Abstract
Purpose of Review
Helicobacter pylori (H. pylori) infections cause various gastric diseases in humans, such as gastritis, peptic ulcerations, and even gastric cancer. Bismuth-based triple or quadruple therapies have been commonly recommended for the treatment of H. pylori infections. Up to now, the molecular mechanisms by which bismuth inhibits the growth of H. pylori are far from fully clear.
Recent Findings
The present concise review intends to cover the most recent reports and discoveries, especially in the past 10 years ever since our previous review on the inhibitory mechanism of bismuth-containing drugs against H. pylori. The proteome work and in vitro studies all supported that enzyme inhibition, attenuated ROS defense, disruption of the intracellular iron metabolism, and reduced bacterium-host cell adhesion are the principal mechanisms underlying the actions of bismuth against H. pylori.
Summary
The review presented here will help us to understand further the molecular mechanisms underlying the actions of metal-based drugs and stimulate further development of effective anti-bacterial drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
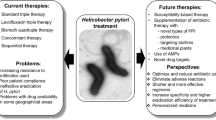
The Gram-negative microaerophilic bacterium Helicobacter pylori infects around half of the population worldwide. The long-term colonization of H. pylori in the stomach causes various gastric diseases, including gastritis, peptic ulcerations, gastric carcinoma, and mucosa-associated lymphoid tissue lymphoma [1], and more recently established, gall bladder cancer, diabetes mellitus, and iron deficiency anemia (IDA) [2]; the latter of which is closely related to the polymorphism of NapA of H. pylori [3, 4]. Complete eradication of H. pylori from the stomach is the method to treat the various kinds of gastric diseases related to H. pylori infections. For example, proton-pump inhibitor (PPI) or bismuth-based drugs have been routinely used together with two antibiotics (triple therapy) for the treatment of H. pylori infections [5]. However, the failure rate of H. pylori treatment has been increasing primarily due to the antibiotic resistance to clarithromycin, metronidazole, or levofloxiacin [6], leading to point mutations in the genome of H. pylori to attenuate the efficiency of antibiotic drugs [7]. Bismuth-based drugs, such as colloidal bismuth subcitrate (CBS) and ranitidine bismuth citrate (RBC), could be used to reduce antibiotic resistance levels when co-administered with PPI and antibiotics [8, 9], because bismuth salts act as an antiseptic rather than an antibiotic [7]. Especially in areas with high rates of clarithromycin and metronidazole resistance, bismuth-containing quadruple therapy is the preferred treatment option [10]. The regimen can be administered by using a single capsule formulation (three-in-one, Pylera®) containing bismuth subcitrate potassium, metronidazole, and tetracycline (triple, Pylera®) in combination with PPI or oramizole. An evaluative work, which tallied the results of relevant studies, confirmed that bismuth-based quadruple therapy using Pylera is an easy, efficacious regimen that achieves cure rates above 90% and is well tolerated [11]. Several reports have also demonstrated the effectiveness of bismuth-containing quadruple therapy through trials. In a multicenter, open-label, single-arm, multinational study, H. pylori eradication rates ranged from 93.2 to 93.8% in the ITT (intention-to-treat analysis) population and 94.7 to 95.0% in the PP (per-protocol) population, indicating that bismuth-based quadruple therapy produced high eradication rates in subjects who had previously failed to eradicate H. pylori [12]. Another randomized, open-label, non-inferiority phase 3 trial conducted in Europe also provided clinicians with assurance of the safety and tolerability of bismuth through a large comparative study of standard and quadruple combination therapies [13].
