Abstract
Purpose of Review
Elderly population represents the fastest growing population segment and target group of many expert organizations developing strategies for successful aging. The substantial increase in man’s lifespan together with implementation of preventive dental programs resulted in decreased tooth loss and increased prevalence of periodontal disease. Hence, the present review focused on positioning periodontal health within aging process, characteristic of the senescent periodontium, periodontitis-aging interplay, clinical characteristics, and implications for periodontal care in geriatric patients.
Recent Findings
The senescent periodontium undergoes degenerative changes that are unrelated to progressive destruction in lack of inflammation. The pathological interplay periodontitis-systemic aging is evidenced. Geriatric patients demonstrate comparable treatment outcomes to those of patients ≤ 60 years regarding non-surgical and surgical periodontal treatment and implant therapy as well.
Summary
Adequate oral hygiene and appropriate control of risk factors represent the key pre-conditions for successful periodontal and systemic aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Geriatric population comprises of adults of ≥ 65 years old and currently counts some 11% of the world’s population with an estimated increase of up to 22% by 2050 according to the United Nations [1••]. Advances in medical sciences contributed to substantially increased man’s lifespan best depicted by the Center for Disease Control’s Morbidity and Mortality reporting the doubling of the population over 65 years in a 30-year timespan. Additionally, the World Health Organization (WHO) estimates 2.5% annual increase of the population over 65 years [2]. The consequence of prolonged longevity of man is increase in age-related health problems. Thus, life quality in elderly population constitute central priorities of modern medicine [3••]. Medical research often prioritizes and indeed distinguishes aging-related health problems from pathologies since in the first case, health issues occur inevitably in all persons. On the other hand, the processes in the aging organism are altered and differ under both physiological and pathological conditions. Out of these reasons, the WHO identifies elderly population as one of the main targets and defines related priority of successful aging as “low probability of disease and disease-related disability, high cognitive and physical function, and active engagement with life” [4, 5]. Within this concept, oral health was envisaged as one of the priorities the retention of more than 20 teeth after 80 years old as a defined goal. As periodontitis represents the first cause of tooth loss [6] and simultaneously the inflammatory burden able to affect systemic health, periodontal health in elderlies derived priority as well. When considering the increased man’s lifespan from the aspect of periodontal status, as a result of successful actions directed to improve the oral health, global population is currently characterized with substantially decreased tooth loss and subsequently increased rate of severe form of periodontitis [7••]. Therefore, periodontal health in geriatric patients is in the focus of many expert groups working hard to develop strategies for successful periodontal aging that would according to proposed concept [4] be intact dentition with minimal attachment loss and minimal limitation in function [5].
Periodontitis and associated tooth loss provide many disabilities affecting the mastication, speech, and providing co-morbidities such as Costen’s syndrome [8] and possibly having general impact in social interactions of the patient. To improve the epidemiological metrics for oral health in geriatric patients, long time ago, Locker [9••] invented concept of oral-health-related-quality of life (OHRQoL) by applying the International Classification of Impairments, Disabilities and Handicaps proposed by WHO on oral health to measure the impact of poor oral health in overall disability. This index remains particularly important since one of the WHO priorities is the decrease in disability adjusted life years (DALYs) directly determined by number of years lived with disability (YLD) [10], while OHRQoL enables appropriate evaluation of these parameters within context of oral health. Finally, the entire branch of periodontology is devoted to the bi-directional pathological interaction between periodontitis and systemic diseases with common inflammatory biotypes [11]. Interestingly enough, the positive association was established between periodontal inflammation and all major systemic conditions associated with aging including atherosclerosis, diabetes mellitus (DM), and Alzheimer’s disease (AD).
The present review will focus on positioning of the periodontal health within aging process, characteristic of the senescent periodontium, periodontitis-aging interplay and related pathological mechanisms, clinical findings, and implications for periodontal care in geriatric patients.
Periodontitis Part of Aging or Its Consequence?
Whether periodontitis represents a part of physiological aging or its simple consequence was a subject of controversy over the years [12]. The simple fact is that periodontitis is highly prevalent lifelong multifactorial disease and the sixth most common health condition [6] illustrates how delicate it is to separate its accumulated consequences from effects of aging. This is challenged by the fact that periodontitis and aging share inflammation as the main pathological mechanism subsequently leading to the overlapping symptomatology due to syndemic effects. To fully understand such complex relationship, it is worthwhile going back to initial definition of periodontitis and related contributing factors.
Periodontal disease is a chronic inflammatory disease of tooth supportive tissues caused by periodontal infection manifested with destruction of connective tissue attachment and alveolar bone resorption as a pathognomonic sign of the disease [13]. The underlying pathogenetical mechanism of periodontitis is non-resolving chronic inflammation against local disbyosis triggered by periodontal key stone pathogens [14]. Clinically, inflammation occurs in the marginal gingiva in contact with accumulated dysbiotic biofilm initiating gingivitis that further transforms into periodontitis once the pro-inflammatory cytokines achieve the critical concentrations to trigger inflammatory osteoclastogenesis and alveolar bone loss [15].
Because of inflammatory background of the disease as well as due to highly resistant inflammophilic character of the periopathogens [16, 17], any factor able to interpose in inflammatory pattern is able to deteriorate periodontal inflammation. Therefore, the four groups of risk factors capable to modulate periodontal inflammation were identified: genetic background, general inflammatory status, environmental factors, and systemic diseases, and aging is source of all factor groups. The inherent characteristic of aging organism is increased inflammatory status referring to the permanently increased systemic levels of pro-inflammatory markers while genetic alterations represent the hallmarks of aging process. The most important aging-related genetic alterations for periodontium are upregulated gene expression for pro-inflammatory mediators, downregulation of genes implicated in pro-formative processes, and genes involved in apoptosis and decrease in cell proliferative potential particularly in progenitors [3••, 18]. Moreover, it was already mentioned that pathological synergism between periodontitis and hallmark systemic diseases of aging was confirmed which will be elaborated in more detail in the further text. Regarding environmental factors, the aging process provide many deteriorating factors for periodontal condition, but the present review will discuss on two most important factors including neglected oral hygiene and decreased salivary flow.
Neglected oral hygiene occurs as a consequence of neglected oral health, very frequent in elderly population, mainly not only due to preoccupying with general health problems but also due to demotivation characteristic for elderlies. The consequence of neglected oral hygiene (OH) is dental plaque accumulation associated with periodontal inflammation; hence, the tooth brushing once a day or less, lack of interdental care, and smoking were proposed the critical periodontal risk factors in geriatric patients [12].
