in both the cyanobacteria species. In Anabaena cylindrica MDA content increase was 138 % of control, and in Synechocystis PCC 6803 the increase was 110 % of control after 72 h of UV-B radiation. Increased MDA content in UV-B treated cyanobacteria was due to oxidative degradation of polyunsaturated fatty acids in cyanobacterial membrane (Girotti 1990; Chis et al. 2006). In the present study, UV-B radiation enhanced the content of MDA in both species (Table 3), indicating oxidative stress in Anabaena cylindrica and Synechocystis PCC 6803.
Proline content
Proline is an amino acid essential for primary metabolism. Its accumulation occurs under unfavorable conditions and abiotic stresses, including UV-B radiation condition. In both Anabaena cylindrica and Synechocystis PCC 6803, proline content increased significantly with increasing doses of UV-B radiation. Proline content in Anabaena cylindrica increased by 40 % of control, where as in Synechocystis PCC 6803 increase was 110 % of control after 72 h of UV-B exposure (Table 3). The accumulation of proline has been reported in many cyanobacteria and also in higher plants due to increase in synthesis and decrease in degradation under stress condition, including UV-B radiation. The accumulation of proline under stress conditions is probably due to decrease in the activity of electron transport system (Venekemp 1989).
Protein content
UV-B exposure was found to have significant alterations on protein content in both the cyanobacteria species, Anabaena cylindrica and Synechocystis PCC 6803 as compared to un-exposed cyanobacteria. In Anabaena cylindrica protein content continuously decreased up to 46 % of control with increasing duration of UV-B exposure up to 72 h. On the other hand in Synechocystis PCC 6803 the protein content increased up to 48 h of UV exposure, followed by decrease in concentration (52 %) under 72 h UV-B exposure (Table 3). Results showed that protein content of both the species of cyanobacteria differed following exposure of UV-B radiation. This is because several proteins are damaged and few new proteins are produced under stress condition, including under short and long term UV-B exposure (Gao et al. 2009). This may be because many defence mechanisms get activated under stress environment to enable the cyanobacteria adapt to stress conditions.
Conclusion
In the present study along with the conventional parameters such as growth measurement, photosynthetic pigment content, antioxidant enzyme activity, lipid peroxidation, proline and protein content, we also studied the effect on excitation energy transfer of photosynthetic apparatus by laser-induced pigment fluorescence spectroscopy to quantify the effect of UV-B radiation on two cyanobacterial strains. Laser-induced pigment fluorescence emission spectra showed unique pattern of pigment fluorescence emission spectra and confirms the effect of UV-B radiation on excitation energy transfer. This biophysical technique clearly showed the sensitivity of cyanobacterial photosynthetic apparatus to the UV-B radiation and sensitivity of this nondestructive technique towards the study of photosynthetic excitation energy transfer.
References
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126.
Bates, L. S., Wadern, R. P., & Teare, I. D. (1973). Rapid estimation of free proline for water stress determination. Plant and Soil, 39, 205–207.
Bennet, A., & Bogorad, L. (1973). Complementry chomatic adaptation in filamentous blue gree algae. The journal of cell biology, 58(2), 419–435.
Britt, A. B. (1995). Repair of DNA damage induced by ultraviolet radiation. Plant Physiology, 108, 891–896.
Chis, A., Zeeshan, M., Abraham, G., & Prasad, S. M. (2006). Proline accumulation in Cylindrospermum sp. Environmental and Experimental Botany, 57, 154–159.
Fridovich, I. (1986). Biological effects of the superoxide radical. Archives of Biochemistry and Biophysics, 247(1), 1–11.
Friso, G., Spetea, C., Giacometti, G. M., Vass, I., & Barbato, R. (1994). Degradation of PS II reaction center D1 protein induced by UV-B radiation in isolated thylakoids: Identification and characterization of C and N terminal break down products. Biochimica et Biophysica Acta, 1184(1), 78–84.
Friso, G., Vass, I., Spetea, C., Barber, J., & Barbato, R. (1995). UV-B induced degradation of D1 protein in isolated reaction centre of PS II. Biochem Biophysics Acta, 1231, 41–46.
