Abstract
Diabetes is one of the most common metabolic disorders often associated with hyperglycemia, altered carbohydrate, lipid, and protein metabolism. Type 2 diabetes is the most common type of diabetes associated with the disturbance of the normal level of insulin secretion from pancreatic β-cell. The current treatment of diabetes is done with semi-synthetic and synthetic drugs, but it is associated with adverse effects. Now, scientific community searches for new herbal bioactive as a replacement for successful management of the disease. Primitively bioactive compounds from herbs served as the backbone of medical therapy. The significant scientific facts and profitable dormant of ancient medicines are directed to increased global attention for herbal remedies. Herbal remedies are composed of an intricate blend of several bioactive molecules with accountable pharmacological action. Numerous published reports claim the pharmacological action of herbal remedies of the exact phytoconstituents. It is imperative to understand the pharmacokinetics of such phytoconstituents, with only a few phytoconstituents whose pharmacokinetic properties have been reported, and it requires to explore the pharmacokinetic property of other phytoconstituents. There are many bioactive plants that have antidiabetic properties such as Capsicum (chili pepper), Vitis vinifera (grape vine), Glycyrrhiza, Cinnamomum extract, Ervatamia microphylla, Trigonella foenum-graecum, and Moringa oleifera. This review highlights comprehensive information on pharmacokinetics and clinical efficacy of different bioactive constituents which is obtained from various plants that may afford as antidiabetic therapeutics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is a metabolic disorder, and it contributes to a serious trouble in modern society due to its life-long health complications. Type 2 diabetes mellitus (T2DM) is the commonest among all types of diabetes (>80% of the total cases) [1,2,3]. The major factor associated with T2DM is a disturbance in the glucose metabolism (glucose homeostasis disturbance) which is regulated by β-cell of the pancreas [4, 5]. It is a peptide hormone insulin secreted from pancreatic β-cells and regulates the uptake of glucose from the systemic circulation through activation of hepatocytes and myocytes as energy source or stored as glycogen in liver/skeletal muscle cells. Destabilization of the pancreatic β-cell leads to an inability of the cell to uptake the blood glucose because of the insulin resistance [6, 7]. The estimated 463 million people survived in 2019 from diabetes, and it is expected to rise to 578 million by 2030 and 700 million by 2045. Approximately 422 million adult people survived with diabetes in 2014 than 108 million in 1980. About 1.5 million people deaths take place in 2012 with diabetes and associated risk diseases. This data reveals an increased risk associated with factors such as hypertension and obesity [8].
Herbal medicines have been used as first-line therapy for the treatment of various diseases. It is a well-established type of prescription for the welfare of humanity, as it holds a reputation in traditional medicinal systems (TMSs), i.e., Ayurveda (India) and customary Chinese medicine (China). Herbal remedies are an umbrella of the traditional system of medicine that encompasses a wide variety of treatment alternatives. These approaches were different from present-day customary medicine.
Many plants and common therapeutic herbal bioactive molecules have been explained/proved earlier as an antidiabetic activity. The mechanism of action of herbal bioactive compounds was not well known, but most of the research work clarified it. About 75–90% of developed countries and 80% of developing countries of the world rely on the utilization of herbal medicine for the treatment of various illnesses [9].
The herbal active constituent exhibited a more complex pharmacokinetic profile, and this explains the relationship like efficacy, duration of therapeutic action, and toxicological impacts on the human being. Additionally, it defines the scope and acceptance by regulatory agencies. As pointed by the WHO, the information like pharmacokinetics, pharmacodynamics, and metabolism of medicinal plants is less available. The information about authentication, efficacy, and safety of the therapeutic constituents (herbal) is well known and meets the required criteria for worldwide use.
Bioactive compounds and their derivatives from a natural source can be better options for treating T2DM and their complications to avoid adverse effects. Many bioactive molecules isolated from plants were reported to have antidiabetic effects [9]. Since ancient times, diabetes and its comorbidities have been prevented and controlled by the use of medicinal plants [10]. This review provides a detailed account of preclinical and clinical evidence of some natural bioactive compounds in the management of diabetes.
Potential Medicinal Plants and Their Bioactive Compounds
There are more than 400 in vitro and in vivo reports of plants and their compounds claimed to have antidiabetic activity. Here, we are mentioning about those selected plants, chemical, and extract with the ability to regulate blood glucose level (BGL) along with pavement and to maintain BGL through any mechanism: (a) insulin-sensitizing effect, (b) enhanced glucose transport by glucose transporter 4 (GLUT-4), (c) p-glycoprotein induced, (d) increased glucose oxidation, and (e) increased insulin secretion by activating β-cell. These plants have also exhibited a synergistic effect with a synthetic or semi-synthetic antidiabetic drug. Hypoglycemic actions in cells, experimental animals, and therapeutic outcomes of the plant extracts and their compounds in patients with type 2 diabetes mellitus have been illustrated in the next sections.
Vaccinium myrtillus (Bilberry)
Vaccinium myrtillus (bilberry) which belongs to the family Ericaceae (European blueberry, huckleberry, whortleberry, or blueberry) has potential antidiabetic property. It contains many constituents like anthocyanoside flavonoid (anthocyanins), vitamins, sugars, and pectins. There are other compounds like quercetin, catechins, tannins, iridoids, and acids that have been extracted from bilberry leaves. Decoctions of the leaf have been extensively used for management of hyperglycemia. It was reported that myrtillin anthocyanoside is the main active compound having the antihyperglycemic activity [11].
Preclinical and Clinical Evidence for Antidiabetic Activity
There are numerous preclinical research studies claiming the hypoglycemic effect of bilberry in different streptozotocin (STZ)-induced diabetic models in rats, mice, and rabbits [12, 13] (Table 1). Hoggard et al. [37] studied the important outcome of a regular bilberry extract on glucose metabolic rate in patients with T2DM. Double-blind crossover interposition followed 14 days for washouts, given one capsule orally (bilberry extract = 0.47 g, ≈36% w/w anthocyanins) equivalent to about 50 g fresh bilberries after a drink containing 75 g of glucose. Administration of the bilberry extract caused a substantial decline in the rise in AUC of glucose (p < 0.003) and insulin (p < 0.03) matched with placebo. It can be concluded that the administration of concentrated bilberry extract decreases postprandial glycemia and insulin in T2DM subjects. The hypoglycemic effects may be attributed to reduced digestion and/or absorption of carbohydrates [37].
Aloe vera
It is the most commonly used herbal source for the treatment of DM worldwide. It contains polysaccharides with a large proportion of mannose, galactose, and galacturonic acid as major active constituents. Reports from the human and animal studies showed that Aloe vera can reduce the chronic hyperglycemia and alter lipid profile which are usually observed in DM patients. It was reported that the antidiabetic effect of Aloe vera is due to the induction of insulin secretion from β-cells of the pancreas [38].
