Abstract
Berberine (BBR) is a naturally occurring alkaloid compound found in various plant parts linked to numerous health benefits. Its use in traditional Ayurvedic health practices dates back to ancient civilizations. The aim of this review is to provide an overview of current studies that examine the development and increased potency of BBR’s therapeutic effects. This review highlights the potential therapeutic properties of this phytochemical compound and its potential use as an alternative to conventional treatment for chronic ailments such as diabetes, cardiovascular diseases, and cancer. Both animal and clinical researches have demonstrated the therapeutic effects of BBR on these diseases, and studies on its toxicity are also included. According to the studies reviewed, BBR has a positive effect on several physiological processes, including glucose and lipid metabolism, inflammation, oxidative stress, and endothelial function. It can also inhibit tumor growth and promote apoptosis in cancer cells. Furthermore, BBR has exhibited the capacity to ameliorate cardiac performance, diminish arterial blood pressure and serum cholesterol concentrations, and augment insulin responsiveness. Collectively, the extant body of knowledge posits that BBR holds therapeutic promise across a spectrum of pathological conditions. Nevertheless, an augmented investigation effort is requisite to ascertain its optimally efficacious dosage, administration modality, and potential untoward reactions. Moreover, extended examinations are imperative to appraise its enduring safety profile and efficaciousness, particularly when employed concomitantly with other pharmacotherapies. Despite a predominantly affirmative outcome within prevailing investigations, it remains imperative to conduct further exhaustive scrutiny, particularly via clinical trials, in order to substantiate its therapeutic security and efficacy in the management of ailments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, pharmaceutical companies are dedicating their time and resources towards researching and developing herbal medication formulations that can be used to manage chronic diseases [1,2,3,4]. The World Health Organization also supports the use of conventional treatments that have natural origins because they are easily accessible and relatively inexpensive to produce [1, 5, 6]. This shift towards natural remedies is mainly due to the fact that some synthetic pharmaceutical drugs may have harmful side effects when used for long-term treatment of chronic diseases. The use of plant-based products as natural medication to treat chronic disorders is a traditional method that has been supported by Ayurveda, an ancient medical system originating from the Indian subcontinent that is now used as an alternative treatment worldwide [7,8,9,10].
Natural products have gained popularity as an alternative to conventional medicine due to their high effectiveness and minimal adverse effects, according to studies [11,12,13,14]. Berberine (BBR) which possesses potent antibacterial, antiprotozoal, antidiarrheal, and anti-trachoma effects, has been used in Ayurveda, Chinese medicine, and Middle Eastern folk medicine for more than 3000 years [15, 16]. In recent years, clinical research has revealed BBR’s effectiveness against various disorders, including hypertension, arrhythmia, hyperglycemia, cancer, depression, inflammation, analgesia, and hypolipidemia [17, 18]. BBR has proven itself to be a promising drug with multispectral activities (Fig. 1).
This review focuses on recent studies to show the drug’s delivery on therapeutic effects to increased potency rather than summarizing earlier findings on pharmacology of BBR.
BBR Chemical Structure and Plant Sources
BBR has a molecular weight of 336.37 g/mol and a chemical formula of C20H18NO4+(16,17-dimethoxy-5,7-dioxa-13-azoniapentacyclo[11.8.0.02,10.04,8.015,20] henicosa-1(13),2,4 (8),9,14,16,18,20-octaene) [19, 21]. It is a stable compound with a bitter taste and appears as a yellow crystalline powder. BBR is highly soluble in polar solvent like water and ethanol, but less soluble in organic solvents such as acetone and ether [22]. The hydrochloride form of BBR, which has a lower water solubility rate compared to its sulfate and phosphate forms, dissolves more readily in hot water. Ethanol and methanol are commonly used agents for obtaining BBR from different plant sources [19]. BBR extraction techniques have evolved in recent years, with both traditional methods like Soxhlet extraction, percolation, and maceration, as well as modern techniques like ultrahigh pressure method, supercritical fluid extraction, and microwave-assisted extraction being employed [15, 19, 23]. Raw extracted BBR is now used to make oral pills or capsules for clinical use. BBR is readily available from medicinal herbs conventionally used and is found in abundance in different parts of medicinally significant plants, such as the stem, tuber, and bark [19] (Table 1).
BBR has been used in normal diet as traditional medicine for centuries, particularly in Chinese medicine as antimicrobial, anti-fungal, antiprotozoal, and anti-effective against different viral, parasitic, and other infections [25]. Sometimes, Chinese people used it as dietary supplement to treat common cold, influenza, and other respiratory infections [26].
Therapeutic Applications of BBR
Anticancer Activity
BBR is one of the powerful substances utilized in anticancer therapy which is derived from a variety of plants. It is shown to have an effective activity against a wide range of cancer including breast cancer, lung cancer, cervical cancer and gastric cancer [21].
Breast Cancer
Breast cancer has become a major concern for women worldwide, claiming numerous lives over the years [27]. Studies have revealed that BBR can effectively reduce cell viability and inhibit the multiplication of cancerous cells [28]. Another study also indicated that BBR can lower the levels of metadherin, which acts as a catalyst for the growth of cancerous cells, thus preventing their proliferation [29]. BBR was found to inhibit the growth of MDA-MB-468 cells by blocking cyclin D/cyclin-dependent kinase 4 (CDK4) complexes and stimulating the cell growth inhibitor p38, leading to the arrest of the cell cycle at the G1/S stage. BBR also reduced the proliferation of MDA-MB-231 cells by blocking the cyclin A/CDK1 cell cycle kinase complex and the AKT/ERK pathways, inhibiting the cell cycle at the G2/M phase [28, 30]. BBR can help in reducing abnormal growth of the cell, apoptosis, and inflammation. It can also be used in coordination with other chemotherapy medicines like cisplatin, 5-fluorouracil, methyl methanesulfonate, and camptothecin to prevent and treat breast cancer [29, 30].
Lung Cancer
Lung cancer is a dangerous and widespread form of cancer that results in a significant number of deaths worldwide [31]. Studies have shown that BBR can reduce the growth of non-small cell lung cancer (NSCLC) xenograft tumors in humans via the SWI-independent-3 transcription regulator. Additionally, BBR can cause DNA damage by downregulating TOP2 levels, leading to NSCLC cell death. BBR also stimulates the miR19a/TF/MAPK signaling pathway, which further promotes NSCLC cell death [31, 32]. BBR has great potential to prevent lung cancer by reducing cell growth, self-destruction of cancer cells, and boosting the anticarcinogenic impact of tyrosine kinase inhibitors. However, future research is necessary to explore other potential mechanisms of BBR-based lung cancer prevention [31,32,33].
Ovarian Cancer
Ovarian cancer is a significant health hazard and prevalent malignancy in women worldwide [30, 34]. BBR has shown promise in enhancing the effectiveness of many anticancer drugs in preventing the growth of ovarian cancer cells. For example, in in-depth analysis of caspase-dependent and RIPK3-MLKL pathways, the conjunction with BBR and cisplatin can effectively induced the ovarian cancer cell to be inactivated, ceased cellular mechanism and gradually program cell death [35]. In another study, BBR was found to inhibit the independent phospholipase A2 (iPLA2)-arachidonic acid (AA)-cyclooxygenase-2 (COX-2)-prostaglandin E2 (PGE2) pathway and reverse the phosphorylation-induced growth of focal adhesion kinase (FAK) caused by the chemotherapeutic drug VP16 [36]. It was also known that BBR in association with drug niraparib has great potential to use as anticancer against ovarian cancer by inducing DNA damage [35, 36]. BBR has great potential to enhance the effectiveness of drugs in conjugation with anticancer drugs especially in ovarian cancer cells by following different cell mechanism and pathways. BBR-based ovarian cancer prevention and treatment need to be explored more with focus to the potential mechanisms of [35, 36].