The molecular mechanisms for the actions of bismuth against H. pylori have attracted many researchers worldwide ever since its successful medical usage. Up to now, the established mechanisms suggest that bismuth inhibits the bacterium in a complicated way and have been reviewed by Lambert and Midolo [14], the Sun group [15], and ourselves [16]. The major in vivo biological targets for bismuth seem to be proteins and enzymes [17, 18], possibly through interference with zinc/iron cofactors in significant enzymes and/or iron/nickel metabolism. Previously established mechanisms of actions for bismuth include (1) inhibition of enzymes, such as urease, catalase, lipase/phospholipase [19], and fumarase [20],; (2) inhibition of adhesion of H. pylori to surface epithelial cells [21]; (3) inhibition of ATP synthesis [22] and inhibition of the activity of F1-F0 ATPase; and (4) inhibition of protein, cell wall synthesis, and membrane function [23]. In a proteome work by our group, we found that the major molecular mechanisms of bismuth’s action against H. pylori seem to be enzyme inhibition, interference with nickel homeostasis and the induced reactive oxidative stress [16, 18, 24]. Bismuth-induced oxidative stress was confirmed by the presence of higher levels of lipid hydroperoxide and hemin in bismuth-treated H. pylori cells [24]. The protease activities were also found to decrease eight-fold upon bismuth treatment [24], indicating a heavy load for the intracellular metabolism due to the accumulation of unwanted proteins intracellularly. A recent temporal kinetic analysis showed that upon entering H. pylori, bismuth initially interferes with the TCA cycle, followed by urease activity, and subsequently induces oxidative stress and suppresses energy production, while triggering a broad downregulation of metabolic levels [25]. Therefore, the molecular mechanisms of bismuth against H. pylori seem to be a systematic action on quite a variety of metabolisms and biological pathways. The present review intends to cover the most recent reports and discoveries in the field of inhibitory mechanisms of bismuth against H. pylori (Fig. 1) especially in the past ten years to act as an update to our last review [16].
Proteome-wide Identification of Potential Targets of Bismuth in H. pylori
About 15 years ago, we published a proteomic work with an aim to identify potential intracellular targets of bismuth in H. pylori in one kick [24]. Eight significantly up- or downregulated proteins upon bismuth treatment were identified, among which four proteins (HspA, HspB, NapA, and TsaA) can be bound to the immobilized bismuth affinity chromatography (Bi-IMAC). In a follow-up proteome work by Sun et al. using Bi-IMAC and MS, 166 potential Bi-binding proteins from H. pylori were identified [26]. The Sun group relied on continuous-flow gel electrophoresis plus ICP-MS to simultaneously identify proteins and the bound metal ions [27]. With bismuth-treated H. pylori 26,695 as a model strain, they detected several bismuth-binding proteins [27], such as UreA/B, CeuE, TsaA/AhpC, cell-binding factor 2, and Hp1286. Among them, Hp1286 is involved in isoprenoid quinone metabolism/transport/storage [28]; CeuE is the periplasmic iron-binding protein in an iron(III) ABC transporter [29]; and cell binding factor 2 is a secreted peptidyl-prolyl cis/trans-isomerase [30]. The Sun group further developed an in-house metalloprteomic method to couple a newly developed fluorescent probe with continuous-flow gel electrophoresis plus ICP-MS [31]. With this method, they identified 63 Bi-binding and 119 Bi-regulated proteins in H. pylori and the proteins are mainly involved in pH buffering and reactive oxidative stress (ROS) defense.
Based on these four proteomic studies, several potential mechanisms of actions of bismuth were proposed. At first, bismuth represses the bacterial capability of fighting against ROS, possibly by inhibiting the ROS resistance system of peroxiredoxins, thioredoxin, TsaA/AhpC, NapA, and so on. In the previous work, H. pylori TsaA was found to be cleaved or degraded upon bismuth treatment [24]. TsaA is a major component of the thiol specific antioxidant (Tsa) that catalyzes the reduction of hydroperoxides and peroxynitrite [32, 33]. NapA is named to reflect its ability to mediate neutrophil adhesion to endothelial cells [34], and is identified as a 150 kDa DNA-binding dodecamer that protects cells from DNA damage [35]. NapA is found to be a Bi3+-binding protein and its expression is upregulated by bismuth [24], which suggests that the upregulation of NapA expression is a response of H. pylori toward bismuth-induced ROS. The binding between NapA and bismuth was confirmed by a laser ablation-ICP-MS [36]. The polymorphism in NapA gene is closely related to the occurrence of IDA [4]. Thr70-type NapA is significantly higher in IDA patients than the wild-type standard Ser70-type. The in vitro study by our group suggested that Thr70-type NapA has much stronger ability to sequester iron(II) compared with the Ser-70 type [3].