Decreased salivary flow normally occurs in linear manner with increasing age due to degenerative changes in major and minor salivary glands characterized with atrophy of acinar tissue and proliferation of ductal elements [19] followed by reduced excretion of saliva. Such type of decreased salivation should be distinguished from another typical condition referring to the syndrome of xerostomia defined as patient-reported mouth dryness [20]. Xerostomia is reported in 25–50% of older people and is associated with systemic diseases (such as Sjögren syndrome, parkinsonism, or DM) [21], use of medications, or psychological conditions but is not considered inherent part of physiological aging. Decreased salivary flow apart from reducing lubrication of oral structures making them vulnerable to mechanical insults, it compromises the auto-cleaning and decreases the local protective capacity while increasing the susceptibility for periodontal infection. Finally, more than 400 medications cause reduced salivary flow as a side effect [12], where drugs frequently used in older populations such as antihypertensives, anticholinergic drugs, psychotropic drugs, and diuretics belong to this group as well [22, 23]. Another frequent pathology related to medical treatment in geriatric patients is drug-induced gingival overgrowth that is most frequently caused by calcium channel blockers in the treatment of hypertension.
Biological and Clinical Characteristics of the Senescent Periodontium
Senescent periodontium is clinically characterized with loss of connective tissue attachment and concomitant bone loss frequently associated with gingival recessions. As the hallmarks of aging represent the loss of physiological integrity with subsequent functio laesa, within research on periodontal aging, the particular focus was on changes in connective tissue. Regarding histological characteristics of connective tissue, the reduced number of elastic fibers, dis-regulation of collagen bundles, and reduced cellular content were observed [24, 25]. Regarding cellular senescence, it was also confirmed that gingival fibroblasts undergo functional and structural alterations with aging as well [26, 27]. In addition to that, it was reported that the decrease in collagen production by gingival fibroblasts with age increases [28] presumably due to methylation of the collagen alpha-1 gene [27] responsible for its biosynthesis as well as expressed intracellular collagen phagocytosis [29]. This might explain the frequent occurrence of gingival recessions in older population since despairing between collagen formation and degradation is a pathogenetical mechanism behind gingival recessions [30]. Additionally, the keratinized gingiva reduces with aging [31], thus contributing to development of gingival recessions. The alteration in quantitative composition of the extracellular matrix between young and old fibroblasts was observed as well [32].
Within repercussions of above-mentioned connective tissue alterations, the structure of periodontal ligament derives irregularly, while its function remains altered as well [31]. The reduction and perturbation in fiber conformation is followed by enlargement of intracellular spaces that subsequently increases vulnerability on infective insults, decreases the amortizing capacity against biomechanical forces, and contributes to tooth mobility. Indeed, it was observed that periodontal ligament fibroblasts release great amounts of [26] pro-inflammatory mediators on biomechanical stress suggesting the possible outcome of wear associated to lifelong amortization of occlusal forces. From the clinical aspect, this should be considered as potential cause of increased susceptibility on periodontal traumatism in geriatric patients. Furthermore, the reduction of cellular content in periodontal ligament has particular importance since this structure represents the source of progenitor cells responsible for regeneration/reparation of the alveolar bone, periodontal ligament, and cementum. The manifestation of decline in stem cells of periodontal ligament is decrease in alveolar bone volume and atrophy of periodontal ligament [33]. Cementum with age derives acellular and demonstrates unusual alternation in resorption and reposition responsible for expressed irregularity of the cementum surface [25] but without important clinical effects.
The integrity of the alveolar bone is maintained on dynamic homeostasis between osteoblastic and osteoclastic activity within physiological bone remodeling. The balance between these processes is pre-condition for bone integrity but is at the same time highly susceptible on numerous factors such as hormonal status, inflammatory status, calcium-phosphates concentration, nutrition, and other. However, the aging process provides many negative factors that compromise the equilibrium between these processes, having as a consequence predomination of pro-resorptive component in elderly persons. Additionally, the decrease in pre-osteoblasts and decline in collagen-1 synthesis should be also considered as possible reasons for bone loss in older persons.
Generally, it is accepted that periodontium undergoes degenerative changes that are unrelated with progressive periodontal destruction [31, 34••], while the senescent periodontium was characterized by clinical attachment loss with concomitant alveolar bone loss qualified as modest in lack of inflammation [34••]. On the other hand, aging process provides a variety of factors contributing to the periodontal disbyosis and related inflammation but it still remains difficult to reliably distinguish the consequence of aging from lifelong periodontitis insults. Therefore, two hypotheses were proposed for periodontal disease in aging persons, the hypothesis of cumulative effect hypothesis that emphasizes the role of accumulated lifelong periodontal insults and age-related susceptibility hypothesis proposing the role of aging in alteration of immuno-inflammatory status of periodontal tissues in course of increased susceptibility to periodontitis [34••].
Interplay Between Periodontal Disease and Aging Within Inflammaging
Aging comprises the physiological processes that cause functio laesa and subsequent loss of physiological integrity leading to death. Those processes start at the molecular level and include nine hallmarks of aging: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication [3••]. At the systemic level, this process is followed by chronic progressive increase in the pro-inflammatory status that contributes in occurrence and enhancement of the inflammatory pathologies referring to the inflammaging [35]. In brief, it was demonstrated that systemic inflammation exacerbates vascular pathology, thus contributing to cardiovascular diseases, increases secretion of cortisol causing the insulin resistance and DM2, and promotes bone resorption and neurodegenerative diseases such as AD [36]. Such permanent systemic inflammation simultaneously increases the susceptibility for other inflammatory pathologies and their syndemic acting, while the best example for that is periodontitis.