Gastric Cancer
Stomach cancer is a prevalent disease across the world [30, 37]. Several studies have reported that BBR can prevent stomach cancer through various mechanisms. For example, BBR can inhibit the proliferation of SGC-7901 cells by blocking the cell cycle at G1 phase [38]. In in vitro and in vivo experiments, BBR can inhibit the growth of cancer cells and reduce the release of IL-8 by inactivating the MAPK signaling pathways [39]. Moreover, it is also known that the BBR can suppress and inhibit the STAT3 stimulation and phosphorylation of the epidermal growth factor receptor (EGFR) in the prevention of stomach cancer [37, 38].
Effect Against Diabetes
BBR has been found to have a significant impact on lipid and carbohydrate metabolism, particularly on glucose homeostasis, according to recent preclinical and clinical investigations [1]. It stimulates the insulin receptor in the liver and skeletal muscle by promoting kinase C–dependent protein, as reported in some studies [40]. Yin and colleagues demonstrated that BBR can be used to treat type 2 diabetes in humans [41]. According to their research findings, BBR had a significant impact on reducing both fasting blood glucose levels and postprandial blood glucose throughout the entire experiment, starting from the first week until the end. The study also revealed that fasting plasma insulin decreased by 28.1%, and the index for assessing insulin resistance (known as homeostasis model assessment) dropped by 44.7% (p 0.001). Additionally, the hemoglobin A1C level decreased from 8.1 ± 0.2 to 7.3 ± 0.3% (p 0.001). The trial showed that 20 (34.5%) of the participants experienced temporary gastrointestinal side effects, while there were no observations of liver or renal impairment. These findings suggested that BBR has a potent oral hypoglycemic effect and is effective in lipid metabolism, making it a promising drug for diabetes treatment [1, 41, 42].
Kong et al. carried out a study aimed at investigating the cellular mechanism by which BBR enhances insulin sensitivity, and they observed that it activates the insulin receptor (InsR), as reported in their publication [43]. Specifically, they found that BBR administration to human liver cells in vitro resulted in a dose- and time-dependent increase in InsR expression, InsR mRNA, and protein expression. When administered to diabetic mice with insulin-deficient type 2 diabetes, BBR reduced blood glucose levels; however, it was not effective against NOD/LtJ type 1 diabetes mellitus. From this, it can be concluded that BBR is a unique phytochemical component that could be useful for treating insulin resistance especially in type 2 diabetes mellitus [42, 44, 45].
Cardiovascular
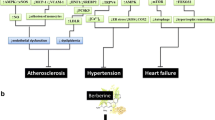
Cardiovascular disease (CVD), encompassing atherosclerosis, hypertension, and heart failure, is the most prevalent cause of mortality globally [46,47,48,49]. Including the developed nations, cardiovascular diseases have greatly impact in mortality despite its preventive measures to mitigate its incidence; numerous patients remain vulnerable to it [50]. And moreover, in the last two decades, the prevalence of morbidity and mortality due to CVD is still alarmingly high [46,47,48,49].
Effect of BBR on Atherosclerosis
Atherosclerosis is a primary disorder of lipid metabolism, which is also associated with numerous cardiovascular and brain diseases [51,52,53]. The progression of atherosclerosis often goes unnoticed for several years, and patients typically become aware of their condition only after experiencing cardiovascular diseases, such as stroke or heart attack. Atherosclerosis is initiated due to the accumulation of low-density lipoprotein (LDL) particles in endothelial cells [54]. Therefore, effective strategies to prevent the progression of atherosclerosis include reducing endothelial dysfunction, reversing dyslipidemia, and suppressing inflammation. BBR has been shown to protect against atherosclerosis by lowering cholesterol levels [51, 52, 55, 56]. The other aspect of BBR includes atheroprotective effects, such as its ability to reduce inflammation, prevent vascular smooth muscle cell proliferation, and act as an antioxidant. Researchers Zimetti and colleagues have demonstrated that BBR also has a dual protective effect on cholesterol homeostasis and on the inflammatory phenotype in macrophages from both mice and humans [57]. In their study, treatment with BBR (150 mg/kg/d, p.o., 12 weeks) resulted in a significant reduction in atherosclerotic plaque area, as well as the suppression of inflammatory and oxidative markers [58]. By controlling different pro-atherogenic cellular events, BBR can effectively prevent the progression of atherosclerosis.
BBR on Hypertension
Hypertension is a widespread chronic medical condition worldwide and a major preventable risk factor for early mortality. Failure to detect hypertension early and provide effective treatment can lead to an increased risk of cardiovascular diseases, including ischemic heart disease, stroke, peripheral vascular disease, and heart failure. Nevertheless, decreasing blood pressure can considerably diminish the likelihood of fatality due to heart disease and stroke. A clinical study conducted in 1993 revealed the efficacy of BBR in treating hypertension in 42 cases [59, 60]. While the precise mechanism responsible for the modulatory impact of BBR on hypertension is currently under investigation, both clinical and experimental researches indicate that BBR and its derivatives exhibit properties that can counteract hypertension [40]. Control of high blood pressure is associated with a reduced risk of developing complications such as stroke, myocardial infarction, heart failure, and peripheral vascular disease [61]. BBR has potential effects to use as drugs for antihypertensive treatment as evidence in both clinical and experimental studies. According to a meta-analysis, taking BBR in combination with lifestyle changes is more effective in reducing blood pressure than just lifestyle changes or a placebo for treating hypertension. From a scientific standpoint, it has been observed that the synergistic utilization of BBR alongside an oral antihypertensive medication yields superior outcomes in blood pressure reduction compared to the singular administration of the antihypertensive drug. Notably, the customary daily consumption of BBR falls within the range of 0.6 to 2.7 g. Furthermore, it is significant to highlight that 27 randomized controlled clinical trials employing sole BBR treatment have reported no significant adverse effects [40].
BBR combined with amlodipine has been shown to have a significant clinical impact on elderly patients with gout and hypertension by effectively lowering blood pressure and UA levels [62, 63]. While the exact mechanism of how BBR affects hypertension is not yet fully understood, clinical and experimental studies have indicated that BBR and its derivatives may possess antihypertensive properties. Hypertension pathogenesis is linked to endothelial cell oxidative stress and cell death caused by endoplasmic reticulum (ER) stress activation. In SHR carotid arteries, Liu et al. found that BBR could inhibit ER stress, activate AMPK, scavenge ROS, and reduce endothelium-dependent contractions by downregulating COX-2 [64]. A recent study revealed that BBR directly relaxes blood vessels to lower blood pressure in old mice while also reducing vascular stiffness [65]. It can lower high blood pressure by affecting vascular stiffness, and high blood pressure is achieved by inhibiting TRPV4, decreasing intracellular calcium levels, reducing MLC phosphorylation, and relaxing VSMCs [65]. Furthermore, in aging ApoE KO mice, BBR may function as a calcium channel blocker to reduce vascular stiffness and high blood pressure. Hypertension awareness and management challenges are widespread, but it is important to recognize that the burden of hypertension varies not only by country but also by ethnicity and socioeconomic status. Controlling blood pressure is associated with a 30–35% reduction in strokes, a 20% reduction in myocardial infarction, and a decreased risk of other hypertension complications such as heart failure, atrial fibrillation, and ventricular arrhythmias [66].