Pseudoprototinosaponin and prototinosaponin AII are a chemical compound of Aloe vera which provoke the glucose acceptance as well as insulin discharge. The hypoglycemic effect of these chemicals by the gluconeogenesis/glycogenolysis process in the liver was studied. Regular administration of Aloe vera extract significantly improves the total blood lipid in alloxan-induced diabetic rats [38]. Also, Aloe vera gel has antioxidant property as well as in vitro pancreatic lipase inhibitory action [39]. There are many reports which claim a reduction in fasting blood glucose (FBG), glycosylated hemoglobin A1c (HbA1c), low/high-density lipoprotein, total cholesterol, triglyceride among pretreated and early non-treated diabetes groups [40].
Preclinical and Clinical Evidence for Antidiabetic Activity
Many preclinical reports are showing the hypoglycemic effect of Aloe vera extract and its bioactive compounds in different animal models [14, 17, 41]. The details of these studies are summarized in Table 1. Administration of Aloe vera leaf extract into sixty hyperlipidemic patients in a 3-month-organized clinical trial showed significantly reduced cholesterol, triglycerides, and LDL levels in serum. It revealed that introductory information gives the valuable impact of Aloe vera in hyperglycemia and hyperlipidemia. Yongchaiyudha and his associates [42] studied the antidiabetic effect of Aloe vera juice for 14 days in patients with diabetes mellitus. The significant reduction (p < 0.05) in BGL and triglyceride (TG) was recorded in the treated groups without any significant alteration in serum cholesterol level supporting the antidiabetic activity of Aloe vera juice [42]. Can and associates [43] evaluated the Aloe vera juice clinically and determined its potential antidiabetic activity. This research proved the reduction in BGL (48%) and triglycerides (52%) while cholesterol remained unchanged. The combined glibenclamide and Aloe vera treatment gives a significant reduction in BGL as compared to Aloe vera juice alone [43].
Panax ginseng and Panax quinquefolium (Ginseng)
Around 200 AD, Shen-Nong first introduced the Panax ginseng for medical purpose. The active constituent quinquefolium was first included in “Essential of Material Medica” (1694, China). Other significant bioactive constituents found in Panax ginseng and Panax quinquefolium are saponins in nature (also known as ginseng saponins (GSs)).
Preclinical and Clinical Evidence for Antidiabetic Activity
Numerous preclinical research studies documented the hypoglycemic effect of Panax ginseng extract and its bioactive compounds in the different STZ- and alloxan-induced diabetic animal models [18, 21]. The details of this study are summarized in Table 1. Anoja and associates [18] extracted and assessed antihyperglycemic and anti-obese properties of Panax ginseng berry extract and its chief component, ginsenoside Re in fat diabetic ob/ob mice and their slim littermates. Panax ginseng extract administered intraperitoneally in ob/ob mice (150 mg/kg, 137 mg/dl) daily exhibited remarkable improvements in glucose tolerance up to the 12th day. The maximum reduction of glucose was found in 2 h by 46% (p < 0.01). An overactive insulinemic-euglycemic clamp study exhibited double increments of insulin level, and the glucose clearance significantly reduced from 112 to 52 mg/dl (p < 0.01) in the vehicle-treated group. Besides, the weight of extract-treated ob/ob mice significantly (p < 0.05) decreases from 51 (0 day) to 45 g (12th day) compared with the vehicle-treated ob/ob mice because of a substantial decrease in food consumption (p < 0.05) with an extensive rise in energy escape (p < 0.01) as well as body temperature (p < 0.01). Usage of the extract also exhibited remarkably decreased plasma cholesterols. Other studies noted that ginsenoside Re plays an important role in hyperglycemia that is not linked with bodyweight alterations [18]. Its clinical evidence for antidiabetic activity is reported in the literature, and the details are summarized in Table 2. Esra and associates [76] studied the ginseng for its glycemic effect on a healthy and diabetic patient using randomized precise trial method for 1 month, and the test was comprised of sixteen trials. The findings suggested that ginseng considerably decreased FBG (p < 0.03 at 95% CI). This research also reported a non-significant effect on fasting plasma insulin and glycated hemoglobin. A subclass showed significant (p < 0.01, CI = 95%) drops in glycated hemoglobin in equivalently matched crossover trials. It was also established that ginseng considerably enhanced FBG in diabetic and healthy persons [76].
Kim et al. [77, 78] studied the arbitrary placebo-measured test to conclude if the fermented red ginseng intake is able to modulate the blood glucose (BG) and insulin fight in T2DM. A total of 38 patients with diabetes mellitus was chosen, and testing was done arbitrarily using fermented red ginseng or placebo crowd. Seven hundred eighty milligrams of fermented ginseng and cellulose was given to the patient every day for 12 weeks. It was observed that the FBG level considerably reduced from 136 to 127 mg/dl and HbA1c reduced with fermented red ginseng crowd at the end of 12 weeks (7.7 to 6.4%). The low-density lipoprotein was reduced by fermented red ginseng crowd and matching to the placebo crowd. These outcomes established that supplement of fermented red ginseng could be helpful to decline the BGL by breaking insulin resistance in T2DM [77].
Shin and colleagues [79] investigated the effect of cheonggukjang and red ginseng on plasma lipids and glucose levels in 45 people (27:18, men:women; average age, 44.9 years). The volunteers were arbitrarily divided into 3 groups comprised of control (starch, 2 g/day), cheonggukjang (20 g/day), and cheonggukjang plus red ginseng (20 g/day). The studies were conducted for 8 weeks. The intake with cheonggukjang and cheonggukjang plus red ginseng considerably reduced the plasma cholesterol (30 mg/ml and 37 mg/ml) individually and restored it to the initial level. The low-density lipoprotein and cholesterol level was also considerably decreased by 29% and 23% with cheonggukjang and cheonggukjang plus red ginseng, respectively, and reached the initial level. The plasma high-density lipoprotein and cholesterol level remarkably declined (107.9 mg/ml) with cheonggukjang plus red ginseng and was brought back to the initial level (139.1 mg/ml). The erythrocyte thiobarbituric acid–reactive constituents were considerably reduced in cheonggukjang (6.5 nmol/ml) and cheonggukjang plus red ginseng (6.6 nmol/ml) and maintained towards normal (7.9 nmol/ml and 8.0 nmol/ml, correspondingly). The FBG was considerably reduced with cheonggukjang and cheonggukjang plus red ginseng administered group and maintained towards normal [79]. Yoon et al. [22] studied the dual-blind, arbitrary placebo-controlled evaluation of ginsam, a vinegar extract of Panax ginseng in patients with T2DM. It was observed that ginsam significantly (p < 0.05) decreased the HbA1c level to 0.56%, 0.31%, and 0.29% with 1500 mg-, 2000 mg-, and 3000 mg-treated groups, respectively. The HbA1c value reduced considerably among the placebo (11.1%), 1500 mg (27.8%), and 2000 mg (27.8%) groups. No serious adversarial actions were seen in any sections. It was established that 8-week administration of ginsam reasonably decreased HbA1c level and well tolerated in T2DM and HbA1c level [22]. Mucalo and his colleagues [80] evaluated the Panax ginseng using a dual-blind, placebo-controlled method on 64 individuals having critical hypertension (controlled) and T2DM. The average age of the participant is 63 years with blood pressure 145/84 mmHg, HbA1C (7%), and FBG (8.1 mmol/l). Each participant was randomized for American ginseng extract and placebo and given a 3 g/day dose for 84 days. It was found that ginseng significantly (p < 0.001) declined systolic BP (11.7%) but had no significant effect on diastolic BP. It was concluded that American ginseng extract requires regular treatment in diabetes-associated hypertension, and it enhanced the arterial rigidity and lowered systolic BP, thus permitting further examination on the long-term trial before being suggested as supplement therapy [80].