The main pathological process of periodontitis is inflammatory osteoclastogenesis regulated by nuclear factor kappa-B (Nf-kB), the transcription factor coding the waste majority of pro-inflammatory cytokines implicated in this process [15, 37]. The process is orchestrated by interaction of his receptor (receptor activator nuclear factor kappa-B RANK) and ligands (receptor activator nuclear factor kappa ligand—RANKL, osteoprotegerin—OPG) and so-called RANK-RANKL-OPG triade [38]. In brief, under inflammation, the RANKL remains expressed on the membranes of osteoblasts, stromal cells, fibroblasts, B cells, and T cells and tends to bind RANK expressed on the membranes of pre-osteoclast and osteoclasts to efferently provide maturation of pre-osteoclasts and/or enhancement of the activity of maturated osteoclasts. The critical mediators implicated in upregulation of RANKL apart bacterial lipopolysaccharide (LPS) are parathormone, epinephrine, 17β-estradiol and glucocorticoids, and pro-inflammatory cytokines (IL-1, IL-6, IL-8, IL-11, IL-17, TNF-α, PGE2, and IFN-γ) [39]. This pattern provides the link for detrimental interaction with aging since the most common hallmarks of immunosenescence are increased systemic levels of pro-inflammatory cytokines such as PG22 and TNF-α, impaired phagocytosis, and increased periodontal levels of Nf-kB itself [34••, 40]. When considering this, initially increased baseline levels of Nf-kB together with PGE2 and TNF-α as confirmed main up-regulators of RANKL-orchestrated osteoclastogenesis induced by Porphyromonas gingivalis (P. gingivalis), Treponema denticola, and Treponema socranskii; this clearly depicts the origin of bone destruction severity in geriatric patients [41]. Indeed, even for RANK RANKL-independent auto-activation, the microbiological challenge needs to exist.
Syndemic Acting Among Periodontal Inflammation and Aging-Related Systemic Diseases
Since systemic diseases and their respective treatment in geriatric patients represent the specificity requiring appropriate modification of the routine clinical protocols, this issue was considered individually.
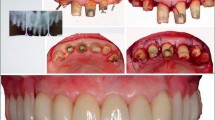
Generally, systemic diseases in elderlies induce multitude of periodontal destruction by contributing to microbiological disbyosis and excessive immunological reactions associated with severe forms of periodontitis. In turn, periodontitis can exacerbate systemic health [42] since periodontal pockets derive inflammatory burden (Fig. 1) and potent enhancer of systemic inflammation and related pathologies [43,44,45]. The histological characteristic of oral vasculature is lack of one entire layer making them extremely permeable being the reason why many systemic diseases give first manifestations in the mouth and reason why the mouth is called the mirror of the health. Under periodontal inflammation, the permeability of vascular elements additionally increases and ulcerates which leads to penetration of periopathogens and rich inflammatory content into systemic circulation [46,47,48]. The periopathogens express pathogenicity on many distant organ systems [49,50,51,52,53,54,55] via two proposed mechanisms, by directly infecting or indirectly by triggering and/or enhancing local inflammation [56]. It is worth mentioning that such a mechanism does not contribute only to the primary pathology but also to poorer response to performed treatment. Regarding scientific evidence on pathological relation between periodontitis and aging-related systemic diseases, it was reported that dissemination of oral bacteria notably contributes to promotion of atherosclerosis and impaired glycemic control within insulin resistance and DM [57••].
Atherosclerosis represents the underlying mechanism of coronary heart disease (CHD) and ischemic stroke and is based on formation of atherosclerotic plaques on inner arterial walls, thus decreasing blood flow and compromising the nutrition of heart and brain. Hence, the thickening of the atherosclerotic plaques represents the critical factor since hypoxia caused by obstruction of nutritive arteries induces infarction and subsequent necrosis of the organ area vascularized by affected arterial branch. The thickening of the atherosclerotic plaque is mostly associated with inflammatory reactions in the arterial wall, calcification, and ruptures in plaques creating the predilection locus for thrombosis. Periodontal disease was reported to directly affect atherosclerosis since periopathogens were identified in intrasurgically retrieved atherosclerotic plaques form arteries of patients suffering CHD [58, 59••]. Although the mechanism is still not defined fully, the periopathogens in atherosclerotic plaque clearly contributes to local inflammation, instability of atherosclerotic plaque, and dysregulation of autophagy [60]. Moreover, periodontal disease affects atherosclerosis via indirect mechanism by increasing systemic inflammation [61]. Such pathological correlation between periodontitis and atherosclerosis can be depicted on the example of the myocardial infarction being the greatest cause of mortality in western countries [62]. In a recent study reported by Marfil-Alvarez et al. [55], it was demonstrated that the extent and severity of periodontitis is positively associated with acute myocardial infarct size, and the effect was measured using troponin as a gold standard biochemical marker of the infarct and myoglobin, a nonspecific myocardial necrosis marker. In turn, periodontal treatment provides decrease in systemic inflammation and improvement of endothelial function [63].The positive association was also reported for periodontitis and ischemic stroke [64] as well as with recurrent vascular events in stroke/transient ischemic attack patients [54]. It was proposed that periodontitis plays as an inflammatory burden that negatively affects the disease via increase of systemic inflammatory markers [54].
Diabetes mellitus (DM) and periodontal disease has a well-established bidirectional pathological link [65, 66••, 67]. In brief, DM negatively contributes to periodontitis by increasing susceptibility to infection and upregulating local inflammation, periodontal inflammation impairs glycemic control, and treatment response in diabetic patients via increase in systemic inflammation while scaling and root planing improves glycemic control in DM2 [67, 68]. It was proposed that DM deteriorate periodontal condition by increasing the local levels of RANKL, OPG, TNFα, IL-1β, and IL-6 while interaction between advanced glycation end products (AGEs) seems to play in severe tissue destruction and impaired repair in diabetes-associated periodontitis [69].
The Alzheimer disease (AD) represents the growing problem in public health that according to the WHO contributes with 60–70% cases out of total 35.6% million people affected by dementia [70]. The disease is characterized with accumulation of the amyloid-β (aβ) plaques and neurofibrillary tangles of hyperphosphorilated tau protein that subsequently interrupt signal neurotransmission and neural function in the brain. The exact cause and underlying mechanism of AD is unfortunately still not established; however, as aβ represents antimicrobial substance, the hypothesis of microbiological disease etiology followed by inflammatory tissue destruction is under strong consideration. In that context, the association between periodontitis and AD was examined. It was demonstrated that serum levels of TNFα and antibodies on Aggregatibacter actinomycetemcomitans, Tannerella forsythia, and P. gingivalis were significantly elevated in AD patients compared to healthy controls while estimated antibodies for periopathogens showed an odd ratio of 6.1 in AD patients [53]. Additionally, the negative correlation between high serum levels on P. gingivalis and low cognitive function was demonstrated [71]. The mechanistic relation between periodontitis and AD is not established, but it was assumed that periodontal bacteria might contribute directly and indirectly to accumulation of aβ. Briefly, it was demonstrated that spirochetes contain amyloidogenic proteins while in vitro and in vivo exposure to bacterial LPS causes deposition of aβ and tau phosphorylation [72]. Hence, it was assumed (plausible mechanism would be) that upstream in systemic inflammation associated with periodontal burden would contribute to damage and increased permeability of blood-brain barrier, thus facilitating penetration of the periopathogens toward brain to finally contribute in formation of amyloid plaques. Related to that, the study was conducted to compare levels of aβ-precursor protein (APP) between gingival tissues from periodontitis-affected patients and periodontally healthy individuals and periodontitis patients demonstrated significantly increased levels of APP, thus suggesting a relationship between periodontitis and AD pathogenesis [73]. Regarding the indirect mechanism and upregulation of systemic inflammation by the periodontal burden, as both microglia and astrocytes are in a pro-inflammatory state in AD, there is a strong therapeutic recommendation for tipping the balance toward an anti-inflammatory state for both types of glia and the first step presents the eradication of inflammatory burdens in the body [74]. With this in relation, the serum levels of the TNFα, a common marker of periodontal and AD destruction, and antibodies on periodontal bacteria were estimated and the significantly higher serum levels of measured parameters were showed in AD patients [75].