The second mechanism is to disturb iron homeostasis through Fur (an iron regulatory factor) inhibition and the ROS-mediated destruction of [Fe-S] clusters [37, 38]. Iron is essential for almost all living organisms and is closely involved in a wide range of metabolic and cellular pathways [39]. Bacteria rely on various complex strategies to maintain intracellular iron homeostasis: to satisfy its own requirement for the iron and to prevent the toxic effects owing to the hydroxyl radicals produced by Fenton reaction in the presence of excessive iron [40]. Fur is a global regulator to control the intracellular iron content to achieve a balance between iron uptake, storage, iron-protein maturation, and excretion [39]. HpFur contains three binding sites: a structural zinc-binding site S1, a regulatory binding site S2, and a co-regulatory binding site S3 [41]. In an in vitro study, bismuth was found to bind to HpFur protein at the S1 site and induce oligomerization state changes, resulting in disrupted DNA-binding capability [42]. The disruption of the iron regulation through HpFur is thus a direct target of bismuth-based drugs.
The third mechanism is to directly inhibit the urease activity by the disruption of urease maturation function of several nickel binding proteins, such as HspA/B, HypB, UreG, and the urease subunit UreB [43], Hpn [44, 45], and Hpn-like proteins [46]. Hpn and Hpn-like are similar His-rich protein [44, 47], and HspA exhibits a unique His/Cys-rich C-terminal extension which other GroES-like proteins do not have [48]. These proteins are supposed to be involved in the intracellular nickel storage [49, 50]. Bismuth binds to these proteins and disrupts their relevant functions in the maturation of urease, and as a result, the ability of H. pylori for the colonization in the acidic gastric environment is diminished [51].
Bismuth Disrupts the Function of Several Key Proteins in H. pylori
Bismuth-based drugs are used for decades in the treatment of H. pylori infections and they are believed to be safe to the human bodies if administrated properly. The reason is that bismuth can bind to antioxidant tripeptide glutathione (GSH) [52]. Bismuth was passively absorbed, bound to GSH, and transported into cells via the multidrug resistance protein (MRP) [52]. GSH is one of the two key thiols, besides metallothionein [53] and protects mammalian cells from severe cell vacuolation, degradation, and death as in H. pylori [16].
Inhibition of enzymes is an important way for bismuth-containing drugs to work. For stable survival and colonization, H. pylori has developed a few methods to cope with the harsh acidic environment in the gastric mucosa. For example, H. pylori can produce several enzymes essential for gastric colonization, including alcohol dehydrogenase [54], urease [55], protease [56], and phospholipase [57]. Once inside the cells of H. pylori, bismuth may bind to various proteins/enzymes and in turn perturb a variety of biological pathways. Although the antimicrobial mechanism involved in bismuth is not thoroughly defined, much current evidence suggests that Bi3+ interacts with the cysteine residues of the target protein and interferes with the sulfur-containing metal-binding site [58]. A metallomics study used laser ablation inductively coupled plasma mass spectrometry to search for bismuth compound targets in H. pylori [36], and as a result, seven candidate bismuth-binding proteins were screened, including four enzymes, whose biological significance deserved further exploration.
H. pylori synthesizes a membrane-bound Ni–Fe hydrogenase which catalyzes reversible oxidation of molecular hydrogen and permits respiratory-based energy production for the bacteria in the mucosa [59]. The assembly of the Ni–Fe hydrogenase relies on the chaperone proteins HypA/B. Knockout of either hypA or hypB gene leads to complete loss of the hydrogenase activity and reduced bacterial habitation in the gastric environment [59]. Bi(III) binds equal molar equivalent of HypB with a dissociation constant of 9.4 (± 2.5) × 10−24 M and induces the protein dimerization in equal molar equivalent or further oligomerization in excess Bi(III) [60]. The formation of higher order oligomers completely abolishes the GTPase activity of HypB, which may pose a threat to H. pylori to coordinate its biological processes and metabolisms.
Proteases are widely present in organisms and are one of the largest functional groups of proteins. Bacterial proteases are known to be essential in maintaining homeostasis, nutrient uptake, and infection of the host [61]. Proteolytic enzymes produced by invasive bacterial species often play an important role in their viability and pathogenicity. A comparative proteomic analysis showed that treatment of H. pylori with CBS resulted in an approximately eightfold decrease in total protease activity [24], confirming that these enzymes are potential targets for bismuth-based anti-ulcer drugs [56, 62]. In addition, the phospholipase secreted by H. pylori is able to degrade the lipids of the gastric mucosa, thereby damaging the protective layer of mucus gel in the stomach [63, 64]. There is evidence suggesting that bismuth salts inhibit the activity of phospholipases A2 and C, and the inhibition is supposed to be due to the binding of bismuth to the calcium site of phospholipase [57, 65].