Momordica charantia (Bitter Gourd)
It is also known as bitter melon because of its taste. The large variety of active chemicals is present in its juice, along with sterols, glucoside blends, and charantin polypeptides [81]. The hypoglycemic action is due to the presence of steroid, alkaloid, triterpene, inorganic lipid, and phenolic compounds [82]. Specifically, the 4-triterpenoids show hypoglycemic activity by activating the AMP-activated protein kinase [83]. Karela is the only one of medicinal plants in which clinical study has been done in blend with normal hypoglycemic agents. Its drupe is also used for diabetic therapy and associated disorder in Asia, South America, India, and East Africa. A large number of preclinical studied have been reported about hypoglycemic properties of Momordica charantia by several hypothesized mechanisms, i.e., stimulation of glycogenesis and hepatic glycolysis. But, clinical trial reports on human volunteers are inadequate and inconsistent by meager study design and statistical support. This review is an effort to underscore the antidiabetic action along with the phytochemical and pharmacological records on Momordica charantia and suggests for superior-designed clinical trials to substantiate its possible healing properties on diabetes.
Preclinical and Clinical Evidence for Antidiabetic Activity
There are many preclinical reports showing the antidiabetic effect of Momordica charantia extract, powder, and their bioactive compounds in the STZ- and alloxan-induced diabetic animals [23, 84]. The details of these studies are summarized in Table 1. Its clinical evidence for the antidiabetic activity is also reported in the literature, and the details are summarized in Table 2. Dans et al. [85] studied the potential outcome of Momordica charantia in 40 patients with T2DM (20 individuals separate in trial and control) in double-blind, randomized controlled trial. Blood serum was collected for 3 months, and HbA1c was checked, but no significant changes in blood HbA1c level were recorded [85].
Uebanso et al. [86] extracted the Momordica charantia extract with different solvents, i.e., water and methanol, and evaluated it for the glucose tolerance. The findings reported considerably reduced plasma glucose values at 30 min as compared to the control. The plasma insulin level at 30 min was also considerably reduced with methanolic extract as compared to control in the oral sucrose fight evaluation. The mode of action of bitter melon extracts is to inhibit the sucrose action in a dose-dependent manner. These outcomes substantiated that Momordica charantia reduced postprandial elevated BGL by suppression of alpha-glucosidase action [86].
Cummings and his colleagues [87] studied the antihyperglycemic effect of Momordica charantia (karela fruit) in aerating T2DM. The in vitro model of action of Momordica charantia as antidiabetic is not well known. The action of Momordica charantia extract is either 3H-2-deoxyglucose or N-methyl-amino-a-isobutyric acid (14C-Me-AIB) acceptance in L-6 rat muscle grown up to myotube level. The L-6 muscle fiber was mixed with different proportions of juice (1–10 μg/ml) or its chloroform extract and stand for 1 h. The 3H-deoxyglucose and 14C-Me-AIB acceptances by L-6 muscle fiber after 1 h of incubation were approximately 32 pmol/min and 13 pmol/min, respectively. The incubation of L-6 myotubes with insulin (100 nM) for 1 h showed a remarkable (p < 0.05) rise in 3H-deoxyglucose and 14C-Me-AIB uptakes, respectively. The 3H-deoxyglucose and 14C-Me-AIB uptakes with insulin were found to be approximately 58.57 pmol/min and 29.52 pmol/min, respectively. The cultivation of L-6 muscle fiber with 3 different levels (1 μg/ml, 5 μg/ml, and 10 μg/ml) of either the lyophilized juice or its chloroform extract showed a rise in 3H-deoxy-d-glucose and 14C-Me-AIB uptakes, with peak uptakes at 5 μg/ml. The incubation of insulin or juice in the presence of wortmannin (phosphatidylinositol 3-kinase inhibitor) exhibited a noticeable suppression of 3H-deoxyglucose by L-6 myotubes. The outcomes of Momordica charantia fruit juice have a similar action to insulin as an antidiabetic action [87].
Tongia and associates [88] carried out a study to evaluate the antihyperglycemic property of Momordica charantia in DM (non-insulin-dependent diabetes mellitus (NIDDM)). The methanolic extract was administered orally for 1 week. The blood sample was collected, and it was found that the extract showed significant (p < 0.05) reduction in fasting postprandial BGL. Ahmad and his fellows [53] evaluated the potential antidiabetic effect of Momordica charantia, on fasting and postprandial (2 h following 75 g oral administration of glucose) BGL. The increase in serum glucose was evaluated in 100 subjects with modest non-insulin-dependent diabetes mellitus. Intake of the aqueous homogenized vegetable pulp dispersed in water resulted in a considerable reduction (p < 0.001) of fasting and post-food intake serum glucose values [88].
Allium sativum
Allium sativum L. is also known as garlic and belongs to Liliaceae family. This is a persistent species that initiated before 5000 years in Central Asia. It is used to fight off wicked spirits and for health promotion and currently grown all around the globe [53, 89]. The bulb of this plant is usually used as medicine. Numerous studies and clinical evaluations have testified several positive properties of Allium and its recipes. These are like (i) lessening of cancer threat; (i) lessening of threat aspects for cardiovascular ailments; (iv) action against bacteria, viruses, fungi, and parasites; (ii) antioxidant effect; (iii) purification of foreign compounds and liver protection; and (iv) reduction in platelet accumulation and atherosclerotic plaques [90, 91].
Preclinical and Clinical Evidence for Antidiabetic Activity
There are various preclinical research studies available showing the hypoglycemic effect of Allium sativum extract, powder, and their bioactive compound in STZ- and alloxan-induced diabetic animals [24, 25, 92]. The favorable properties of Allium are largely due to the existence of volatile sulfur complexes; i.e., allicin, alliin, diallyl sulfide, S-allylcysteine, and triallyl trisulfide are major volatile sulfur compounds present in the Allium sativum. Allium and its extracts have been proven to be active in preventing insulin resistance. Allium is significant in managing diabetes mellitus problems, making Allium to have been patented [26].