Besides the described pathway of dissemination via systemic circulation, the aspiration of periodontal pathogens presents a pathogenic pattern particularly important for geriatric population. In fact, this is a causative mechanism of pneumonia in hospitalized patients and elderly population [70, 76] and it was demonstrated that oral care reduces pneumonia in older patients in nursing homes [77].
Periodontal Care for Geriatric Patients
When considering all aforementioned, it is evident that neglected oral hygiene presents the major threat for periodontal health in geriatric patients. It was elaborated that senescent organism could be considered a “bomb” of threats for periodontal health while development of periodontal inflammation in this setting means opening of the “Pandora’s box” for systemic health. However, in lack of dental biofilm, any of these might occur, meaning that impeccable oral hygiene is the key imperative for prevention of adverse effects within successful aging. Related to that, it was demonstrated that older people receiving regular dental care tend to retain more teeth than those without a dental maintenance program [78]. Additionally, it was proposed that the number of dental visits seems not to affect periodontal parameters while the intervention of periodontists seems to be beneficial [78].
As previously said, the main reasons for neglecting oral hygiene in elderly population is their focus on frequent medical problems and demotivation as a manifestation of psychological alterations accompanying aging. This indicates the need for adjusting the approach of both medical and dental professionals to geriatric patients. The medical doctors dealing with geriatric patients should regularly explain and remind the patients on the importance of oral hygiene and its impact to general health, while dental professionals should particularly focus on motivation and education on OH and possibly adapt the approach for older persons. In this clinical phase, the psycho-physical disability should be estimated properly, in order to propose the appropriate devices for OH such as tooth brushes with softer ergometric handles and floss holding devices for patients with disabilities or electronic brushes particularly suitable for patients with decreased dexterity. It was reported that this kind of adapted home care regimen may improve oral health in the elderly [79]. Furthermore, dental practitioners should carefully perform the risk assessment in older persons to disclose the presence of any systemic diseases and drug consumption that would require adjustment in preventive strategy for optimal disease prevention. The preventive strategy for elderlies should consider the reduced salivary flow that subsequently increases the susceptibility on periodontal infection and caries, thus use of chlorhexidine rinses and fluorides is strongly recommended for better plaque control and better resistance on infection.
Periodontal Treatment for Geriatric Patients
Periodontal treatment in geriatric patients is oriented toward elimination of dental biofilms and associated periodontal inflammation, recontouring and/or restoring of the soft and bone tissues when indicated to enable functional teeth support and particularly conditions for proper cleaning and good OH. In general, there is a large body of evidence showing that both non-surgical and surgical periodontal treatment is successful independently of age [80]. However, scientific evidence on periodontal treatment outcome in elderlies is not tremendous since the effect of age was rarely considered in data analysis [80, 81].
Initial step in treatment planning is determination of treatment goals and criteria for treatment success. In that context, the adjustment of periodontal diagnostic criteria for geriatric patients is of great importance. Although it was concluded that periodontal aging is unrelated to periodontal destruction seen in periodontitis [82], the periodontium undergoes some alterations requiring adjustment of the clinical criteria for periodontal diagnostics. Therefore, it was proposed that increase in crown-to-root ratio, probing depths ≤ 4 mm, moderate clinical attachment loss associated with physiological tooth mobility, and sporadic inflammation that is not pronounced can be considered a physiological finding in aging persons.
Two studies estimated the outcomes of surgical and non-surgical periodontal treatment between older and “younger” patients with moderately advanced or advanced periodontitis and showed comparable results while less bleeding on probing following surgical treatment in older population was even demonstrated [83, 84]. Regarding guided tissue regeneration, there is only one reported study that estimated the effect of aging on class II buccal furcation defects treatment and demonstrated a better treatment outcome in older population independently of treatment protocol using membrane alone or combined with enamel matrix derivate [85].
Regarding gingival recessions as intrinsic clinical manifestation of aging, there is no evidence on gingival recession treatment outcomes in older population but the appropriate treatment of gingival recessions is necessary to prevent the root caries highly prevalent in the older population [86]. However, it is expected that treatment outcome might be challenged by aging-related alterations in connective tissue and its metabolism. In brief, decline in progenitor cells, diminishing in keratinization of gingival epithelium, and perturbation of periodontal fibers provide susceptibility for gingival recessions. Such susceptibility might additionally impact the outcomes of surgical periodontal treatment since it can increase the risk for development of post-surgical gingival recessions. Hence, this should be carefully considered in treatment planning and prognosis.
Dental implants in geriatric patients present a particularly attractive issue in the current dentistry. The restoration of tooth loss using dental implants is the optimal solution for disabilities associated with tooth loss, thereby perfectly fitting requests addressed by WHO. However, what is the impact of aging in occurrence of biological complications in the short and long term was questioned. This concern was well elaborated in the recent review reported by Bartold et al. [87]. In general, the scientific evidence shows that the success rate in elderlies is similar to that of younger patients regarding both functional and esthetic outcomes [84,85,86]. In the study on long-term survival of rough-surface implants, it was reported that age was not related to implant survival time [88]. Regarding risk factors, similarly like to periodontitis, neglected OH, and systemic diseases and related medicaments are the main risk factors for dental implants. The small retrospective study demonstrated that patients over 70 years with well-controlled systemic diseases do not present high risk for implant-related complications while another group of researchers failed to demonstrate the relationship between osteoporosis and peri-implantitis [89, 90]. Indeed, it was demonstrated that use of bisphosphonates reduces survival and success rates of dental implants [91]. Regarding incidence of peri-implant diseases in older patients, it was reported that patients older than 65 years were more likely to develop such peri-implant mucositis or peri-implantitis while the positive association of increased prevalence of peri-implant disease with increased function time was additionally confirmed [92, 93]. Hence, geriatric patients can be considered suitable candidates for successful implant treatment while the control of numerous risk factors remains to be a necessity. From the biological standpoint, osseointegration presents the mechanism of implant anchorage in the bone molecularly regulated by Nf-kB signalization [87]. Related to that, a successful osseointegration is characterized by equilibrium between pro-formative and pro-resorptive processes within a balanced RANKL/OPG ratio. As previously suggested, the increased baseline levels of Nf-KB and crucial RANKL up-regulators senescent periodontium might play in disequilibration of RANKL/OPG within osseointegration and cause subsequent predomination of bone resorption. It is less possible that this would occur spontaneously in a successfully osseointegrated implant without signs of inflammation, but this might suggest that elderlies are at higher risk to develop severe peri-implant defect in presence of infective threats. Moreover, a good control of systemic conditions such as DM is essential for implant treatment success [94], since diseases with a familiar inflammatory pattern might contribute to development of the peri-implant disease. Finally, within implant treatment planning for geriatric patients, it is particularly important to disclose the use of drugs with detrimental effects on periodontal tissues and supportive bone with special emphasis on bisphosphonates [95, 96].