Urease is highly expressed in H. pylori, up to 10% of total proteins expressed. The initially synthesized protein is in its inactive apo-form, which still requires a nickel-bound maturation process. The mechanism for the translocation of nickel to apo-urease in H. pylori has been identified, the process with a collaboration of several accessory proteins, UreE, UreF, UreG, and UreH [66]. Among the accessory proteins, UreG was found to have the potential to interact with bismuth [67]. Each UreG monomer was observed to bind to approximately two Bi(III) ions with an apparent dissociation constant of 3.1 × 10−24 M. Mutagenesis of the metal-binding site of UreG demonstrated that the binding of Bi3+ was associated with Cys66 and Cys46, where Cys66 is also involved in the binding of Ni2+ [68]. This study suggests that once Bi3+ occupies the binding site of UreG, Ni2+ will no longer be able to insert. Both the tertiary structure of UreG and the complex with other accessory proteins are also disturbed by the binding of Bi3+, which severely interferes with Ni2+ transport [43, 69].
Carbon metabolism is essential in maintaining cellular structure and supplying energy [70]. Proteomic analysis revealed that nearly 10 enriched pathways of carbohydrate metabolism, such as glycolysis, the pentose phosphate pathway, and the citric acid cycle (TCA), were significantly inhibited upon treatment with bismuth-based drugs [71]. Glucose appears to be the main carbohydrate utilized by H. pylori [72, 73]. H. pylori is able to metabolize glucose via glycolysis, pentose phosphate pathway, and Entner-Doudoroff pathway. In the presence of CBS, a downregulation of gene expression associated with glycolysis as well as the pentose phosphate pathway was observed, and the binding of bismuth to the glycolytic enzymes fructose-diphosphate aldolase (FbpA) and enolase (Eno) was also detected [31], suggesting that bismuth-based drugs inhibit glucose catabolism. The TCA cycle has a dual function in cellular metabolism as a hub for the metabolic linkage of sugars, lipids, and amino acids. It provides both precursors for a variety of biosyntheses, such as ketoglutarate, succinate, and oxaloacetate, and a source of energy for cellular metabolism through the production of reduced nucleotides [74]. Bioassays have confirmed that CBS may impede central carbon metabolism and disrupt the TCA cycle: the activity of enzymes in the TCA cycle usually decreases in a dose-dependent manner after CBS treatment [74]. The expression of enzymes involved in the TCA cycle, such as aconitic acid hydratase (Acna), isocitrate dehydrogenase (Icd), and fumarase, is inhibited by bismuth [71]. Fumarase has been most thoroughly studied. It catalyzes the reversible hydration of fumarate to malate in the TCA cycle and generates reducing equivalents to facilitate oxidative ATP synthesis, which is essential for cellular energetics [75]. A proteomic work showed that fumarase is able to bind bismuth and is one of the major targets of intracellular bismuth-containing drugs [56]. Fluorescence quenching assays on bismuth titration showed that each fumarase binds one molar equivalent of Bi3+ and results in an apparently non-competitive near stoichiometric inactivation of fumarase [20].
Conclusion
Bismuth-based triple or quadruple therapies have been commonly recommended for the treatment of H. pylori infections–associated gastric diseases. The proteome work and the in vitro validation studies in the past 5 years supported that enzyme inhibition, attenuated ROS defense capability, disruption of the intracellular iron metabolism, and abolished bacterium-host interactions are supposed to be the major molecular mechanisms of bismuth’s action against H. pylori. Despite the information gathered so far, many aspects of the molecular mechanisms underlying the actions of bismuth toward H. pylori in vivo are still far from clear. Therefore, as stated 5 years ago,(16) cell- and tissue-wide advanced nuclear techniques, such as neutron activation analysis, X-ray emission/fluorescence spectroscopy, isotope dilution and tracing, X-ray absorption, neutron scattering, electron paramagnetic resonance, and nuclear magnetic resonance [76, 77], can be used to clarify in several ways the inhibitory effects of bismuth against H. pylori: the cellular localization of bismuth inside the bacterium; the structure of bismuth-containing metalloenzymes, implying the possible inhibitory ways; bismuth-DNA species inside the bacterium and the proteins binding to these adducts; and so on.