Atkin and associates [93] examined the theory that aged Allium extract (AAE) can increase endothelial utility, oxidative tension, vascular inflammation, and insulin conflict in persons with cardiovascular threat and T2DM. A dual-blind, placebo-organized crossover trial was done in 26 volunteers with T2DM who took 1200 mg of AAE or placebo a day for 28 days followed by 28-day washout time. The 17 males and 9 females with an average age of 61 years, HbA1c of 7.2%, BP of 130/75 mmHg, overall cholesterol of 4.2 mmol/l, triglyceride of 2.11 mmol/l, and HDL cholesterol of 1.04 mmol/l participated in the study. Nearly all volunteers were being administered with metformin (59%), aspirin (50%), and statin (96%) treatments. No alterations were made in this treatment during the whole study. The treatment with AAE exhibited a non-considerable effect on the aforementioned metabolic factors along with insulin resistance. AAE did not show considerable action on endothelial marker utility (plethysmography), oxidative tension, or inflammation (HsCRP). After 28 days, treatment with AAE did not show considerable recovery of endothelial utility, vascular inflammation, oxidative tension, or insulin resistance [94].
Huang and teammates [95] examined the antihyperglycemic action of the plant’s gallic acid (GA) on glucose acceptance in an insulin-resistant cell culture medium and carbohydrate metabolism (liver) in rats with high-fructose diet (HFD)-induced diabetes. Gallic acid augmented glucose uptake action by 19.2% at 6.25 μg/ml in insulin-resistant mouse liver cell (FL83B). GA considerably declined the elevated glucose levels in HFD-induced diabetic rats and decreased the AUC for glucose in oral glucose uptake. GA also remarkably decreased the serum C-peptide, fructosamine, and cardiovascular threat index in HFD-induced diabetic rats. Further, GA significantly monitored the sign of insulin transduction signal of the liver like insulin receptor, insulin receptor substrate 1, phosphatidylinositol 3-kinase, Akt/protein kinase B, and glucose transporter 2, in HFD rats. GA also downregulated the liver gluconeogenesis-linked proteins, like fructose 1 and 6-bisphosphatase, and upregulated the liver glycogen synthase and glycolysis-linked proteins, along with hexokinase and aldolase in HFD rats. These outcomes direct that GA has effectiveness as a healthy diet component to avoid DM [93].
Padiya and Banerjee [94] explain the applications and importance of Allium and its pharmacological elements in regulating diabetes and associated disease. Allium and its active biological compound have been largely recognized for its hypoglycemic actions in either drug-persuaded or genetic animal prototypes of diabetes. Its extract is a powerful remedy for decreasing insulin resistance [94]. Chourey and his fellows [96] studied changes in absorption, distribution, metabolism, and excretion (ADME) of metformin (320 mg/kg) with Allium sativum. The garlic extract (500 mg/kg) was given with and without metformin orally to rats, and the BG-lowering effect as well as an alteration in ADME were measured. It was found that Allium sativum altered the pharmacokinetics with a significant increase in Cmax, AUC of 0–12 h, and enhanced half-life of metformin. The wide clinical pharmacokinetic examinations are needed to instigate this drug-drug interaction in upper species [95]. Ashraf et al. [97] studied the potential antidiabetic effects of Allium in patients with T2DM (n = 60) at the FBG level of more than 126 mg/dl. The subjects were divided into two groups randomly. The first group (n = 30) was administered with tablet garlic (KWAI, 300 mg) three times a day with metformin 500 mg twice a day. The second group (n = 30) was administered with placebo and metformin 500 mg twice a day for 168 days. The plasma lipid and FBG were checked at a predetermined time interval (0 day, 56 days, and 168 days). The first group exhibited a substantial (p < 0.005) decrease in FBG in 168 days (−3.12%) as matched to the second group (0.59%). At the end of day 168, the first group exhibited a significant reduction in averaged whole cholesterol (6.2 mg/dl, −2.82%, p < 0.005), low-density lipoprotein C (−3 mg/dl, 2.2%, p < 0.005), and triglycerides (−5.2 mg/dl, 3.12%, p < 0.005) while HDL cholesterol was considerably increased (2.36 mg/dl, 6.72%, p < 0.005), compared with the second group. It was concluded that concurrent therapy of Allium with distinctive antidiabetic therapy exhibits glycemic control along with antihyperlipidemic action [96].
Ashraf and his teammates [98] examined the action of Allium sativum on cardiovascular parameters, i.e., dyslipidemia in subject with T2DM in 70 subjects for 84 days. The subjects are grouped into two arbitrarily, in a single-blind way. The garlic was given to a patient in tablet dosage form (300 mg, 1.3% allicin) twice daily and alike placebo tablets correspondingly. A considerable decrease in whole cholesterol (−28 mg/dl, −12%, p < 0.001) and LDL-C (−30 mg/dl, −18%, p < 0.001) was reported. The placebo-administered group (n = 32) had a non-considerable reduction in whole cholesterol and LDL-C. The HDL cholesterol considerably rose in subjects administered with garlic (3.35 mg/dl, 8.81%, p = <0.05) than placebo, but no notable change in triglyceride was detected between both groups. So, the necessity of long-term controlled clinical trials is suggested to estimate the value of Allium on vascular and circulatory ailment pathways [97].
Trigonella foenum-graecum (Fenugreek)
It is a traditional medicinal herb in Bangladesh and has different medicinal and pharmacological activities. The seeds of fenugreek have been used in the treatment of diabetes and also in elevated cholesterol, and gastrointestinal disorder [98]. It contains saponins, fenugreekine, nicotinic acid, trigonelline, scopoletin, and coumarin active constituents [99].
Preclinical and Clinical Evidence for Antidiabetic Activity
Various preliminary preclinical studies on fenugreek extract and powder and its bioactive compounds reported the antidiabetic action in a different diabetic animal model [27,28,29, 100,101,102,103]. Many clinical trials proposed conceivable antidiabetic and lipid-lowering effects of orally administered powdered fenugreek seeds [31, 98]. The whole fenugreek seed powder and its dilutions were tried for their antidiabetic action on ordinary and diabetic rats. The powder, methanolic extract, and their residue had a significant antihyperglycemic effect when administered concurrently with glucose. The aqueous extract of seed powder indicated marked hypoglycemic activity at various prandial conditions. The soluble dietary fiber (SDF) had not shown hypoglycemic action on the FBG of normal or rats with NIDDM. On the other hand, on administration at the same time with glucose, it produced a marked hypoglycemic action (p < 0.05) in NIDDM rats. The synthetic investigation demonstrated that the real component of the SDF is a galactomannan. The outcomes verify the contribution of SDF in the antidiabetic action of fenugreek seeds.