Concluding Remarks
Elderly population is the fastest growing population segment currently counting of about 841 million persons with expected growth of up to 2 billion persons by 2015 [97••], thus qualifying successful aging as the priority goal of leading medical organizations. Regarding the impact of periodontal health within successful aging concept, the senescent periodontium is not physiologically associated with progressive destruction while in turn, an infective challenge transforms the periodontium into potent inflammatory burden associated with severe periodontal destruction negatively affecting systemic aging. The clinical implications for geriatric patients would be the following:
-
Clinical protocols in the periodontal management of geriatric patients require adjustment regarding the preventive approach, diagnostic criteria, and treatment planning
-
Neglected oral hygiene is the major threat for periodontal health in elderlies that might simultaneously trigger many related complications of the systemic health, thus presenting a focus of entire clinical concept for periodontal management of these patients
-
Education and motivation of the older persons to establish and maintain the optimal oral hygiene should be the main objective of the preventive approach for geriatric patients
-
Careful assessment of the risk factors is required for this patient group since the specific treatment plan must be tailored to match unique needs of individual geriatric patients
-
Medical anamnesis of these patients should be attentively carefully assessed to disclose the presence of systemic pathologies and/or related treatments requiring intervention delaying, premedication or modification of the periodontal treatment plan
-
Appropriate home periodontal care regimen should be designed and prescribed against identified risk factors and in relation to the present disabilities that patient might demonstrate
-
Probing depths ≤ 4 mm, moderate clinical attachment loss associated with physiological tooth mobility and sporadic inflammation that is not pronounced can be considered a physiological finding in aging person
-
Treatment plan should focus on elimination of dental biofilms, reduction of periodontal inflammation, and improvement of the conditions for optimal plaque control as ultimate priorities while the esthetic aspect might be considered secondary priority when it is required for better achievement of aforementioned priority goals
-
Gingival recessions are highly prevalent in geriatric patients due to aging-related alterations in periodontal connective tissues and such susceptibility should be particularly considered in periodontal treatment planning and prognosis as potential risk for post-surgical recessions
-
Non-surgical and surgical treatment provides successful treatment outcomes in geriatric patients
-
Implant therapy has a high survival and success rate in patients with well-controlled systemic diseases while infection control represents an essential pre-condition for that
In summary, efficient plaque control presents the essential pre-condition to prevent bi-directional deteriorating effects between periodontitis and systemic aging and to ensure the successful oral and systemic aging.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance ••Of major importance
•• Newgard CB, Sharpless NE. Coming of age: molecular drivers of aging and therapeutic opportunities. J Clin Invest. 2013;123(3):946–50. https://doi.org/10.1172/JCI68833.
From the Centers for Disease Control and Prevention. Public health and aging: trends in aging—United States and worldwide. JAMA. 2003;289:1371–3.
•• López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217. https://doi.org/10.1016/j.cell.2013.05.039.
Rowe JW, Kahn RL. Successful aging. The Gerontologist. 1997;37(4):433–40. https://doi.org/10.1093/geront/37.4.433.
World Health Statistics 2015. 2015.
Dye BA. Global periodontal disease epidemiology. Periodontol. 2012;58(1):10–25. https://doi.org/10.1111/j.1600-0757.2011.00413.x.
•• Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJL, Marcenes W. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045–53. https://doi.org/10.1177/0022034514552491.
Costen JB. A syndrome of ear and sinus symptoms dependent upon disturbed function of the temporomandibular joint. 1934. Ann Otol Rhinol Laryngol. 1997;106(10):805–19. https://doi.org/10.1177/000348949710601002.
•• Locker D. Measuring oral health: a conceptual framework. Community Dent Health. 1988;5(1):3–18.
Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet Lond Engl. 2012;380(9859):2197–223. https://doi.org/10.1016/S0140-6736(12)61689-4.
Loos BG. Periodontal medicine: work in progress! J Clin Periodontol. 2016;43(6):470–1. https://doi.org/10.1111/jcpe.12550.
Lamster IB, Asadourian L, Del Carmen T, Friedman PK. The aging mouth: differentiating normal aging from disease. Periodontol. 2016;72(1):96–107. https://doi.org/10.1111/prd.12131.
Graves DT, Li J, Cochran DL. Inflammation and uncoupling as mechanisms of periodontal bone loss. J Dent Res. 2011;90(2):143–53. https://doi.org/10.1177/0022034510385236.
Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10(10):717–25. https://doi.org/10.1038/nrmicro2873.
Cochran DL. Inflammation and bone loss in periodontal disease. J Periodontol. 2008;79(8s):1569–76. https://doi.org/10.1902/jop.2008.080233.
Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol. 2014;29(6):248–57. https://doi.org/10.1111/omi.12065.
Hajishengallis G, Lamont RJ. Breaking bad: manipulation of the host response by Porphyromonas gingivalis: HIGHLIGHTS. Eur J Immunol. 2014;44(2):328–38. https://doi.org/10.1002/eji.201344202.
Brunet A, Berger SL. Epigenetics of aging and aging-related disease. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S17–20. https://doi.org/10.1093/gerona/glu042.
Razak PA, Richard KJ, Thankachan RP, Hafiz KA, Kumar KN, Sameer KM. Geriatric oral health: a review article. J Int Oral Health JIOH. 2014;6:110.
Guggenheimer J, Moore PA. Xerostomia: etiology, recognition and treatment. J Am Dent Assoc. 2003;134(1):61–119. https://doi.org/10.14219/jada.archive.2003.0018.