BBR on Heart Failure
Heart failure is associated to various cardiac diseases including cardiac remodeling, cardiac arrest, hypertrophy, and fibrosis [1, 5]. Effective interventions for cardiac remodeling are crucial for the treatment of heart failure [1]. Pathological cardiac hypertrophy is a key transitional stage that leads to heart failure and other cardiac diseases [1]. In the realm of scientific understanding, Pak1, belonging to the serine/threonine protein kinase family, plays a vital role in facilitating adaptive physiological cardiac remodeling. Exhaustive investigations have been conducted to explore its roles in upholding electrophysiological stability and maintaining ventricular Ca2+ homeostasis in the presence of physiological, β-adrenergic, and hypertrophic stress conditions [1]. Interestingly, BBR can be used against the heart failure and has been found to have multiple pharmacological activities [1]. In a clinical investigation involving 12 individuals afflicted with intractable congestive heart failure, berberine (BBR) exhibited a safeguarding influence. The intravenous administration of BBR, dosed at 0.2 mg/kg per minute, yielded noteworthy alterations in hemodynamic parameters [7].
Studies using animal models have demonstrated the potential of BBR to reduce the severity of heart failure (HF). As an illustration, the administration of BBR in canines afflicted with ischemic left ventricular heart failure has demonstrated a propensity to augment cardiac output and the apex rate of ascent of left ventricular pressure (+dp/dt), concomitantly with a reduction in left ventricular end-diastolic pressure (LVEDP), diastolic blood pressure (DBP), and systemic vascular resistance. In a parallel manner, within a rat model simulating heart failure (HF) induced by a heightened dosage of isoproterenol, the simultaneous administration of total saponins extracted from Panax ginseng (20 mg/kg/d), BBR (20 mg/kg/d), and captopril over a continuous period of 12 days showcased a discernible anti-heart failure effect in both experimental cohorts [67, 68].
Effect of BBR on Neurology
BBR plays a pivotal role as neuroprotective against ischemic damage by suppressing Bcl-2 phosphorylation. BBR has been shown to have a neuroprotective effect that improves survival of brain cells that are electrically excitable survive, develop, function, and be protected [69]. Therefore, it can be used as a powerful phytoconstituent in the treatment of a variety of neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease [70,71,72]. BBR has been widely used in therapeutic against neurodegeneration as it has potential to prevent from neurodegeneration in various cases like Alzheimer’s disease [73]. In the studies of administration of BBR on rat model of Alzheimer’s disease, it was discovered after 2 weeks that intra-gastric administration of 50 mg kg of BBR chloride improves memory loss while enhancing the expression of interleukin 1 beta and inducible nitric oxide in the hippocampus of rat suffering from amyloid beta–induced Alzheimer’s diseases [74]. According to reports, the study on BBR prevents glutamate-induced toxicity in astroglial cell cultures, including protein mis-folding, aggregation, mitochondrial fragmentation, and neurodegeneration [75, 76]. Berberine treatment increases the cytotoxicity of 6-hydroxydopamine in PC12 cells, which causes degeneration of dopaminergic neurons in substantia nigra of rats [77]. BBR significantly reduced behavioral functional impairment and artificially induced brain ischemia (caused by permanent occlusion of the middle cerebral artery) [78]. The Akt/GSK3/ERK1/2 survival/apoptotic signaling pathway, Jun amino-terminal kinases (JNK), and caspase-3 activity inhibition are all involved in the neuroprotective effects of BBR [79].
Effect/Role of BBR on Antiviral Activities
In viral replication, BBR interferes to decrease the replication rate in the virus and host interaction. BBR interferes with DNA synthesis and reverse transcriptase activity by intercalating into DNA. It prevents from replication of human cytomegalovirus (HCMV), human papillomavirus (HPV), herpes simplex virus (HSV), and human immunodeficiency virus (HIV) [79]. Hong et al. have demonstrated that BBR inhibits the replication of the hepatitis C virus (HCV) by preventing entry of HCV by targeting the viral E2 glycoprotein. BBR interacts with the HCV E2 glycoprotein, according to molecular docking studies [80]. BBR also demonstrates antiviral activity against infections caused by the dengue virus (DENV) and the Zika virus (ZIKV). BBR shows antiviral activity against infections caused by the dengue virus (DENV) and the Zika virus (ZIKV). The four DENV serotypes—DENV-1, DENV-2, DENV-3, and DENV-4—transmit the virus to people when Aedes aegypti and Aedes albopictus mosquitoes bite. This virus causes a variety of illnesses such as asymptomatic infection, dengue fever, dengue hemorrhagic fever (DHF), and the potentially fatal dengue shock syndrome (DSS) [81].
]The severe acute respiratory syndrome coronavirus (SARS-CoV), which is the cause of the respiratory disease SARS, can also be treated with BBR. Additionally, BBR lowers the risk of ALI/ARDS in COVID-19 patients by preventing the release of inflammatory cytokines and the inflammatory signaling pathway [82]. COVID-19 caused immunological, inflammatory and an oxidative change; on the other hand, SARS-CoV-2 induces acute respiratory distress syndrome (ARDS), endothelial dysfunction (ED), acute lung injury (ALI), and multi-organ failure (MOF) [82,83,84,85] According to Warowicka et al., BBR has strong antiviral activity against various viruses which include coronaviruses, influenza, respiratory syncytial virus (RSV), herpes, and influenza [79]. BBR reduces the spread of SARS-CoV-2 and the inflammatory disorders; it is linked by activating the inflammatory signaling pathway.
Effect/Role of BBR on Antimicrobial Activities
BBR has shown promise potential treatment for a number of infections due to its capacity to inhibit a variety of microorganisms, such as bacteria, fungi, viruses, and protozoa. BBR is normally found as chloride and sulfate salts which are resistant against Staphylococcus epidermidis, Neisseria meningitidis, and Escherichia coli. BBR is found effective against Salmonella, Shigella, Giardia, and cholera in human beings [86]. According to Chinese herbal, Material medica, BBR sulfate exhibits significant antimicrobial activity against a variety of microorganisms, including Salmonella, Candida, Staphylococcus (Staph), Klebsiella, Clostridium, Pseudomonas proteus, Shigella, Vibrio, Cryptococcus, and Entamoeba species [87]. The National Institutes of Health stated that BBR has showed significant antimicrobial activity against bacteria, fungi, viruses, and chlamydia, demonstrating antimicrobial properties [88]. Bacteria, yeasts, and fungi were found to be moderate effects on BBR, but it had little effects on both Gram-positive and Gram-negative bacteria.