Neelakantan and his fellow researchers [104] investigated the clinical actions of Trigonella foenum-graecum with control mediation. The trial was evaluated for FBG, 2 h post-intake glucose, and HbA1c. Trigonella foenum-graecum markedly altered FBG by −0.96 mmol/l, 2 h post-load glucose by −2.19 mmol/l, and HbA1c by −0.85% as matched with control arbitrations. The substantial diversity in research outcomes was partially explicated by diabetes position and dose. The marked changes in fasting and 2 h glucose were only seen in experiments that directed moderate or large doses of Trigonella foenum-graecum. It concluded that fenugreek seed showed a remarkable hypoglycemic effect on diabetes [104].
Yaheya and Ismail conducted the study of potential antidiabetic activity of fenugreek (FG) powder. FG powder was administered orally, a blood sample was analyzed, and the result showed that FG possesses potential antidiabetic activity. This research has shown a new path for a future clinical study for the NIDDM [105].
Gupta et al. [58] evaluated the actions of fenugreek seeds on glycemic control and insulin resistance in moderate T2DM. The 25 patients with T2DM (FBG < 200 mg/dl) were arbitrarily divided into two groups. Set 1 (n = 12) took 1 g/day hydroalcoholic extract of FG seeds, and set 2 (n = 13) took regular care (diet regulation, workout) and placebo capsules for 8 weeks. The FBG (148 to 120 mg/dl vs. 138 to 113 mg/dl) and 2 h post-blood glucose (211 to 181 mg/dl vs. 220 to 241 mg/dl) were the same. However, the AUC of blood glucose (2375 vs. 27597) and insulin (2492 vs. 5631) was markedly reduced (p < 0.001). HOMA model imitative insulin resistance revealed a reduction in % beta-cell secretion in set 1 versus set 2, and a rise in % insulin sensitivity (112 vs. 92, p < 0.05) was observed. Serum TG level significantly decreased as well as HDL, cholesterol level significantly increased (p < 0.05) in set 1 as compared to set 2. It was revealed that the addition of FG seeds increases the glycemic regulation and reduces the insulin resistance in minor T2DM and also has positive action on hypertriglyceridemia. Madar and associates [60] evaluated the action of FG on food glucose and insulin heights using meal tolerance test (MTT). The study was conducted in patients with NIDDM. Water-soaked FG seed powder (15 g) was administered orally, which exhibited a marked decrease in the subsequent postprandial glucose. FG plasma insulin not is significantly reduced in NIDDM. FG had no action on lipid elevations after 3 h of succeeding MTT. So, it was concluded that FG may be useful in treating NIDDM [31].
Cinnamon
Cinnamon is very popular as a spice in the food and beverage and is also known for its medicinal properties. Exploiting cinnamon as a potential antidiabetic agent for T2DM started nearly 20 years ago. The dried barks of cinnamon trees are rich in polyphenolic compound and are used to promote overall health, treating ailments including diabetes. The prime component of cinnamon bark oil is cinnamaldehyde (more precisely trans-cinnamaldehyde or 3-phenyl-2-propenal) and eugenol in the leaf oil. The antidiabetic effect of cinnamon can be attributed to several mechanisms. The numerous in vitro experiments have revealed that cinnamon raises glucose uptake by cells due to increased phosphorylation of the insulin receptor and translocation of GLUT-4 to the plasma membrane [106, 107]. Another feasible mechanism of reduction of glucose level is by activating insulin sensitivity through a rise in the manifestation of α- and β-peroxisome proliferator–activated receptor (PPAR). Furthermore, cinnamon also inhibits intestinal glucosidases and pancreatic amylase. The Ceylon cinnamon is the strongest inhibitor of pancreatic amylase and intestinal sucrase [108]. A clinical study has revealed its power in delaying gastric emptying and reducing the post-food glucose level [109].
Preclinical and Clinical Evidence for Antidiabetic Activity
Various preclinical research studies reported the hypoglycemic effect of cinnamon extract and powder and its bioactive compound in different STZ- and alloxan-induced animal models. The details of these studies are summarized in Table 1. Cinnamon is considered an important antidiabetic agent, and different studies comprising cinnamon administration have shown divergent results. The aim of a randomized, double-blind clinical trial was to attribute the action of powdered cinnamon on FBG, HbA1c, and oxidative condition in Iraqi patients with type 2 diabetes mellitus [32].
Zhu et al. [110] studied cinnamaldehyde, one of the active constituent derivatives of cinnamon as an antidiabetic drug. It was found that cinnamaldehyde reduces glucolipid in diabetic animals by raising cellular uptake of glucose and activating insulin to skeletal muscle and adipose tissue. Cinnamon stimulates glycogenesis, repairing pancreatic islets and delaying the gastric emptying time. Cinnamaldehyde acts through several signaling pathways comprised of PPARs, AMPK, P13K/IRS-1, RBP4-GLUT-4, and NEF2 pathways. Additionally, it appears to control the actions of α-amylase and PTP1B. Moreover, it has the power of metabolizing into cinnamyl alcohol and methyl cinnamate and cinnamic acid inside the body. Briefly, cinnamaldehyde intake maintains glucose and lipid homeostasis in diabetic animals, which may be a new alternative for diabetes [110].
Sahib [75] evaluated the action of cinnamon on FBG, HbA1c, and oxidative stress markers in controlled T2DM. A total of 25 patients with T2DM of either sex, with an average age of 49.1 years, was selected for the treatment with cinnamon for 12 weeks. It was observed that there was remarkable (p < 0.001) lowering in the FBG level (10%) followed by 10% and 17% in 6-week and 12-week treatments than the initial point value and placebo group. The in-between level of glycosylated Hb decreased not significantly (2.6% in 6 weeks, 8.3% in 12 weeks) compared to the initial level. The serum glutathione level increased significantly (p ≤ 0.001) after 3 months than the starting point level and placebo group. Moreover, the cinnamon significantly elevated the superoxide dismutase level in a diabetic patient in 3 months. It was observed that administration of 1 g of cinnamon for 3 months decreases BGL and HbA1c among the patients with T2DM. The report concluded that cinnamon is a good food supplement for controlling diabetes and antioxidant [75].
Kirkham et al. [111] evaluated the clinical strength of cinnamon with diabetics and insulin-resistant diabetes in a randomized manner (311 volunteers). It revealed a significant reduction in blood glucose (FBG) (8–29% and 10.3%; p < 0.05), substantiated by a trial with healthy humans which reported an 8.4% FBG decrease (p < 0.01) versus dummy, and one more showing substantial drops in glucose response following oral glucose tolerance tests (p < 0.05). It revealed that cinnamon has potential antidiabetic and antihyperglycemic activity. Additionally, it needs to authenticate the relation among FBG levels [111].
Khan and associates [112] studied the role of cinnamon on BG and lipid profile in T2DM for a month. A total of 14 patients of either sex, aged 40 years, was selected and divided into two groups. The daily 1.5 g cinnamon was administered in group 1 while 1.5 g placebo dose/day was administered in group 2 for a month. Blood samples were collected at fasting from 0 day to the last day of study. The BG, TG, total cholesterol (TC), HDL, and LDL cholesterol were determined for both groups. It was found that cinnamon markedly lowered BG, TG, and TC levels [112].