References
Blaser MJ. Gastric Campylobacter-like organisms, gastritis, and peptic ulcer disease. Gastroenterology. 1987;93(2):371–83.
Franceschi F, Tortora A, Gasbarrini G, Gasbarrini A. Helicobacter pylori and extragastric diseases. Helicobacter. 2014;19(Suppl 1):52–8.
Shan W, Kung HF, Ge R. Comparison of iron-binding ability between Thr70-NapA and Ser70-NapA of Helicobacter pylori. Helicobacter. 2016;21(3):192–200.
Yokota S, Toita N, Yamamoto S, Fujii N, Konno M. Positive relationship between a polymorphism in Helicobacter pylori neutrophil-activating protein a gene and iron-deficiency anemia. Helicobacter. 2013;18(2):112–6.
Barak R, Eisenbach M. Fumarate or a fumarate metabolite restores switching ability to rotating flagella of bacterial envelopes. J Bacteriol. 1992;174(2):643–5.
Lim SG, Park RW, Shin SJ, Yoon D, Kang JK, Hwang JC, et al. The relationship between the failure to eradicate Helicobacter pylori and previous antibiotic use. Dig Liver Dis. 2016;48:385–90.
Megraud F. The challenge of Helicobacter pylori resistance to antibiotics: the comeback of bismuth-based quadruple therapy. Therap Adv Gastroenterol. 2012;5(2):103–9.
Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59(8):1143–53.
Midolo PD, Lambert JR, Kerr TG, Tee W. In vitro synergy between ranitidine bismuth citrate and tetracycline or clarithromycin against resistant strains of Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1999;18(11):832–4.
Pellicano R, Zagari RM, Zhang S, Saracco GM, Moss SF. Pharmacological considerations and step-by-step proposal for the treatment of Helicobacter pylori infection in the year 2018. Minerva Gastroenterol Dietol. 2018;64(3):310–21.
Saleem A, Qasim A, O’Connor HJ, O’Morain CA. Pylera for the eradication of Helicobacter pylori infection. Expert Rev Anti Infect Ther. 2009;7(7):793–9.
Delchier JC, Malfertheiner P, Thieroff-Ekerdt R. Use of a combination formulation of bismuth, metronidazole and tetracycline with omeprazole as a rescue therapy for eradication of Helicobacter pylori. Aliment Pharmacol Ther. 2014;40(2):171–7.
Malfertheiner P, Bazzoli F, Delchier JC, Celiñski K, Giguère M, Rivière M, et al. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet. 2011;377(9769):905–13.
Lambert JR, Midolo P. The actions of bismuth in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 1997;11(Suppl 1):27–33.
Li H, Sun H. Recent advances in bioinorganic chemistry of bismuth. Curr Opin Chem Biol. 2012;16(1–2):74–83.
Ge R, Chen Z, Zhou Q. The actions of bismuth in the treatment of Helicobacter pylori infections: an update. Metallomics. 2012;4(3):239–43.
Mendis AHW, Marshall BJ. Helicobacter pylori and bismuth. In: Sun H, editor. Biological chemistry of arsenic, antimony and bismuth. Hoboken, NJ: John Wiley & Sons; 2011. p. 241–62.
Ge R, Sun H. Bioinorganic chemistry of bismuth and antimony: target sites of metallodrugs. Acc Chem Res. 2007;40(4):267–74.
Slomiany BL, Kasinathan C, Slomiany A. Lipolytic activity of Campylobacter pylori: effect of colloidal bismuth subcitrate (De-Nol). Am J Gastroenterol. 1989;84(10):1273–7.
Chen Z, Zhou Q, Ge R. Inhibition of fumarase by bismuth(III): implications for the tricarboxylic acid cycle as a potential target of bismuth drugs in Helicobacter pylori. Biometals. 2012;25(1):95–102.
Goodwin CS, Armstrong JA, Cooper M. Colloidal bismuth subcitrate inhibits the adherence of H. pylori to epithelial cells. Ital J Gastroenterol. 1991;23 (Suppl. 2):40.