Mechanism of Action of BBR
On weight loss
Obesity represents a persistent illness distinguished by abnormal accumulation of fat that may have a negative impact on health. Globally, the rise in the proportion of overweight and obese people has caused great concern and is estimated to have resulted in 3.4 million deaths [89]. The primary repository for lipids and fatty acids is the adipocyte, which causes obesity, diabetes, and other related diseases. According to a new study conducted by Wu et al. (2019), therapy with BBR at a dose of 100 mg per day for 1 month increased the mass and activity of brown adipose tissue (BAT) in both humans and mice (n = 10) [90]. Thus, in modestly overweight individuals who have non-alcoholic fatty liver disease, weight loss will increase their sensitivity to insulin. Contrarily, in diet-induced obese mice and chow-fed mice, BBR promotes BAT growth by activating brown adipogenic genes, which increase BAT thermogenesis and overall consumption of energy. Han et al. [91] reported that the diabetic patients (n = 10) and mice (n = 10) who received BBR 500 mg/day for 10 weeks saw a significant decrease in the amounts of Firmicutes and Clostridia whereas an increase in the relative abundance of Bacteroidetes and Betaproteobacteria. For the NOD mice, however, oral probiotic therapy caused them to develop diabetes. BBR can modify gut flora without having any systemic anti-infective effects. By reducing the expression of LXRs, PPARs, and SREBPs in visceral white adipose tissue, BBR counteracts the effects of streptozotocin and aids in weight loss [92]. Peroxisome proliferator–activated receptors (PPARs) and liver X receptors (LXRs) are expressed more when BBR is present. The expression of PPAR2, C/EBP, adiponectin, and leptin mRNA is down regulated in pre-adipocytes after BBR administration on human fat in vitro [93].
On Cholesterol Metabolism
Cholesterol is a type of lipid (fat) molecule that is essential for various physiological functions in the body, such as building cell membranes and producing hormones. However, having excessively high levels of cholesterol in the bloodstream, particularly low-density lipoprotein (LDL) cholesterol, is associated with an increased risk of cardiovascular diseases like heart attacks and strokes. The cholesterol homeostasis is regulated by LDL receptor mediated in the liver [94]. Since the initial study showing that BBR reduced cholesterol through LDL receptor–mediated liver LDL cholesterol clearance [95], numerous studies have been carried out in vitro and in vivo to investigate this specific mechanism of action [96, 97]. Numerous studies have demonstrated that BBR increases the expression of hepatic LDL receptors [98]. BBR promotes LDL receptor expression in hepatocytes by stabilizing LDL receptor mRNA through activation of the ERK pathway [99].
The liver achieves cholesterol clearance by transforming cholesterol into bile acids, which are subsequently secreted either as free cholesterol in bile or as bile acids. Research has provided evidence that the utilization of BBR (berberine) amplifies the process of cholesterol excretion from the liver into bile, which is then eliminated from the body through fecal excretion. Supplementation with BBR induces notable alterations in the composition of bile acids, characterized by an increase in primary bile acids and a simultaneous decrease in secondary bile acids within both liver tissue and serum. Moreover, an in-depth analysis of gene expression patterns in liver tissue samples obtained from hamsters subjected to BBR treatment reveals a substantial surge in the expression of mitochondrial sterol 27-hydroxylase. This enzyme plays a pivotal role in regulating the synthesis of bile acids from cholesterol within the liver. When all these findings are considered together, it becomes evident that BBR contributes to the augmentation of both cholesterol breakdown and the excretion of bile acids. Consequently, this multifaceted impact culminates in the reduction of total cholesterol (T-C) and low-density lipoprotein cholesterol (LDL-C) levels in the bloodstream [100, 101].
On Tumor Suppression
According to research by Turner et al. (2008), BBR had been demonstrated to hinder mitochondrial respiratory complex I that may cause AMP levels to rise and AMPK to become activated [102]. However, a recent study by Xu et al. (2014) reveals that BBR blocks complex I, increasing glucose intake and lactate release independently of AMPK. The addition of BBR may induce cellular stress responses, such as activation of the p38 and JNK pathways, which may or may not be dependent on activation of AMPK. This is because blockage of the respiratory chain results in stress conditions. Additionally, we cannot rule out extra-mitochondrial processes induced by this medication [103].
On Endothelium and the Heart
An essential early stage in the development of atherosclerosis is endothelial dysfunction. Numerous factors that contribute to endothelium damage are connected to metabolic changes. The rise in secretion of proinflammatory cytokines and a reduction in the production of adiponectin, the increased levels of free fatty acids in the blood, and hyperglycemia in people with obesity and insulin resistance may affect gene expression and cell signaling in the vascular endothelium and alter the release of endothelium-derived factors. NADPH oxidase activation, eNOS uncoupling, increased endothelin-1 expression, an imbalance in the generation of vasodilators and vasoconstrictor mediators, and the induction of adhesion molecules are all signs of a dysfunctional endothelium [104]. In turn, the altered endothelium homeostasis aids in the formation and growth of plaque. According to Endemann and Schiffrin (2004), it is linked to the majority of cardiovascular diseases, including hypertension, coronary artery disease, chronic heart failure, peripheral artery disease, diabetes, and chronic kidney failure [105]. Due to LDLc’s stimulation of endothelial eNOS downregulation, endothelial cells exposed to hypercholesterolemia exhibit a decreased capacity to release endothelium-derived relaxing factors [106]. Endothelial function appears to be promoted by decreasing cholesterol levels [107].
Xu et al. (2009) have highlighted additional processes that underlie BBR’s actions on the endothelium [108] showed that BBR triggers an upregulation of endothelial progenitor cells (EPC-CFUs) through NO generation in healthy people. Following BBR treatment, the quantity of EPC-CFUs increased and the functions, including proliferation, adhesion, and migration, were improved. Additionally, the very same study group noticed that after BBR therapy, circulating endothelial microparticles (EMPs), which are typically linked to endothelial dysfunction, decreased. This finding demonstrates a clear connection between the fall of EMPs and an enhancement in flow-mediated vasodilation (FMD) in healthy patients. Additionally, the EMPs caused decreased eNOS protein expression in vitro, a negative consequence that BBR prevented [109]. A clinical, double-blind, placebo-controlled research in which BBR was given in combination with policosanols and RYR also recently showed the therapeutic effects of BBR treatment on FMD. In a population of hypercholesterolemic patients, this investigation showed that the medication resulted in a considerable improvement of FMD [110].
Toxicity
The Singapore government prohibited the use of BBR in 1976 due to concerns that it could cause hemolytic hepatitis in newborn babies if taken during pregnancy or nursing, as it may lead to a lack of glucose-6-phosphate dehydrogenase (G6PD) [111]. There were no sex-specific effects of BBR toxicity that were well-established and widely acknowledged. But the toxicity varies depending upon factors such as dosage, sensitivity of individuals, and its interaction with other medication.
BBR has been found to be the toxic component in mice, with the median acute oral lethal dose of the total RC extract being 2.95 g/kg [112]. However, when combined with other natural plants, BBR exhibited minimal toxicity and side effects [113]. When BBR is combined with other natural plants or compounds, it can exhibit synergistic effects. It may be possible to use lower individual doses of each plant while still getting the desired therapeutic result when BBR is combined with other plants. BBR may be less toxic and have fewer side effects at lower doses. The toxicity of BBR is dependent on the administration method and dose, with intravenous and intraperitoneal injections resulting in LD50 of 9.0386 g/kg and 57.6103 g/kg, respectively, while intra-gastric administration had no LD50 [114]. Administration of BBR sulfate at a dose of 50 mg/kg in rats resulted in diarrhea in 40% of cases [115]. Oral administration of BBR sulfate at a dose of 100 mg/kg in cats induced vomiting within 6–8 h and resulted in death after 8–10 days, with hemorrhagic inflammatory issues observed in the small and large intestines in cats given 50/100 mg/kg doses of BBR sulfate orally for 10 days [115]. Dogs administered low doses of BBR, and its compounds showed mild signs of poisoning, such as salivation, nausea, diarrhea, emesis, muscle tremor, and occasionally paralysis [116].