Nagler RM. Salivary glands and the aging process: mechanistic aspects, health-status and medicinal-efficacy monitoring. Biogerontology. 2004;5(4):223–33. https://doi.org/10.1023/B:BGEN.0000038023.36727.50.
Astor FC, Hanft KL, Ciocon JO. Xerostomia: a prevalent condition in the elderly. Ear Nose Throat J. 1999;78(7):476–9.
Scelza MFZ, Silva Dde F, Ahiadzro NK, Da Silva LE, Scelza P. The influence of medication on salivary flow of the elderly: preliminary study. Gerodontology. 2010;27(4):278–82. https://doi.org/10.1111/j.1741-2358.2009.00326.x.
Severson JA, Moffett BC, Kokich V, Selipsky H. A histologic study of age changes in the adult human periodontal joint (ligament). J Periodontol. 1978;49(4):189–200. https://doi.org/10.1902/jop.1978.49.4.189.
van der Velden U. Effect of age on the periodontium. J Clin Periodontol. 1984;11(5):281–94. https://doi.org/10.1111/j.1600-051X.1984.tb01325.x.
Abiko Y, Shimizu N, Yamaguchi M, Suzuki H, Takiguchi H. Effect of aging on functional changes of periodontal tissue cells. Ann Periodontol. 1998;3(1):350–69. https://doi.org/10.1902/annals.1998.3.1.350.
Takatsu M, Uyeno S, Komura J, Watanabe M, Ono T. Age-dependent alterations in mRNA level and promoter methylation of collagen alpha1(I) gene in human periodontal ligament. Mech Ageing Dev. 1999;110(1-2):37–48. https://doi.org/10.1016/S0047-6374(99)00041-X.
Johnson BD, Page RC, Narayanan AS, Pieters HP. Effects of donor age on protein and collagen synthesis in vitro by human diploid fibroblasts. Lab Investig J Tech Methods Pathol. 1986;55:490–6.
Lee W, McCulloch CA. Deregulation of collagen phagocytosis in aging human fibroblasts: effects of integrin expression and cell cycle. Exp Cell Res. 1997;237(2):383–93. https://doi.org/10.1006/excr.1997.3802.
Perunovic N, Rakic M, Jankovic S, Aleksic Z, Struillou X, Cakic S, et al. MMP-9-1562 C> T (rs3918242) promoter polymorphism as a susceptibility factor for multiple gingival recessions. Int J Periodontics Restorative Dent. [Internet]. 2015 [cited 2016 Mar 15];35. Available from: http://search.ebscohost.com/login.aspx?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=01987569&AN=102660917&h=69iPx9DyoSwxUuW%2BRj9mJQBsw6PxsEKVEkQ0FvUA1GR73xNOhAq0C7OEbS9snCU6jRdJqt9yc22UEgaIPdG%2BOQ%3D%3D&crl=c
Huttner EA, Machado DC, de Oliveira RB, Antunes AGF, Hebling E. Effects of human aging on periodontal tissues. Spec Care Dentist. 2009;29(4):149–55. https://doi.org/10.1111/j.1754-4505.2009.00082.x.
Bartold PM, Boyd RR, Page RC. Proteoglycans synthesized by gingival fibroblasts derived from human donors of different ages. J Cell Physiol. 1986;126(1):37–46. https://doi.org/10.1002/jcp.1041260106.
Huang L, Salmon B, Yin X, Helms JA. From restoration to regeneration: periodontal aging and opportunities for therapeutic intervention. Periodontol. 2016;72(1):19–29. https://doi.org/10.1111/prd.12127.
•• Hajishengallis G. Aging and its impact on innate immunity and inflammation: implications for periodontitis. J Oral Biosci. 2014;56(1):30–7. https://doi.org/10.1016/j.job.2013.09.001.
Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54.
Salminen A, Kaarniranta K, Kauppinen A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging. 2012;4(3):166–75. https://doi.org/10.18632/aging.100444.
Rakic M, Lekovic V, Nikolic-Jakoba N, Vojvodic D, Petkovic-Curcin A, Sanz M. Bone loss biomarkers associated with peri-implantitis. A cross-sectional study. Clin Oral Implants Res. 2013;24(10):1110–6. https://doi.org/10.1111/j.1600-0501.2012.02518.x.
Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004;15(6):457–75. https://doi.org/10.1016/j.cytogfr.2004.06.004.
Tsutsumi R, Xie C, Wei X, Zhang M, Zhang X, Flick LM, et al. PGE2 signaling through the EP4 receptor on fibroblasts upregulates RANKL and stimulates osteolysis. J Bone Miner Res Off J Am Soc Bone Miner Res. 2009;24(10):1753–62. https://doi.org/10.1359/jbmr.090412.
Wu Y, Dong G, Xiao W, Xiao E, Miao F, Syverson A, et al. Effect of aging on periodontal inflammation, microbial colonization, and disease susceptibility. J Dent Res. 2016;95(4):460–6. https://doi.org/10.1177/0022034515625962.
Choi B-K, Moon S-Y, Cha J-H, Kim K-W, Yoo Y-J. Prostaglandin E(2) is a main mediator in receptor activator of nuclear factor-kappaB ligand-dependent osteoclastogenesis induced by Porphyromonas gingivalis, Treponema denticola, and Treponema socranskii. J Periodontol. 2005;76(5):813–20. https://doi.org/10.1902/jop.2005.76.5.813.
Ellen RP. Periodontal disease among older adults: what is the issue? Periodontol. 1998;16(1):7–8. https://doi.org/10.1111/j.1600-0757.1998.tb00111.x.
Cockburn AF, Dehlin JM, Ngan T, Crout R, Boskovic G, Denvir J, et al. High throughput DNA sequencing to detect differences in the subgingival plaque microbiome in elderly subjects with and without dementia. Investig Genet. 2012;3(1):19. https://doi.org/10.1186/2041-2223-3-19.
Shoemark DK, Allen SJ. The microbiome and disease: reviewing the links between the oral microbiome, aging, and Alzheimer’s disease. J Alzheimers Dis JAD. 2015;43(3):725–38. https://doi.org/10.3233/JAD-141170.
Zapata HJ, Quagliarello VJ. The microbiota and microbiome in aging: potential implications in health and age-related diseases. J Am Geriatr Soc. 2015;63(4):776–81. https://doi.org/10.1111/jgs.13310.
Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67(10s):1123–37. https://doi.org/10.1902/jop.1996.67.10s.1123.
Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol. 1998;3(1):51–61. https://doi.org/10.1902/annals.1998.3.1.51.
Van Dyke TE, van Winkelhoff AJ. Infection and inflammatory mechanisms. J Periodontol. 2013;84(4-s):S1–7. https://doi.org/10.1902/jop.2013.1340018.
Perunovic ND, Rakic MM, Nikolic LI, Jankovic SM, Aleksic ZM, Plecas DV, et al. The association between periodontal inflammation and labor triggers elevated cytokine levels in pre-term birth: a cross-sectional study. J Periodontol. 2015:1–13.
Akcalı A, Güven Yılmaz S, Lappin DF, Eğrilmez S, Buduneli N. Effect of gingival inflammation on the inflammatory response in patients with idiopathic uveitis. J Clin Periodontol. 2016;43(8):637–45. https://doi.org/10.1111/jcpe.12555.
Gümüş P, Buduneli E, Bıyıkoğlu B, Aksu K, Saraç F, Nile C, et al. Gingival crevicular fluid, serum levels of receptor activator of nuclear factor-κB ligand, osteoprotegerin, and interleukin-17 in patients with rheumatoid arthritis and osteoporosis and with periodontal disease. J Periodontol. 2013;84(11):1627–37. https://doi.org/10.1902/jop.2013.120595.
Retzepi M, Donos N. The effect of diabetes mellitus on osseous healing: the effect of diabetes mellitus on osseous healing. Clin Oral Implants Res. 2010;21(7):673–81. https://doi.org/10.1111/j.1600-0501.2010.01923.x.
Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ. Inflammation and Alzheimer’s disease: possible role of periodontal diseases. Alzheimers Dement. 2008;4(4):242–50. https://doi.org/10.1016/j.jalz.2007.08.004.
Sen S, Sumner R, Hardin J, Barros S, Moss K, Beck J, et al. Periodontal disease and recurrent vascular events in stroke/transient ischemic attack patients. J Stroke Cerebrovasc Dis. 2013;22(8):1420–7. https://doi.org/10.1016/j.jstrokecerebrovasdis.2013.06.024.
Marfil-Álvarez R, Mesa F, Arrebola-Moreno A, Ramírez-Hernández JA, Magán-Fernández A, O’Valle F, et al. Acute myocardial infarct size is related to periodontitis extent and severity. J Dent Res. 2014;93(10):993–8. https://doi.org/10.1177/0022034514548223.
Sanz M, Kornman K, Working group 3 of joint EFP/AAP workshop. Periodontitis and adverse pregnancy outcomes: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013;40(Suppl 14):S164–9. https://doi.org/10.1111/jcpe.12083.
•• Jepsen S, Stadlinger B, Terheyden H, Sanz M. Science transfer: oral health and general health—the links between periodontitis, atherosclerosis and diabetes. J Clin Periodontol. 2015;42(12):1071–3. https://doi.org/10.1111/jcpe.12484.
Pucar A, Milasin J, Lekovic V, Vukadinovic M, Ristic M, Putnik S, et al. Correlation between atherosclerosis and periodontal putative pathogenic bacterial infections in coronary and internal mammary arteries. J Periodontol. 2007;78(4):677–82. https://doi.org/10.1902/jop.2007.060062.
•• Figuero E, Sánchez-Beltrán M, Cuesta-Frechoso S, Tejerina JM, del Castro JA, Gutiérrez JM, et al. Detection of periodontal bacteria in atheromatous plaque by nested polymerase chain reaction. J Periodontol. 2011;82(10):1469–77. https://doi.org/10.1902/jop.2011.100719.
Reyes L, Herrera D, Kozarov E, Roldán S, Progulske-Fox A. Periodontal bacterial invasion and infection: contribution to atherosclerotic pathology. J Clin Periodontol. 2013;40:S30–50. https://doi.org/10.1111/jcpe.12079.
Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Clin Periodontol. 2013;40:S51–69. https://doi.org/10.1111/jcpe.12060.
Meurman JH, Sanz M, Janket S-J. Oral health, atherosclerosis, and cardiovascular disease. Crit Rev Oral Biol Med Off Publ Am Assoc Oral Biol. 2004;15(6):403–13. https://doi.org/10.1177/154411130401500606.
D’Aiuto F, Orlandi M, Gunsolley JC. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J Clin Periodontol. 2013;40:S85–105. https://doi.org/10.1111/jcpe.12061.
Palm F, Lahdentausta L, Sorsa T, Tervahartiala T, Gokel P, Buggle F, et al. Biomarkers of periodontitis and inflammation in ischemic stroke: a case-control study. Innate Immun. 2014;20(5):511–8. https://doi.org/10.1177/1753425913501214.
Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol. 2001;6(1):99–112. https://doi.org/10.1902/annals.2001.6.1.99.
•• Chapple ILC, Genco R, Working group 2 of the joint EFP/AAP workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013;40:S106–12. https://doi.org/10.1111/jcpe.12077.
Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Clin Periodontol. 2013;40:S135–52. https://doi.org/10.1111/jcpe.12080.
Sgolastra F, Severino M, Pietropaoli D, Gatto R, Monaco A. Effectiveness of periodontal treatment to improve metabolic control in patients with chronic periodontitis and type 2 diabetes: a meta-analysis of randomized clinical trials. J Periodontol. 2013;84(7):958–73. https://doi.org/10.1902/jop.2012.120377.
Taylor JJ, Preshaw PM, Lalla E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol. 2013;40:S113–34. https://doi.org/10.1111/jcpe.12059.
Scannapieco FA, Cantos A. Oral inflammation and infection, and chronic medical diseases: implications for the elderly. Periodontol. 2016;72(1):153–75. https://doi.org/10.1111/prd.12129.
Noble JM, Borrell LN, Papapanou PN, Elkind MSV, Scarmeas N, Wright CB. Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES-III. J Neurol Neurosurg Psychiatry. 2009;80(11):1206–11. https://doi.org/10.1136/jnnp.2009.174029.
Novaes AB Jr, Souza SLS, de Barros RRM, KKY P, Iezzi G, Piattelli A. Influence of implant surfaces on osseointegration. Braz Dent J. 2010;21(6):471–81. https://doi.org/10.1590/S0103-64402010000600001.
Kubota T, Maruyama S, Abe D, Tomita T, Morozumi T, Nakasone N, et al. Amyloid beta (A4) precursor protein expression in human periodontitis-affected gingival tissues. Arch Oral Biol. 2014;59(6):586–94. https://doi.org/10.1016/j.archoralbio.2014.03.004.