Sox TE, Olson CA. Binding and killing of bacteria by bismuth subsalicylate. Antimicrob Agents Chemother. 1989;33(12):2075–82.
Armstrong JA, Wee SH, Goodwin CS, Wilson DH. Response of Campylobacter pyloridis to antibiotics, bismuth and an acid-reducing agent in vitro−an ultrastructural study. J Med Microbiol. 1987;24(4):343–50.
Ge R, Sun X, Gu Q, Watt RM, Tanner JA, Wong BC, et al. A proteomic approach for the identification of bismuth-binding proteins in Helicobacter pylori. J Biol Inorg Chem. 2007;12(6):831–42.
Han B, Zhang Z, Xie Y, Hu X, Wang H, Xia W, et al. Multi-omics and temporal dynamics profiling reveal disruption of central metabolism in Helicobacter pylori on bismuth treatment. Chem Sci. 2018;9(38):7488–97.
Wang Y, Tsang CN, Xu F, Kong PW, Hu L, Wang J, et al. Bio-coordination of bismuth in Helicobacter pylori revealed by immobilized metal affinity chromatography. Chem Commun (Camb). 2015;51(92):16479–82.
Hu L, Cheng T, He B, Li L, Wang Y, Lai YT, et al. Identification of metal-associated proteins in cells by using continuous-flow gel electrophoresis and inductively coupled plasma mass spectrometry. Angew Chem Int Ed Engl. 2013;52(18):4916–20.
Handa N, Terada T, Doi-Katayama Y, Hirota H, Tame JR, Park SY, et al. Crystal structure of a novel polyisoprenoid-binding protein from Thermus thermophilus HB8. Protein Sci. 2005;14(4):1004–10.
Rees DC, Johnson E, Lewinson O. ABC transporters: the power to change. Nat Rev Mol Cell Biol. 2009;10(3):218–27.
Pathak SK, Basu S, Bhattacharyya A, Pathak S, Banerjee A, Basu J, et al. TLR4-dependent NF-kappaB activation and mitogen- and stress-activated protein kinase 1-triggered phosphorylation events are central to Helicobacter pylori peptidyl prolyl cis-, trans-isomerase (HP0175)-mediated induction of IL-6 release from macrophages. J Immunol. 2006;177(11):7950–8.
Wang Y, Hu L, Xu F, Quan Q, Lai YT, Xia W, et al. Integrative approach for the analysis of the proteome-wide response to bismuth drugs in Helicobacter pylori. Chem Sci. 2017;8(6):4626–33.
Baker LM, Raudonikiene A, Hoffman PS, Poole LB. Essential thioredoxin-dependent peroxiredoxin system from Helicobacter pylori: genetic and kinetic characterization. J Bacteriol. 2001;183(6):1961–73.
Bryk R, Griffin P, Nathan C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature. 2000;407(6801):211–5.
Yoshida N, Granger DN, Evans DJ Jr, Evans DG, Graham DY, Anderson DC, et al. Mechanisms involved in Helicobacter pylori-induced inflammation. Gastroenterology. 1993;105(5):1431–40.
Cooksley C, Jenks PJ, Green A, Cockayne A, Logan RP, Hardie KR. NapA protects Helicobacter pylori from oxidative stress damage, and its production is influenced by the ferric uptake regulator. J Med Microbiol. 2003;52(Pt 6):461–9.
Tsang CN, Bianga J, Sun H, Szpunar J, Lobinski R. Probing of bismuth antiulcer drug targets in H. pylori by laser ablation-inductively coupled plasma mass spectrometry. Metallomics. 2012;4(3):277–83.
Bland MV, Ismail S, Heinemann JA, Keenan JI. The action of bismuth against Helicobacter pylori mimics but is not caused by intracellular iron deprivation. Antimicrob Agents Chemother. 2004;48(6):1983–8.
Ge R, Sun X. Iron trafficking system in Helicobacter pylori. Biometals. 2012;25(2):247–58.
Andrews SC, Robinson AK, Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27(2–3):215–37.
Stadtman ER, Berlett BS. Fenton chemistry. Amino acid oxidation J Biol Chem. 1991;266(26):17201–11.