There have been some preliminary results regarding effect of BBR on the gut microbiome, but more extensive research is required to fully comprehend its effects. However, in some studies, BBR can change the gut microbiota’s makeup by encouraging the growth of good bacteria while preventing the growth of harmful bacteria. The following are some possible BBR effects on the gut microbiome: (1) Change in the composition of the microbiome: BBR may affect the relative abundance of specific bacterial species in the gut, resulting in a more advantageous microbiome. (2) BBR has been shown to have antimicrobial properties, which suggests that it could aid in the fight against some pathogenic bacteria in the gut. (3) Anti-inflammatory properties: According to some studies, BBR may lessen gut inflammation, which might have an indirect effect on the gut microbiome by reducing the inflammatory environment [117]. (4) Metabolic effects: BBR may also affect the metabolic processes of the gut microbiome, which may have an impact on how nutrients are absorbed and processed. BBR has also been found to have some immunotoxic effects, leading to a decrease in leukocyte, neutrophil, and lymphocyte levels, as well as decreased generation and differentiation of B- and T-cells and splenic CD19+ B-cells, CD4+, and CD8+ T-cells. Exposure to BBR can also cause uterine contractions and have teratogenic effects [111,112,113,114,115,116, 118].
Mitigation of Side Effect of BBR
-
Start with a low dose: It is good to start with a low dose of BBR and gradually increase it over time if we are new to supplementation. This strategy enables our body to get used to the substance and may lower your risk of experiencing serious side effects.
-
Take with meals: BBR should be taken with meals to lessen its effects on the digestive system and the likelihood of stomach discomfort. BBR can be more easily absorbed if we eat.
-
Divide the dose: Take the daily dose in two or three smaller doses as opposed to one larger dose. This may lessen side effects by lowering the amount of BBR present in the digestive tract at one time.
-
By taking special precaution when applied to skin especially to pregnant women.
-
Drink plenty of water to stay hydrated and to support our digestive system overall while taking BBR. This will help us avoid constipation.
Conclusion
BBR shows promising potential as a medication for a wide range of clinical applications. Evidence suggests that BBR and its derivatives may have positive outcomes for various medical conditions. The main objective of pharmaceutical research is to enhance a medication’s bioavailability while minimizing harm. This review focuses on the potential therapeutic applications of BBR. Although various pharmacological effects of BBR have been discovered through clinical research over the years, further investigation is necessary to understand its practical application in treating diseases. Numerous studies have demonstrated the efficacy of BBR in treating hypertension, hyperglycemia, cancer, depression, inflammation, pain, and hyperlipidemia. Therefore, it is reasonable to consider BBR as a medication with a broad spectrum of activity that could emerge as a promising therapeutic agent.
Data Availability
Authors declared that data may be shared upon request.
References
Vuddanda PR, Chakraborty S, Singh S. Berberine: a potential phytochemical with multispectrum therapeutic activities. Expert Opin Investig Drugs. 2010;19(10):1297–307.
Dash S, Panda MK, Singh MC, Jit BP, Singh YD, Patra JK. Bioactive molecules from the alpinia genus: A comprehensive review. Curr Pharm Biotechnol. 2020;21(14):1412–21.
Lambert G, Ancellin N, Charlton F, Comas D, Pilot J, Keech A, Patel S, Sullivan DR, Cohn JS, Rye KA, Barter PJ. Plasma PCSK9 concentrations correlate with LDL and total cholesterol in diabetic patients and are decreased by fenofibrate treatment. Clin Chem. 2008;54(6):1038–45.
Haokip SW, Sheikh KA, Das S, Devi OB, Singh YD, Wangchu L, Heisnam P. Unraveling physicochemical profiles and bioactivities of citrus peel essential oils: a comprehensive review. Eur Food Res Technol. 2023:1–4.
World Health Organization. General guidelines for methodologies on research and evaluation of traditional medicine (No. WHO/EDM/TRM/2000.1). World Health Organization; 2000.
Singh YD, Panda MK, Satapathy KB. Ethnomedicine for drug discovery. Advances in Pharmaceutical Biotechnology: Recent Progress and Future Applications. 2020:15–28
Patil S. A case series sharing novel experience of treating viral pandemic cases of morbid, mid aged, mild, moderate & severe grade with only Ayurvedic medicines. J Ayurveda Integr Med. 2022;13(1):100420.
Sharifi-Rad J, Quispe C, Patra JK, Singh YD, Panda MK, Das G, Adetunji CO, Michael OS, Sytar O, Polito L, Živković J. Paclitaxel: application in modern oncology and nanomedicine-based cancer therapy. Oxid Med Cell Longevity. 2021;2021.
Das G, Shin HS, Leyva-Gómez G, Prado-Audelo ML, Cortes H, Singh YD, Panda MK, Mishra AP, Nigam M, Saklani S, Chaturi PK. Cordyceps spp.: A review on its immune-stimulatory and other biological potentials. Front Pharmacol. 2021;11:2250
Panda S, Sahoo S, Tripathy K, Singh YD, Sarma MK, Babu PJ, Singh MC. Essential oils and their pharmacotherapeutics applications in human diseases. Adv Tradit Med. 2020:1-5.
Mohd Zaid NA, Sekar M, Bonam SR, Gan SH, Lum PT, Begum MY, Mat Rani NNI, Vaijanathappa J, Wu YS, Subramaniyan V, Fuloria NK. Promising natural products in new drug design, development, and therapy for skin disorders: an overview of scientific evidence and understanding their mechanism of action. Drug Des Devel Ther. 2022;16:23–66.
Salehi B, Del Prado-Audelo ML, Cortés H, Leyva-Gómez G, Stojanović-Radić Z, Singh YD, Patra JK, Das G, Martins N, Martorell M, Sharifi-Rad M. Therapeutic applications of curcumin nanomedicine formulations in cardiovascular diseases. J Clin Med. 2020;9(3):746.
Singh YD, Jena B, Ningthoujam R, Panda S, Priyadarsini P, Pattanayak S, Panda MK, Singh MC, Satapathy KB. Potential bioactive molecules from natural products to combat against coronavirus. Adv Tradit Med. 2020:1–2.
Singh YD, Das D, Das S, Swain KD, Pradhan S, Babu PJ. Pharmacological activities of limonin from Khasi Mandarin as therapeutic applications. Pharmacological Research-Modern Chinese Medicine. 2022:100181.
Neag MA, Mocan A, Echeverría J, Pop RM, Bocsan CI, Crişan G, Buzoianu AD. Berberine: botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front Pharmacol. 2018;9:557.
Kumar A, Chopra K, Mukherjee M, Pottabathini R, Dhull DK. Current knowledge and pharmacological profile of berberine: an update. Eur J Pharmacol. 2015;761:288–97.