Gahagen, H. E., Holm, R. E., & Abeles, F. B. (1968). Effect of ethylene on peroxidase activity. Physiologia Plantarum, 21, 12–70.
Gao, Y., Xiong, W., Gao, Li. X., Zhang, C. F., Li, Y. L. H., & Wu, Q. (2009). Identification of the proteomic changes in Synechocystis sp. PCC 6803 following prolonged UV-B irradiation. Journal of Experimental Botany, 60, 1141–1154.
Gao, K., Yu, H., & Brown, M. T. (2007). Solar PAR and UV radiation affects the physiology and morphology of cyanobacterium Anabaena sp. PCC 7120. Journal of Photochemistry and Photobiology B, 89, 117–124.
Giannopolitis, C. N., & Ries, S. K. (1977). Superoxide dismutases: I. Occurrence in higher plants. Plant Physiology, 59(2), 309–314.
Girotti, A. W. (1990). Photodynamic lipid peroxidation in biological systems. Photochemistry and Photobiology, 51, 497–509.
Govindjee, (2004). Chlorophyll a fluorescence: A bit of basics and history. In G. C. Papageorgiou & Govindjee (Eds.), Chlorophyll fluorescence: A signature of photosynthesis (pp. 1–42). Dordrecht: © Kluwer Academic Publishers.
Govindjee, & Shevela, D. (2011). Adventures with cyanobacteria: A personal perspective. Frontiers in Plant Science, 2, 1–17.
Häder, D.-P., Helbling, E. W., Williamson, C. E., & Worrest, R. C. (2011). Effects of UV radiation onaquatic ecosystems and interactions with climate change. Photochemical & Photobiological Sciences, 10, 242–260.
Hader, D.-P., Kumar, H. D., Smith, R. C., & Worrest, R. C. (2007). Effect of Solar UV radiation on aquatic ecosystem and interaction with climate change. Photochemical & Photobiological Sciences, 6, 267–285.
Halliwell, B., & Gutteridge, J. M. C. (2006). Free redicals in biology and medicine. New York: Oxford University Press Inc.
He, Y. Y., & Häder, D.-P. (2002). Reactive oxygen species and UV-B: Effect on cyanobacteria. Photochemical and Photobiological Science, 1, 729–736.
Heath, R. L., & Packer, L. (1968). Photoperoxidation on in isolated chloroplasts, I. Stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125(1), 189–198.
Hidge, E., & Vass, I. (1996). UV-B induced free radical production in plant leaves and isolated thylakoid membranes. Plant Science, 115, 251–260.
Jensen, A. (1978). Chlorophyll and carotenoids. In J. A. Hellebust & J. S. Craige (Eds.), Handbook of physiological methods (pp. 60–70). Cambridge: Cambridge University Press.
Kaňa, R., Prášil, O., Komárek, O., Papageorgiou, G. C., & Govindjee, (2009). Spectral characteristic of fluorescence induction in a model cyanobacterium, Synechococcus sp. (PCC 7942). Biochimica et Biophysica Acta, 1787, 1170–1178.
Kim, S. T. (1995). Sancer a photorepair of nonadjacent pyrimidine dimmers by DNA photolyase. Photochemistry and Photobiology, 61, 171–174.
Kondo, N., & Kawashima, M. (2000). Enhancement of the tolerance to oxidative stress in cucumber (Cucumis sativus) seedlings by UV-B irradiation: Possible involvement of phenolic compounds and antioxidative enzymes. Journal of Plant Research, 113, 311–317.
Lesser, M. P. (2006). Oxidative stress in merine environments: Biochemistry and physiological ecology. Annual reviews of Physiology, 68, 253–278.
Lesser, M. P. (2008). Effects of ultraviolet radiation on productivity and nitrogen fixation on the cyanobacterium, Anabaena sp. (Newton’s strain). Hydrobiologia, 598, 1–9.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Mackinney, G. (1941). Absorption of light by chlorophyll solutions. Journal of Biology and Chemistry, 140, 315–322.