Dian C, Vitale S, Leonard GA, Bahlawane C, Fauquant C, Leduc D, et al. The structure of the Helicobacter pylori ferric uptake regulator Fur reveals three functional metal binding sites. Mol Microbiol. 2011;79(5):1260–75.
He X, Liao X, Li H, Xia W, Sun H. Bismuth-induced inactivation of ferric uptake regulator from Helicobacter pylori. Inorg Chem. 2017;56(24):15041–8.
Yang X, Li H, Lai TP, Sun H. UreE-UreG complex facilitates nickel transfer and preactivates GTPase of UreG in Helicobacter pylori. J Biol Chem. 2015;290(20):12474–85.
Ge R, Watt RM, Sun X, Tanner JA, He QY, Huang JD, et al. Expression and characterization of a histidine-rich protein, Hpn: potential for Ni2+ storage in Helicobacter pylori. Biochem J. 2006;393(1):285–93.
Ge R, Zhang Y, Sun X, Watt RM, He QY, Huang JD, et al. Thermodynamic and kinetic aspects of metal binding to the histidine-rich protein. Hpn J Am Chem Soc. 2006;128(35):11330–1.
Chang YY, Lai YT, Cheng T, Wang H, Yang Y, Sun H. Selective interaction of Hpn-like protein with nickel, zinc and bismuth in vitro and in cells by FRET. J Inorg Biochem. 2015;142:8–14.
Zeng YB, Zhang DM, Li H, Sun H. Binding of Ni2+ to a histidine- and glutamine-rich protein. Hpn-like J Biol Inorg Chem. 2008;13(7):1121–31.
Suerbaum S, Thiberge JM, Kansau I, Ferrero RL, Labigne A. Helicobacter pylori hspA-hspB heat-shock gene cluster: nucleotide sequence, expression, putative function and immunogenicity. Mol Microbiol. 1994;14(5):959–74.
Benoit SL, Miller EF, Maier RJ. Helicobacter pylori stores nickel to aid its host colonization. Infect Immun. 2013;81(2):580–4.
Dosanjh NS, Michel SL. Microbial nickel metalloregulation: NikRs for nickel ions. Curr Opin Chem Biol. 2006;10(2):123–30.
Ge RG, Wang DX, Hao MC, Sun XS. Nickel trafficking system responsible for urease maturation in Helicobacter pylori. World J Gastroenterol. 2013;19(45):8211–8.
Hong Y, Lai YT, Chan GC, Sun H. Glutathione and multidrug resistance protein transporter mediate a self-propelled disposal of bismuth in human cells. Proc Natl Acad Sci U S A. 2015;112(11):3211–6.
Sadler PJ, Sun H, Li H. Bismuth(III) complexes of the tripeptide glutathione (r-L-Glu-L-Cys-Gly). Chemistry (Weinheim an der Bergstrasse, Germany). 1996;2(6):701–8.
Lan J, Szeto KY, Li Z, Du W, Sun H. Inhibition of alcohol dehydrogenase by bismuth. J Inorg Biochem. 2004;98(8):1331–7.
Zhang L, Mulrooney SB, Leung AF, Zeng Y, Ko BB, Hausinger RP, et al. Inhibition of urease by bismuth(III): implications for the mechanism of action of bismuth drugs. Biometals. 2006;19(5):503–11.
Ge R, Sun X, Gu Q, Watt RM, Tanner JA, Wong B, et al. A proteomic approach for the identification of bismuth-binding proteins in Helicobacter pylori. J Biol Inorg Chem. 2007;12(6):831–42.
Ottlecz A, Romero JJ, Hazell SL, Graham DY, Lichtenberger LM. Phospholipase activity of Helicobacter pylori and its inhibition by bismuth salts Biochemical and biophysical studies. Digest Dis Sci. 1993;38(11):2071.
Keogan DM, Griffith DM. Current and potential applications of bismuth-based drugs. Molecules. 2014;19(9):15258–97.
Olson JW, Maier RJ. Molecular hydrogen as an energy source for Helicobacter pylori. Science. 2002;298(5599):1788–90.
Xia W, Li H, Sun H. Functional disruption of HypB, a GTPase of Helicobacter pylori, by bismuth. Chem Commun (Camb). 2014;50(13):1611–4.