Ghavipanje N, Fathi Nasri MH, Vargas-Bello-Pérez E. An insight into the potential of berberine in animal nutrition: current knowledge and future perspectives. J Anim Physiol Anim Nutr. 2022;107:808–29.
Beba M, Djafarian K, Shab-Bidar S. Effect of berberine on C-reactive protein: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2019;46:81–6.
Xu X, Yi H, Wu J, Kuang T, Zhang J, Li Q, Du H, Xu T, Jiang G, Fan G. Therapeutic effect of berberine on metabolic diseases: both pharmacological data and clinical evidence. Biomed Pharmacother. 2021;133:110984.
Imenshahidi M, Hosseinzadeh H. Berberine and barberry (Berberis vulgaris): a clinical review. Phytother Res. 2019;33(3):504–23.
Rokade M, Vichare V, Neve T, Parande B, Dhole S. A review on anticancer potential of Berberis aristata and berberine with focus on quantitative methods. J Prev Diagnos Treat Strat Med. 2022;1(2):65–7.
Singh S, Pathak N, Fatima E, Negi AS. Plant isoquinoline alkaloids: advances in the chemistry and biology of berberine. Eur J Med Chem. 2021;226:113839.
Katare AK, Singh B, Shukla P, Gupta S, Singh B, Yalamanchili K, Kulshrestha N, Bhanwaria R, Sharma AK, Sharma S, Sneha. Rapid determination and optimisation of berberine from Himalayan Berberis lycium by Soxhlet apparatus using CCD-RSM and its quality control as a potential candidate for COVID-19. Nat Prod Res. 2022;36(3):868–73.
Singla RK, Joon S, Sinha B, Kamal MA, Simal-Gandara J, Xiao J, Shen B. Current trends in natural products for the treatment and management of dementia: computational to clinical studies. Neurosci Biobehav Rev. 2023;147:105106.
Tang J, Feng Y, Tsao S, Wang N, Curtain R, Wang Y. Berberine and Coptidis rhizoma as novel antineoplastic agents: a review of traditional use and biomedical investigations. J Ethnopharmacol. 2009;126(1):5–17.
Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109(suppl 1):69–75.
Sun Y, Wang W, Tong Y. Berberine inhibits proliferative ability of breast cancer cells by reducing metadherin. Med Sci Monit. 2019;25:9058–66.
Berlin IG, Jennings CC, Shin S, Kenealey J. Utilizing mixture design response surface methodology to determine effective combinations of plant derived compounds as prostate cancer treatments. Cancer Rep. 2023;6:e1790.
Peng B, Zhang SY, Chan KI, Zhong ZF, Wang YT. Novel anti-cancer products targeting AMPK: natural herbal medicine against breast cancer. Molecules. 2023;28(2):740.
Xiong RG, Huang SY, Wu SX, Zhou DD, Yang ZJ, Saimaiti A, Zhao CN, Shang A, Zhang YJ, Gan RY, Li HB. Anticancer effects and mechanisms of berberine from medicinal herbs: an update review. Molecules. 2022;27(14):4523.
Devarajan N, Nathan J, Mathangi R, Mahendra J, Ganesan SK. Pharmacotherapeutic values of berberine: a Chinese herbal medicine for the human cancer management. J Biochem Mol Toxicol. 2023;37:e23278.
Ni L, Li Z, Ren H, Kong L, Chen X, Xiong M, Zhang X, Ning B, Li J. Berberine inhibits non-small cell lung cancer cell growth through repressing DNA repair and replication rather than through apoptosis. Clin Exp Pharmacol Physiol. 2022;49(1):134–44.
Chen J, Huang X, Tao C, Wang L, Chen Z, Li X, Zeng Q, Ma M, Zhang R, Wu Z. Berberine chloride suppresses non-small cell lung cancer by deregulating Sin3A/TOP2B pathway in vitro and in vivo. Cancer Chemother Pharmacol. 2020;86:151–61.
Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384(9951):1376–88.
Ionescu OM, Frincu F, Mehedintu A, Plotogea M, Cirstoiu M, Petca A, Varlas V, Mehedintu C. Berberine—a promising therapeutic approach to polycystic ovary syndrome in infertile/pregnant women. Life. 2023;13(1):125.
Zhao Y, Yang X, Zhao J, Gao M, Zhang M, Shi T, Zhang F, Zheng X, Pan Y, Shao D, Li J. Berberine inhibits chemotherapy-exacerbated ovarian cancer stem cell-like characteristics and metastasis through GLI1. Eur J Pharmacol. 2021;895:173887.
Mao QQ, Xu XY, Shang A, Gan RY, Wu DT, Atanasov AG, Li HB. Phytochemicals for the prevention and treatment of gastric cancer: effects and mechanisms. Int J Mol Sci. 2020;21(2):570.
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–48.
Li HL, Wu H, Zhang BB, Shi HL, Wu XJ. MAPK pathways are involved in the inhibitory effect of berberine hydrochloride on gastric cancer MGC 803 cell proliferation and IL-8 secretion in vitro and in vivo. Mol Med Rep. 2016;14(2):1430–8.
Lan J, Zhao Y, Dong F, Yan Z, Zheng W, Fan J, Sun G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J Ethnopharmacol. 2015;161:69–81.
Yin J, Ye J, Jia W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharm Sin B. 2012;2(4):327–34.
Xie W, Su F, Wang G, Peng Z, Xu Y, Zhang Y, Xu N, Hou K, Hu Z, Chen Y, Chen R. Glucose-lowering effect of berberine on type 2 diabetes: a systematic review and meta-analysis. Front Pharmacol. 2022;13:4734.
Kong WJ, Zhang H, Song DQ, Xue R, Zhao W, Wei J, Wang YM, Shan N, Zhou ZX, Yang P, You XF. Berberine reduces insulin resistance through protein kinase C–dependent up-regulation of insulin receptor expression. Metabolism. 2009;58(1):109–19.
Li A, Lin C, Xie F, Jin M, Lin F. Berberine ameliorates insulin resistance by inhibiting IKK/NF-κB, JNK, and IRS-1/AKT signaling pathway in liver of gestational diabetes mellitus rats. Metab Syndr Relat Disord. 2022;20(8):480–8.
Zhang L, Han L, Ma J, Wu T, Wei Y, Zhao L, Tong X. Exploring the synergistic and complementary effects of berberine and paeoniflorin in the treatment of type 2 diabetes mellitus by network pharmacology. Eur J Pharmacol. 2022;919:174769.
Chandirasegaran G, Elanchezhiyan C, Ghosh K. Effects of berberine chloride on the liver of streptozotocin-induced diabetes in albino Wistar rats. Biomed Pharmacother. 2018;99:227–36.
Xu S, Xu Y, Yin M, Zhang S, Liu P, Koroleva M, Si S, Little PJ, Pelisek J, Jin ZG. Flow-dependent epigenetic regulation of IGFBP5 expression by H3K27me3 contributes to endothelial anti-inflammatory effects. Theranostics. 2018;8(11):3007.
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492.
Fang J, Little PJ, Xu S. Atheroprotective effects and molecular targets of tanshinones derived from herbal medicine danshen. Med Res Rev. 2018;38(1):201–28.