Melis, A., Nemson, J. A., & Harrison, M. A. (1992). Damage to functional components and partial degradation of PS II reaction centre proteins upon chloroplast exposure to ultraviolet-B radiation. Biochimica et Biophysica Acta, 1100(3), 312–320.
Middleton, E. M., & Teramura, A. H. (1993). The role of flavonol glycosides and carotenoids in protecting soybean from ultraviolet-B damage. Plant Physiology, 103, 741–752.
Mittler, R., & Tel-Or, E. (1991). Oxidative stress responses in the unicellular cyanobacterium Synechococcus PCC 7942. Free Radical Research Communication, 12, 845–850.
Nultsch, W., & Agel, G. (1986). Fluence rate and wave length dependence of photobleaching in the cyanobacterium, Anabaena variabilis. Archives of Microbiology, 144, 268–271.
Pandey, J. K., Dubey, G., & Gopal, R. (2014). Study the effect of insecticide dimethoate on photosynthetic pigments and photosynthetic activity of pigeon pea: Laser-induced chlorophyll fluorescence spectroscopy. Journal of Photochemistry and Photobiology B: Biology,. doi:10.1016/j.jphotobiol.2014.08.014.
Pandey, J. K., & Gopal, R. (2011). Laser-induced chlorophyll fluorescence: A technique for detection of dimethoate effect on chlorophyll content and photosynthetic activity of wheat plant. Journal of Fluorescence, 21, 785–791.
Rippka, R., Deruelles, J., Waterbury, J. B., Herdman, M., & Stanier, R. (1979). Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Journal of General and Applied Microbiology, 111, 1–61.
Sinha, R. P., & Hader, D. P. (2008). UV-protectants in cyanobacteria. Plant Science, 174, 278–289.
Stork, T., Michel, K. P., Pistorius, E. K., & Dietz, K. J. (2005). Bioinformatic analysis of the genomes of the cyanobacteria Synechocystis sp. PCC 6803 and Synechococcus elongatus PCC 7942 for the presence of peroxiredoxins and their transcript regulation under stress. Journal of Experimental Botany, 56, 3193–3206.
Tyagi, R., Srinivas, G., Vyas, D., Kumar, A., & Kumar, H. D. (1992). Differential effect of ultraviolet-B radiation on certain metabolic process in a chromatically adapting Nostoc. Photochemistry and Photobiology, 55, 401–407.
Tyystjärvi, E. (2008). Photoinhibition of PS II and photodamage of the oxygen evolving manganese cluster coordination. Chemical Reviews, 252, 361–376.
Venekemp, J. H. (1989). Regulation of cytosolic acidity in plants under condition of drought. Plant Physiology, 76, 112–117.
Wua, H., Abasova, L., Cheregi, O., Deák, Z., Gao, K., & Vass, I. (2011). D1 protein turnover is involved in protection of PS II against UV-B induced damage in the cyanobacterium Arthospira (Spirulina) platensis. Journal of Photochemistry and Photobiology B: Biology, 104, 320–325.
Xue, L., Zhang, T., An, L., & Wang, X. (2005). Effect of enhanced ultraviolet- B radiation on algae and cyanobacteria. Critical Reviews in Microbiology, 31, 79–89.
Zlatko, S., Fernando, Zlatev., Lidon, J. C., & Kaimakanova, M. (2012). Plant physiological responses to UV-B radiation. Emirates Journal of Food and Agriculture, 24(6), 481–501.
Acknowledgments
Md. Akhlaqur Rahman is thankful to University Grants Commission, New Delhi, India for financial assistance under Maulana Azad National Senior Research Fellowship to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahman, M.A., Pandey, J.K., Sundaram, S. et al. Response of growth, photosynthetic pigments, laser-induced pigment fluorescence, antioxidant enzymes and lipid peroxidation to ultraviolet-B radiation in two cyanobacteria. Ind J Plant Physiol. 20, 240–248 (2015). https://doi.org/10.1007/s40502-015-0169-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-015-0169-0