Miklossy J. Chronic inflammation and amyloidogenesis in Alzheimer’s disease—role of Spirochetes1. J Alzheimers Dis. 2008;13(4):381–91. https://doi.org/10.3233/JAD-2008-13404.
Kamer AR, Craig RG, Pirraglia E, Dasanayake AP, Norman RG, Boylan RJ, et al. TNF-α and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J Neuroimmunol. 2009;216(1-2):92–7. https://doi.org/10.1016/j.jneuroim.2009.08.013.
Shay K. Infectious complications of dental and periodontal diseases in the elderly population. Clin Infect Dis Off Publ Infect Dis Soc Am. 2002;34(9):1215–23. https://doi.org/10.1086/339865.
Yoneyama T, Yoshida M, Ohrui T, Mukaiyama H, Okamoto H, Hoshiba K, et al. Oral care reduces pneumonia in older patients in nursing homes. J Am Geriatr Soc. 2002;50(3):430–3. https://doi.org/10.1046/j.1532-5415.2002.50106.x.
Renvert S, Persson RE, Persson GR. A history of frequent dental care reduces the risk of tooth loss but not periodontitis in older subjects. Swed Dent J. 2011;35(2):69–75.
Gonsalves WC, Wrightson AS, Henry RG. Common oral conditions in older persons. Am Fam Physician. 2008;78(7):845–52.
Renvert S, Persson GR. Treatment of periodontal disease in older adults. Periodontol. 2016;72(1):108–19. https://doi.org/10.1111/prd.12130.
Trombelli L, Rizzi A, Simonelli A, Scapoli C, Carrieri A, Farina R. Age-related treatment response following non-surgical periodontal therapy. J Clin Periodontol. 2010;37(4):346–52. https://doi.org/10.1111/j.1600-051X.2010.01541.x.
Papapanou PN, Lindhe J, Sterrett JD, Eneroth L. Considerations on the contribution of ageing to loss of periodontal tissue support. J Clin Periodontol. 1991;18(8):611–5. https://doi.org/10.1111/j.1600-051X.1991.tb00098.x.
Lindhe J, Socransky S, Nyman S, Westfelt E, Haffajee A. Effect of age on healing following periodontal therapy. J Clin Periodontol. 1985;12(9):774–87. https://doi.org/10.1111/j.1600-051X.1985.tb01403.x.
Abbas F, van der Velden U, Hart AA. Relation between wound healing after surgery and susceptibility to periodontal disease. J Clin Periodontol. 1984;11(4):221–9. https://doi.org/10.1111/j.1600-051X.1984.tb02212.x.
Hoffmann T, Richter S, Meyle J, Gonzales JR, Heinz B, Arjomand M, et al. A randomized clinical multicentre trial comparing enamel matrix derivative and membrane treatment of buccal class II furcation involvement in mandibular molars. Part III: patient factors and treatment outcome. J Clin Periodontol. 2006;33(8):575–83. https://doi.org/10.1111/j.1600-051X.2006.00947.x.
Bharateesh JV, Kokila G. Association of root caries with oral habits in older individuals attending a rural health centre of a dental hospital in India. J Clin Diagn Res JCDR. 2014;8(11):ZC80–2. https://doi.org/10.7860/JCDR/2014/8771.5165.
Bartold PM, Ivanovski S, Darby I. Implants for the aged patient: biological, clinical and sociological considerations. Periodontol. 2016;72(1):120–34. https://doi.org/10.1111/prd.12133.
Becker ST, Beck-Broichsitter BE, Rossmann CM, Behrens E, Jochens A, Wiltfang J. Long-term survival of Straumann dental implants with TPS surfaces: a retrospective study with a follow-up of 12 to 23 years. Clin Implant Dent Relat Res. 2016;18(3):480–8. https://doi.org/10.1111/cid.12334.
Lee H-J, Kim Y-K, Park J-Y, Kim S-G, Kim M-J, Yun P-Y. Short-term clinical retrospective study of implants in geriatric patients older than 70 years. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(4):442–6. https://doi.org/10.1016/j.tripleo.2010.02.019.
Dvorak G, Arnhart C, Heuberer S, Huber CD, Watzek G, Gruber R. Peri-implantitis and late implant failures in postmenopausal women: a cross-sectional study. J Clin Periodontol. 2011;38(10):950–5. https://doi.org/10.1111/j.1600-051X.2011.01772.x.
Ata-Ali J, Ata-Ali F, Peñarrocha-Oltra D, Galindo-Moreno P. What is the impact of bisphosphonate therapy upon dental implant survival? A systematic review and meta-analysis. Clin Oral Implants Res. 2016;27(2):e38–46. https://doi.org/10.1111/clr.12526.
Marrone A, Lasserre J, Bercy P, Brecx MC. Prevalence and risk factors for peri-implant disease in Belgian adults. Clin Oral Implants Res. 2013;24(8):934–40. https://doi.org/10.1111/j.1600-0501.2012.02476.x.
Meijer HJA, Raghoebar GM, de Waal YCM, Vissink A. Incidence of peri-implant mucositis and peri-implantitis in edentulous patients with an implant-retained mandibular overdenture during a 10-year follow-up period. J Clin Periodontol. 2014;41(12):1178–83. https://doi.org/10.1111/jcpe.12311.
Monje A, Catena A, Borgnakke WS. Association between diabetes mellitus/hyperglycaemia and peri-implant diseases: systematic review and meta-analysis. J Clin Periodontol. 2017;44(6):636–48. https://doi.org/10.1111/jcpe.12724.
Paiva-Fonseca F, Santos-Silva A-R, Della-Coletta R, Vargas P-A, Lopes M-A. Alendronate-associated osteonecrosis of the jaws: a review of the main topics. Med Oral Patol Oral Cirugia Bucal. 2014;19:e106–11.
Seymour RA, Thomason JM, Ellis JS. The pathogenesis of drug-induced gingival overgrowth. J Clin Periodontol. 1996;23(3):165–75. https://doi.org/10.1111/j.1600-051X.1996.tb02072.x.
•• World Health Organization, editor. World report on ageing and health. Geneva, Switzerland: World Health Organization; 2015.
Acknowledgments
This work was supported by the Ministry of Education and Science, Republic of Serbia (project references #41008 and #173056).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Systemic Diseases
Rights and permissions
About this article
Cite this article
Rakic, M., Vojvodic, D. & Sculean, A. Periodontology for Geriatric Patients. Curr Oral Health Rep 5, 39–49 (2018). https://doi.org/10.1007/s40496-018-0169-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40496-018-0169-z