Banegas JR, López-García E, Dallongeville J, Guallar E, Halcox JP, Borghi C, Massó-González EL, Jiménez FJ, Perk J, Steg PG, De Backer G. Achievement of treatment goals for primary prevention of cardiovascular disease in clinical practice across Europe: the EURIKA study. Eur Heart J. 2011;32(17):2143–52.
Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci. 2018;132(12):1243–52.
Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852–66.
Nowak WN, Deng J, Ruan XZ, Xu Q. Reactive oxygen species generation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37(5):e41–52.
Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15(5):551–61.
Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–25.
Raggi P, Genest J, Giles JT, Rayner KJ, Dwivedi G, Beanlands RS, Gupta M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis. 2018;276:98–108.
Zimetti F, Adorni MP, Ronda N, Gatti R, Bernini F, Favari E. The natural compound berberine positively affects macrophage functions involved in atherogenesis. Nutr Metab Cardiovasc Dis. 2015;25(2):195–201.
Feng M, Zou Z, Zhou X, Hu Y, Ma H, Xiao Y, Li X, Ye X. Comparative effect of berberine and its derivative 8-cetylberberine on attenuating atherosclerosis in ApoE−/− mice. Int Immunopharmacol. 2017;43:195–202.
Wang CL, He SX, Wei F. A clinical investigation of berberine on treatment of 42 cases with hypertension. Med J Intern Mong. 1993;13(2):54.
Huang Z, Han Z, Ye B, Dai Z, Shan P, Lu Z, Dai K, Wang C, Huang W. Berberine alleviates cardiac ischemia/reperfusion injury by inhibiting excessive autophagy in cardiomyocytes. Eur J Pharmacol. 2015;762:1–10.
Ventura HO, Lavie CJ. Epidemiology and managing aspects of hypertension. Curr Opin Cardiol. 2019;34(4):329–30.
Huang GL. Effect of amlodipine and berberine in mild and midrange hypertension patients complicated with hyperuricemia arthrolithiasis. China Pharm. 2013;22(5):32–3.
Cai SW. Clinical effect and safety of combination medicine for elderly patients with hypertension and gout. China Reflexolocy. 2017;26(11):84–9.
Liu L, Liu J, Huang Z, Yu X, Zhang X, Dou D, Huang Y. Berberine improves endothelial function by inhibiting endoplasmic reticulum stress in the carotid arteries of spontaneously hypertensive rats. Biochem Biophys Res Commun. 2015;458(4):796–801.
Wang J, Guo T, Peng QS, Yue SW, Wang SX. Berberine via suppression of transient receptor potential vanilloid 4 channel improves vascular stiffness in mice. J Cell Mol Med. 2015;19(11):2607–16.
Law MR, Morris JK, Wald N. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. Bmj. 2009;338:b1665.
Huang WM, Yan H, Jin JM, Yu C, Zhang H. Beneficial effects of berberine on hemodynamics during acute ischemic left ventricular failure in dogs. Chin Med J. 1992;105(12):1014–9.
Li Y, Chen X, Liu H, FeiLuo, and Gang Li. Effects of ginseng total saponins with berberine on plasma brain natriuretic peptide and Ca2+ concentration in experimental rats with chronic congestive heart failure. ZhongguoZhongyaozazhi= ZhongguoZhongyaoZazhi= China Journal of Chinese MateriaMedica. 2009;34(3):324–7.
Kysenius K, Huttunen HJ. Stress-induced upregulation of VLDL receptor alters Wnt-signaling in neurons. Exp Cell Res. 2016;340(2):238–47.
Zhang J, Yang JQ, He BC, Zhou QX, Yu HR, Tang Y, Liu BZ. Berberine and total base from rhizoma coptis chinensis attenuate brain injury in an aluminum-induced rat model of neurodegenerative disease. Saudi medical journal. 2009;30(6):760–6.
Kim M, Cho KH, Shin MS, Lee JM, Cho HS, Kim CJ, Shin DH, Yang HJ. Berberine prevents nigrostriatal dopaminergic neuronal loss and suppresses hippocampal apoptosis in mice with Parkinson’s disease. Int J Mol Med. 2014;33(4):870–8.
Jiang W, Wei W, Gaertig MA, Li S, Li XJ. Therapeutic effect of berberine on Huntington’s disease transgenic mouse model. PLoS One. 2015;10(7):e0134142.
Ji HF, Shen L. Molecular basis of inhibitory activities of berberine against pathogenic enzymes in Alzheimer’s disease. Sci World J. 2012;2012:4.
Zhu F, Qian C. Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin-1beta and inducible nitric oxide synthase in the rat model of Alzheimer’s disease. BMC Neurosci. 2006;7(1):1–9.
Campisi A, Acquaviva R, Mastrojeni S, Raciti G, Vanella A, De Pasquale R, Puglisi S, Iauk L. Effect of berberine and Berberis aetnensis C. Presl. alkaloid extract on glutamate-evoked tissue transglutaminase up-regulation in astroglial cell cultures. Phytother Res. 2011;25(6):816–20.
Shin KS, Choi HS, Zhao TT, Suh KH, Kwon IH, Choi SO, Lee MK. Neurotoxic effects of berberine on long-term L-DOPA administration in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Arch Pharm Res. 2013;36:759–67.
Kwon IH, Choi HS, Shin KS, Lee BK, Lee CK, Hwang BY, Lim SC, Lee MK. Effects of berberine on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and a rat model of Parkinson’s disease. Neurosci Lett. 2010;486(1):29–33.
Zou H, Long J, Zhang Q, Zhao H, Bian B, Wang Y, Zhang J, Zhao H, Wang L. Induced cortical neurogenesis after focal cerebral ischemia–three active components from Huang-Lian-Jie-Du decoction. J Ethnopharmacol. 2016;178:115–24.
Warowicka A, Nawrot R, Goździcka-Józefiak A. Antiviral activity of berberine. Arch Virol. 2020;165:1935–45.
Hung TC, Jassey A, Liu CH, Lin CJ, Lin CC, Wong SH, Wang JY, Yen MH, Lin LT. Berberine inhibits hepatitis C virus entry by targeting the viral E2 glycoprotein. Phytomedicine. 2019;53:62–9.
Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10(2):100–3.
Babalghith AO, Al-Kuraishy HM, Al-Gareeb AI, De Waard M, Al-Hamash SM, Jean-Marc S, Negm WA, Batiha GES. The role of berberine in Covid-19: potential adjunct therapy. Inflammopharmacology. 2022;30(6):2003–16.
Behera S, Rana G, Satapathy S, Mohanty M, Pradhan S, Panda MK, Ningthoujam R, Hazarika BN, Singh YD. Biosensors in diagnosing COVID-19 and recent development. Sensors International. 2020;1:100054.
Bharadwaj KK, Srivastava A, Panda MK, Singh YD, Maharana R, Mandal K, Manisha Singh BS, Singh D, Das M, Murmu D, Kabi SK. Computational intelligence in vaccine design against COVID-19. Computational intelligence methods in COVID-19: surveillance, prevention, prediction and diagnosis. 2021:311–29.
Singh YD, Ningthoujam R, Panda MK, Jena B, Babu PJ, Mishra AK. Insight from Nanomaterials and Nanotechnology towards covid-19. Sensors International. 2021;2:100099.
Chevalier A. Encyclopedia of medicinal plants, Revised Edition. Sydney (AUS): Dorling Kindersley; 2001.