Medagama [4] reviewed various articles and critically evaluated the investigational confirmation of existing work for cinnamon in successful glycemic targets in animals and humans. The eight clinical studies of cinnamon were evaluated using powdered and aqueous cinnamon in a dose of 500 mg to 6 g for a period of 40 days to 4 months, and two clinical studies that used cinnamon on treated prediabetics. It revealed that BG level is well controlled in patients with prediabetes (IFG or IGT) and pretreatment of high HbA1c [4].
Pham and colleagues [113] studied the effectiveness of cinnamon in 164 patients with T2DM. No remarkable changes were observed between cinnamon and placebo in reducing BGL. The results authenticated the possible effect of cinnamon in reducing BGL, though clinicians appealed not to recommend cinnamon as a substitute to recognized usual care like change in lifestyle, oral hypoglycemic, and insulin [113]. Mang and his fellow researchers [114] evaluated the aqueous cinnamon extract for glycemic management and lipid profile in patients with T2DM. The HbA1c, FBG, TC, LDL, HDL, and TG levels were evaluated. Three grams of cinnamon powder was administered a day. There was a marked reduction of FBG level in the cinnamon group (10.3%) as compared to the placebo group (3.4%), without any adverse effect. The extract of cinnamon proved to have reasonable influence in lowering fasting FBG levels as compared to controlled diabetics [114].
Suppapitiporn et al. [115] evaluated the hypoglycemic action of cinnamon cassia powder (1.5 g daily) in patients with T2DM. The HbA1c, FBG, creatinine, lipid profile, liver function test, and adverse effects were documented. It was observed that after 12 weeks of treatment, lowering of HbA1c was similar in both groups, i.e., 8.14–7.76% in cinnamon group and 8.06–7.87% in placebo group, respectively, without any statistical significance. Results concluded that cinnamon cassia powder 1.5 g/day did not show a significant effect in lowering FBG level, HbA1c, and serum lipid profile in T2DM with mean the FBG of 154.40 ± 24.72 mg/dl [115]. Khan et al. [116] studied the hypoglycemic effect of cinnamon extract in T2DM. A total of 60 volunteers of either sex (30 male and 30 female, average age 52 years) was selected and allocated randomly into 6 groups. The cinnamon was daily administered (1 g, 3 g, or 6 g) to groups 1, 2, and 3, and placebo capsules to 4, 5, and 6 groups, respectively, for 40 days then a washout period of 20 days. A significant reduction was seen after 40 days, i.e., FBG (18–29%), TG (23–30%), LDL cholesterol (7–27%), and TC (12–26%) level without any significant change in the placebo groups. Findings concluded that cinnamon supplementation to T2DM will lower diabetes and cardiovascular disease risk factors [116].
Abelmoschus esculentus (Okra)
Okra (Abelmoschus esculentus) is a vegetable crop grown in tropical and subtropical regions of the globe [117]. Okra is the sole important vegetable crop belonging to the family Malvaceae. Although it is highly consumed in India, it has originated from Ethiopia, Sudan, and the Northeastern African countries. There are reports about the traditional use of okra seeds to manage elevated blood sugar which mainly contains oligomeric catechins (2.5 mg) and flavone derivatives (3.4 mg) per gram of seeds [118].
Preclinical and Clinical Evidence for Antidiabetic Activity
Various preclinical reports show the hypoglycemic effect of Abelmoschus esculentus, seed powder, and their bioactive compounds in different STZ- and alloxan-induced animal models [119]. The details of these studies are summarized in Table 1. Khosrozadeh et al. [34] evaluated the hypoglycemic activity of Abelmoschus esculentus. It was found that Abelmoschus esculentus extract has BG lowering capacities. It can be used to manage DM, and it inhibits cholesterol absorption, leading to lowering of lipid profile in blood [34].
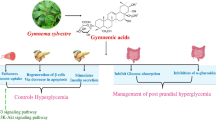
Gymnema sylvestre
Gymnema sylvestre (GS) is a big wooded creeper of family Asclepiadaceae and available in India, Africa, and Australia. From ancient times, it has been used for treating diabetes in Ayurveda.
Preclinical and Clinical Evidence for Antidiabetic Activity
There are reports about the antidiabetic effects of crude or low molecular weight GS in alloxan- or STZ-induced diabetic animals by increasing plasma insulin levels and reducing blood glucose responses in oral glucose/sucrose tolerance tests [120].
Many researchers have revealed the antidiabetic action of GS extract in patients with hyperglycemia [121]. The antihyperglycemic effect of GS extracts is due to a rise in insulin secretion from β-cells of the islets of Langerhans, although this was not well established. Al-Romaiyan and associates [122] evaluated the activity of GS leaf extract in glucose level reduction in T2DM (60 patients). With the 500 g/day administered orally, a marked (p < 0.05) decrease in FBG from 162 to 119 mg/dl and postprandial BGL from 291 to 236 mg/dl was observed, without affecting body weight. It was concluded that Om Santal Adivasi (OSA®), a high molecular weight portion separated from an extract of GS leaf, effectively lowers the BG elevating levels of insulin and C-peptide. The matched in vitro studies suggest that some of the effects of OSA® may be linked to a straight stimulatory action on insulin release from β-cells of the islets of Langerhans. Therefore, OSA® may be a possible substitute for treating hyperglycemia linked with T2DM [122]. Shanmugasundaram and associates [121] studied the hypoglycemic effect of aqueous soluble extract of GS in 27 insulin-dependent diabetes mellitus (IDDM). GS (400 mg/day) was administered with insulin to patients with IDDM. The reduction in insulin dose, FBG, HbA1c, and glycosylated plasma protein levels was observed. The serum lipid level returned to normal whereas HbA1c and glycosylated plasma protein levels were found to be less than control. Patients with IDDM receiving insulin therapy did not show a significant decrease in serum lipids, HbA1c, and glycosylated plasma proteins upon treating after 10–12 months. So, GS treatment seems to raise endogenous insulin, which may be due to revival/regeneration of the remaining beta cells in IDDM [121]. Al-Romaiyan and colleagues [123] documented that oral administration of OSA (500 mg/kg) extract from GS lowered BGL at 1.5–2 h in ob/ob mice. The in vitro study revealed that OSA (0.25 mg/ml) induced insulin release from islets of mouse upon mixing with 2 mmol/l and 20 mmol/l of glucose whereas the preproinsulin (PPI) manifestation rises upon incubation of islets with OSA (0.125 mg/ml). Moreover, the rise in PPI gene manifestation levels was seen with a higher amount of OSA (0.25 mg/ml). However, these levels were markedly lesser than 20 mmol/l glucose. It was concluded that OSA induced insulin release from the islet to manage normal BGL as well as PPI biosynthesis, leading to insulin production [123].