Kaman WE, Hays JP, Endtz HP, Bikker FJ. Bacterial proteases: targets for diagnostics and therapy. Eur J Clin Microbiol Infect Dis. 2014;33(7):1081–7.
Sun H, Zhang L, Szeto K. Bismuth in medicine. Met Ions Biol Syst. 2004;41(7):333.
Slomiany BL, Kasinathan C, Slomiany A. Lipolytic activity of Campylobacter pylori: effect of colloidal bismuth subcitrate (De-Nol). Am J Gastroenterol. 1989;84(10):1273–7.
Slomiany BL, Bilski J, Sarosiek J, Murty V, Dworkin B, Vanhorn K, et al. Campylobacter pyloridis degrades mucin and undermines gastric mucosal integrity. Biochem Biophys Res Commun. 1987;144(1):307–14.
Ottlecz A, Romero JJ, Lichtenberger LM. Effect of ranitidine bismuth citrate on the phospholipase A2 activity of Naja naja venom and Helicobacter pylori: a biochemical analysis. Aliment Pharmacol Ther. 1999;13(7):875–81.
Griffith DM, Li H, Werrett MV, Andrews PC, Sun H. Medicinal chemistry and biomedical applications of bismuth-based compounds and nanoparticles. Chem Soc Rev. 2021;50(21):12037–69.
Yang X, Koohi-Moghadam M, Wang R, Chang Y, Woo P, W J, et al. Metallochaperone UreG serves as a new target for design of urease inhibitor: a novel strategy for development of antimicrobials. Plos Biology. 2008;16(1):e2003887.
Yuen MH, Fong YH, Nim YS, Lau PH, Wong KB. Structural insights into how GTP-dependent conformational changes in a metallochaperone UreG facilitate urease maturation. Proc Natl Acad Sci U S A. 2017;114(51):E10890–8.
Fong YH, Wong HC, Yuen MH, Lau PH, Chen YW, Wong KB, et al. Structure of UreG/UreF/UreH complex reveals how urease accessory proteins facilitate maturation of Helicobacter pylori urease. PLoS Biol. 2013;11(10):e1001678.
Joshua CJ. Metabolomics: a microbial physiology and metabolism perspective. Methods Mol Biol. 2019;1859:71–94.
Yao X, Xiao S, Zhou L. Integrative proteomic and metabolomic analyses reveal the mechanism by which bismuth enables Helicobacter pylori eradication. Helicobacter. 2021;26(6):e12846.
Mendz GL, Hazell SL, Burns BP. Glucose utilization and lactate production by Helicobacter pylori. J Gen Microbiol. 1993;139(12):3023–8.
Chalk PA, Roberts AD, Blows WM. Metabolism of pyruvate and glucose by intact cells of Helicobacter pylori studied by 13C NMR spectroscopy. Microbiology. 1994;140(Pt 8):2085–92.
Pitson SM, Mendz GL, Srinivasan S, Hazell SL. The tricarboxylic acid cycle of Helicobacter pylori. Eur J Biochem. 2010;260(1):258–67.
Ge Z, Feng Y, Dangler CA, Xu S, Taylor NS, Fox JG. Fumarate reductase is essential for Helicobacter pylori colonization of the mouse stomach. Microb Pathog. 2000;29(5):279–87.
Ge R, Chu IK, Sun H. Nuclear-based metallomics in metal-based drugs. In: Chen C, Chai Z, Gao Y, editors. Nuclear analytical techniques for metallomics and metalloproteomics. London: Royal Soc Chem. 2011. p. 265–98.
Ge R, Sun X, He QY. Overview of the metallometabolomic methodology for metal-based drug metabolism. Curr Drug Metab. 2011;12(3):287–99.
Funding
This work was supported by the National Natural Science Foundation of China (21771199).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors received no financial support in the writing of this manuscript. All the authors are either students or faculty of Sun Yat-Sen University. The opinions expressed in this publication are those of the authors and do not necessarily reflect those of the university who employs them.
Huamn and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Li, X., Zhu, Y. et al. Molecular Mechanisms of Bismuth-containing Drugs Against Helicobacter pylori: a Further Update. Curr Pharmacol Rep 9, 59–65 (2023). https://doi.org/10.1007/s40495-022-00305-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-022-00305-9