Bensky, D. and Gamble, A., 1993. Chinese Herbal Materia Medica, Revised Edition.
Sack RB, Froehelich JL. Berberine-one herb in many ways. Altern Med Rev. 2000;5:175–7.
Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9· 1 million participants. Lancet. 2011;377(9765):557–67.
Wu L, Xia M, Duan Y, Zhang L, Jiang H, Hu X, Yan H, Zhang Y, Gu Y, Shi H, Li J. Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans. Cell Death Dis. 2019;10(6):468.
Han J, Lin H, Huang W. Modulating gut microbiota as an anti-diabetic mechanism of berberine. Med Sci Monit. 2011;17(7):RA164.
Li GS, Liu XH, Zhu H, Huang L, Liu YL, Ma CM, Qin C. Berberine-improved visceral white adipose tissue insulin resistance associated with altered sterol regulatory element-binding proteins, liver X receptors, and peroxisome proliferator-activated receptors transcriptional programs in diabetic hamsters. Biol Pharm Bull. 2011;34(5):644–54.
Yang Y, Hu C, Abu-Omar MM. Conversion of glucose into furans in the presence of AlCl3 in an ethanol–water solvent system. Bioresour Technol. 2012;116:190–4.
Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol. 2009;29(4):431–8.
Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H, Wang S. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10(12):1344–51.
Wang Y, Yi X, Ghanam K, Zhang S, Zhao T, Zhu X. Berberine decreases cholesterol levels in rats through multiple mechanisms, including inhibition of cholesterol absorption. Metabolism. 2014;63(9):1167–77.
Gu S, Cao B, Sun R, Tang Y, Paletta JL, Wu XL, Liu L, Zha W, Zhao C, Li Y, Radlon JM. A metabolomic and pharmacokinetic study on the mechanism underlying the lipid-lowering effect of orally administered berberine. Mol BioSyst. 2015;11(2):463–74.
He K, Ye X, Wu H, Wang Y, Zou Z, Ning N, Hu Y, Chen B, Fang X, Li X. The safety and anti-hypercholesterolemic effect of coptisine in syrian golden hamsters. Lipids. 2015;50(2):185–94.
Abidi P, Zhou Y, Jiang JD, Liu J. Extracellular signal-regulated kinase–dependent stabilization of hepatic low-density lipoprotein receptor mRNA by herbal medicine berberine. Arterioscler Thromb Vasc Biol. 2005;25(10):2170–6.
Li XY, Zhao ZX, Huang M, Feng R, He CY, Ma C, Luo SH, Fu J, Wen BY, Ren L, Shou JW. Effect of berberine on promoting the excretion of cholesterol in high-fat diet-induced hyperlipidemic hamsters. J Transl Med. 2015;13(1):1–9.
Wang Y, Jia X, Ghanam K, Beaurepaire C, Zidichouski J, Miller L. Berberine and plant stanols synergistically inhibit cholesterol absorption in hamsters. Atherosclerosis. 2010;209(1):111–7.
Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H, Taketo MM, Cooney GJ, Kraegen EW, James DE, Hu LH. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57(5):1414–8.
Xu M, Xiao Y, Yin J, Hou W, Yu X, Shen L, Liu F, Wei L, Jia W. Berberine promotes glucose consumption independently of AMP-activated protein kinase activation. PLoS One. 2014;9(7):e103702.
Rask-Madsen C, King GL. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3(1):46–56.
Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15(8):1983–92.
Henry PD, Cabello OA, Chen CH. Hypercholesterolemia and endothelial dysfunction. Curr Opin Lipidol. 1995;6(4):190–5.
Martínez-González J, Raposo B, Rodríguez C, Badimon L. 3-Hydroxy-3-methylglutaryl coenzyme a reductase inhibition prevents endothelial NO synthase downregulation by atherogenic levels of native LDLs: balance between transcriptional and posttranscriptional regulation. Arterioscler Thromb Vasc Biol. 2001;21(5):804–9.
Xu MG, Wang JM, Chen L, Wang Y, Yang Z, Tao J. Berberine-induced upregulation of circulating endothelial progenitor cells is related to nitric oxide production in healthy subjects. Cardiology. 2009;112(4):279–86.
Wang JM, Yang Z, Xu MG, Chen L, Wang Y, Su C, Tao J. Berberine-induced decline in circulating CD31+/CD42− microparticles is associated with improvement of endothelial function in humans. Eur J Pharmacol. 2009;614(1-3):77–83.
Affuso F, Ruvolo A, Micillo F, Sacca L, Fazio S. Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr Metab Cardiovasc Dis. 2010;20(9):656–61.
Pang B, Yu XT, Zhou Q, Zhao TY, Wang H, Gu CJ, Tong XL. Effect of Rhizoma coptidis (Huang Lian) on treating diabetes mellitus. Evid-based Complement Altern Med. 2015;2015:10.
Ma BL, Ma YM, Shi R, Wang TM, Zhang N, Wang CH, Yang Y. Identification of the toxic constituents in Rhizoma coptidis. J Ethnopharmacol. 2010;128(2):357–64.
Singh IP, Mahajan S. Berberine and its derivatives: a patent review (2009–2012). Expert Opin Ther Pat. 2013;23(2):215–31.
Kheir MM, Wang Y, Hua L, Hu J, Li L, Lei F, Du L. Acute toxicity of berberine and its correlation with the blood concentration in mice. Food Chem Toxicol. 2010;48(4):1105–10.
Kulkarni SK, Dandiya PC, Varandani NL. Pharmacological investigations of berberine sulphate. Jpn J Pharmacol. 1972;22(1):11–6.
Mahmoudi M, ZamaniTaghizadehRabe S, Balali-Mood M, Karimi G, Memar B, Rahnama M, Tabasi N, Khazaee M, Riahi-Zanjani B. Immunotoxicity induced in mice by subacute exposure to berberine. J Immunotoxicol. 2016;13(2):255–62.
Hitch TC, Hall LJ, Walsh SK, Leventhal GE, Slack E, de Wouters T, Walter J, Clavel T. Microbiome-based interventions to modulate gut ecology and the immune system. Mucosal Immunol. 2022;15(6):1095–113.
Marin-Neto JA, Maciel BC, Secches AL, Gallo L. Cardiovascular effects of berberine in patients with severe congestive heart failure. Clin Cardiol. 1988;11(4):253–60.
Acknowledgements
The corresponding author YDS is thankful to the vice chancellor, Central Agricultural University, Imphal, Manipur, India, for providing facilities.
Funding
The authors would like to thank and acknowledge Department of Biotechnology, New Delhi, Govt. of India (Sanction order no: BT/PR39789/NER/95/1664/2020 dated 09/02/2021, BT/PR44872/SPD/24/849/20, Dated 31/01/2022) for providing financial support for this study.
Author information
Authors and Affiliations
Contributions
TM and BLD: conceptualization, methodology, writing—original draft, and supervision; YDS: data curation, validation, writing—original draft, and writing—review and editing; TM: resources and investigation; and TM, BLD, and YDS: methodology. All authors commented on previous versions of the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mana, T., Devi, O.B. & Singh, Y.D. Therapeutic Application of Berberine: a Consolidated Review. Curr. Pharmacol. Rep. 9, 329–340 (2023). https://doi.org/10.1007/s40495-023-00330-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-023-00330-2