Curcuma longa
The rhizome of Curcuma longa (Linn) or turmeric which belongs to the family Zingiberaceae has a pungent and bitter taste and is extensively used in native medicine and home therapies. Turmeric is also optional in the treatment of diabetes, high cholesterol, abdominal pains, menstrual disorder, wounds, eczema, jaundice, inflammations, cancerous symptoms, and cleansing blood. The rhizome constitutes about 70–76% yellow-colored phenolic pigment curcumin, 16% dimethoxycurcumin, and 8% bisdemethoxycurcumin [124].
Preclinical and Clinical Evidence for Antidiabetic Activity
Many researchers have reported the antidiabetic property of Curcuma longa extract in preclinical studies [124]. Olatunde and associates [125] evaluated the antidiabetic effect of Curcuma longa on alloxan-induced diabetic and non-diabetic rats; 200 mg/kg of oral aqueous extract of rhizome was administered for 4 weeks and showed significantly (p < 0.05) controlled BGL. It was concluded that the aqueous extract of Curcuma longa rhizome has antidiabetic properties and may help to control diabetes and related metabolic disorders [125].
Polyphenols
There are different classes of polyphenols depending on phenol ring numbers and elements that bind them [126]. Roughly, the diet constituted one-third of phenolic acids from total polyphenolic compounds, mainly hydroxybenzoic acid derivatives (protocatechuic acid, gallic acid, p-hydroxybenzoic acid) and hydroxycinnamic acid derivatives (caffeic acid chlorogenic acid, coumaric acid, ferulic acid). The berry fruits, kiwi, cherry, apple, pear, chicory, and coffee are a rich source of phenolic compound [127]. Our diet contains 4000 types of flavonoid (polyphenols) and around six subclasses of flavonoid together with anthocyanins. The family of berries like red wine, red cabbage, cherry, and strawberry contains anthocyanins (cyanidin, pelargonidin, delphinidin, malvidin). The flavonols, along with quercetin, kaempferol, and myricetin, are mainly found in onion, curly kale, leeks, broccoli, and blueberries. Isoflavones are other principal dietary flavonoids that contain genistein and glycitein. Soybeans and soy products primarily contain estrogen-like compounds [128]. The antidiabetic property of polyphenols is due to their capacity to inhibit intestinal absorption of carbohydrates, an inflection of the enzymes linked to glucose metabolism, enhancement of β-cell activity, and prompting the insulin release [129].
Preclinical and Clinical Evidence for Antidiabetic Activity
Kim and associates [78] have reported lowering of BGL and HbA1c upon administration of quercetin in C57BL/KsJ-db/db mice for 49 days. Quercetin is capable of preventing damage to β-cells of the pancreas caused by STZ and normalization of BGL in rats [130].
Kaempferol Glycoside
Kaempferol is a glycon part of kaempferol glycoside (KG) found in different plant sources like soya bean and exhibits potential antidiabetic activity [131].
Preclinical and Clinical Evidence for Antidiabetic Activity
Zang and associates [132] studied the hypoglycemic and anti-obesity effects of KG isolated from immature Jindai soybean Edamame leaves in C57BL/6J mice. KG (0.15 g) was administered in mice for 92 days with a high-fat diet. Administration resulted in a decrease in body weight, adipose tissue, and TG levels compared to those in the high fat–fed control group. It also lowered FBG and HbA1c levels and promoted better insulin resistance. Gene expression study of the liver revealed that KG reduced peroxisome proliferator–activated receptor gamma (PPAR-γ) and sterol regulatory element–binding protein 1c (SREBP-1c) expression. These outcomes propose that KG lowered the accumulation of adipose tissue, taming hyperlipidemia and hyperglycemia in fat mice by rising lipid metabolism and downregulating PPAR-γ and SREBP-1c. Therefore, KG can be used for controlling obesity and diabetes [132]. Apart from the aforesaid studies, there are various preclinical and clinical studies of herbal medicine reporting hypoglycemic activities, fewer from those compiled in Tables 1 and 2.
Conclusion and Future Prospect
Natural herbal preparations, like plant extracts, and their phytochemicals are appealing for their promising therapeutic application in treating several diseases like T2DM. A systemic review on reports about the in vivo and in vitro investigation of numerous plant extracts and natural bioactive molecules for their antidiabetic properties was carried out. Many plant extracts and natural bioactive molecules seem to possess potential hypoglycemic properties, and their strategic inclusion in the diet could be promising in the prevention and control of diabetes. Therapeutic regimens based on phytochemicals could be a novel pharmacological approach in the treatment or to strengthen the available therapies. There are various tasks met in the herbal medicine–based drug discovery which have provided abundant clinically useful compounds and remained as an essential component in the search for new medicines. So, herbal medicine can be exploited successfully to find new herbal bioactive compound for the treatment of diabetes.
Abbreviations
- BGL:
-
Blood glucose level
- DIO:
-
Diet-induced obesity
- DM:
-
Diabetes mellitus
- GLUT-4:
-
Glucose transporter 4
- FBG:
-
Fasting blood glucose
- HDL:
-
High-density lipoprotein
- HFD:
-
High-fructose diet
- IDDM:
-
Insulin-dependent diabetes mellitus
- LDL:
-
Low-density lipoprotein
- NIDDM:
-
Non-insulin-dependent diabetes mellitus
- PPAR-γ:
-
Peroxisome proliferator–activated receptor gamma
- SREBP-1c:
-
Sterol regulatory element–binding protein 1c
- STZ:
-
Streptozotocin
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- WHO:
-
World Health Organization
References
Baby J, Jini D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac J Trop Dis. 2013;3(2):93–102.
Shidfar F, Heydari I, Hajimiresmaiel SJ, Hosseini S, Shidfar S, Amiri F. The effects of cranberry juice on serum glucose, apoB, apoA-I, Lp(a), and paraoxonase-1 activity in type 2 diabetic male patients. J Res Med Sci. 2012;17:355–60.
Frati AC, Jiminez E, Raoul Ariza C. Hypoglycaemic effect of Opuntia ficus indica in non-insulin-dependent diabetes mellitus patients. Phytother Res. 1990;4:195–7.
Medagama AB. The glycaemic outcomes of cinnamon, a review of the experimental evidence and clinical trials. Nutr J. 2015;14:108.
Williams G, Pick JC. Textbook of diabetes II. Oxford: Blackwell; 1991. p. 977–93.
Wainstein J, Ganz T, Boaz M, Dayan YB, Dolev E, Kerem Z, et al. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J Med Food. 2012;15(7):26.
Singh V, Singh SP, Singh M, Gupta AK, Kumar A. Combined potentiating action of phytochemical(s) from Cinnamomum tamala and Aloe vera for their anti-diabetic and insulinomimetic effect using in vivo rat and in vitro NIH/3 T3 cell culture system. Appl Biochem Biotechnol. 2015;175:2542–63.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. 2019;157:107843.
Patel D, Prasad S, Kumar R, Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed. 2012;2:320–30.
Arulselvan P, Ghofar HAA, Karthivashan G, Halim MFA, Ghafar MSA, Fakurazi S. Antidiabetic therapeutics from natural source: a systematic review. Biomed Prev Nutr. 2014;4:607–17.
Allen FM. Blueberry leaf extract. Physiologic and clinical properties in relation to carbohydrate metabolism. JAMA. 1927;89:1577–81.
Asgary S, Rafieian Kopaei M, Sahebkar A, Shamsi F, Goli-malekabadi N. Anti-hyperglycemic and anti-hyperlipidemic effects of Vaccinium myrtillus fruit in experimentally induced diabetes (antidiabetic effect of Vaccinium myrtillus fruit). J Sci Food Agric. 2016;96(3):764–8.
Sidorova Y, Shipelin V, Mazo V, Zorin S, Petrov N, Kochetkova A. Hypoglycemic and hypolipidemic effect of Vaccinium myrtillus L. leaf and Phaseolus vulgaris L. seed coat extracts in diabetic rats. Nutrition. 2017;41:107–12.
Bhaskar S, Sufiyan S, Gurudayal R, Manisha C, Gaurav S. Hypoglycemic and hepatoprotective effects of processed Aloe vera gel in a mice model of alloxan induced diabetes mellitus. J Diabetes Metab. 2013;4:9.
Agarwal V, Sharma AK, Upadhyay A, Singh G, Gupta R. Hypoglycemic effects of Citrullus colocynthis roots. Acta Pol Pharm. 2012;69(1):75–9.
Kim K, Kim H, Kwon J, Lee S, Kong H, Im SA, et al. Hypoglycemic and hypolipidemic effects of processed Aloe vera gel in a mouse model of non-insulin-dependent diabetes mellitus. Phytomedicine. 2009;16(9):856–63.
Rajasekaran S, Sivagnanam K, Ravi K, Subramanian S. Beneficial effects of Aloe vera gel extract on lipid profile status in rats with streptozotocin diabetes. Clin Exp Pharmacol Physiol. 2006;33(3):232–7.
Attele AS, Zhou Y-P, Xie J-T, Wu JA, Zhang L, Dey L, et al. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–8.
Kim JH, Hahm DH, Yang DC, Kim JH, Lee HJ, Shim I. Effect of crude saponin of Korean red ginseng on high-fat diet-induced obesity in the rat. J Pharmacol Sci. 2005;97(1):124–31.
Lee KT, Jung TW, Lee HJ, Kim SG, Shin YS, Whang WK. The antidiabetic effect of ginsenoside Rb2 via activation of AMPK. Arch Pharm Res. 2011;34(7):1201–8.
Amin KA, Awad EM, Nagy MA. Effects of Panax quinquefolium on streptozotocin-induced diabetic rats: role of C-peptide, nitric oxide and oxidative stress. Int J Clin Exp Med. 2011;4(2):136–47.
Yoon JW, Kang SM, Vassy JL, Shin H, Lee YH, Ahn HY, et al. Efficacy and safety of ginsam, a vinegar extract from Panax ginseng, in type 2 diabetic patients: results of a double-blind, placebo-controlled study. J Diabetes Investig. 2012;3:309–17.
Vijaya K, Sunitha SP, Husssain JA, Sandhya P, Sujatha D, Gopireddy G. Synergistic antihyperglycemic, antihyperlipidemic and antioxidant effects of Momordica charantia and metformin in streptozotocin induced diabetic rats. World J Pharm Res. 2014;3:1901–80.
Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006;13(9–10):624–9.
Thomson M, Al-Amin ZM, Al-Qattan KK, Shaban LH, Ali M. Antidiabetic and hypolipidaemic properties of garlic (Allium sativum) in streptozotocin-induced diabetic rats. Int J Diabetes Metab. 2007;15:108–15.
Otunola GA, Afolayan AJ. Antidiabetic effect of combined spices of Allium sativum, Zingiber officinale and Capsicum frutescens in alloxan-induced diabetic rats. 2015;8(4):314–23.
Xue WL, Li XS, Zhang J, Liu YH, Wang ZL, Zhang RJ. Effect of Trigonella foenum-graecum (fenugreek) extracts on blood glucose, blood lipid and hemorheological properties in streptozotocin-induced diabetic rats. Asia Pac J Clin Nutr. 2007;16(S1):422–6.
Annida B, Stanely Mainzen Prince P. Supplementation of fenugreek leaves lower lipid profile in streptozotocin-induced diabetic rats. J Med Food. 2004;7(2):153–6.
Abou El-Soud NH, Khalil MY, Hussein JS, Oraby FSH, Hussein Farrag AR. Antidiabetic effects of fenugreek alkaliod extract in streptozotocin induced hyperglycemic rats. J Appl Sci Res. 2007;3(10):1073–83.
Kim K, Kim H, Kwon J, Lee S, Kong H, Im SA, Lee YL, Lee YR, Oh ST, Jo TH, Park YI, Lee CK, Kim K. Hypoglycemic and hypolipidemic effects of processed Aloe vera gel in a mouse model of non-insulin-dependent diabetes mellitus. Phytomedicine. 2009;16(9):856–63.
Jin Y, Shi Y, Zou Y,Miao C, Sun B, Li C. Fenugreek prevents thedevelopment of STZ-induced diabetic nephropathy in a rat modelof diabetes. Evid Based Complement Alternat Med. 2014; 2014:259368.
Anand P, Murali KY, Tandon V, Murthy PS, Chandra R. Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chem Biol Interact. 2010;186(1):72–81.
Asmena M, Alauddin M, Md Atiar R, Kabir A. Antihyperglycemic effect of Trigonella foenum-graecum (fenugreek) seed extract in alloxan-induced diabetic rats and its use in diabetes mellitus: a brief qualitative phytochemical and acute toxicity test on the extract. Afr J Tradit Complement Altern Med. 2009;6(3):255–61.
Khosrozadeh M, Heydari N, Abootalebi M. The effect of Abelmoschus esculentus on blood levels of glucose in diabetes mellitus. Iran J Med Sci. 2016;41(3):S63.
Kim SH, Hyun SH, Choung SY. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J Ethnopharmacol. 2006;104(1–2):119–23.
Zang Y, Igarashi K, Li Y. Anti-diabetic effects of luteolin and luteolin-7-O-glucoside on KK-A(y) mice. Biosci Biotechnol Biochem. 2016;80(8):1580–6.
Hoggard N, Cruickshank M, Moar KM, Bestwick C, Holst JJ, Russell W, et al. A single supplement of a standardised bilberry (Vaccinium myrtillus L.) extract (36 % wet weight anthocyanins) modifies glycaemic response in individuals with type 2 diabetes controlled by diet and lifestyle. J Nutr Sci. 2013;2:e22.
Shukla K, Narain JP, Puri P, Gupta A, Bijlani RL, Mahapatra SC, et al. Glycaemic response to maize, bajra and barley. Indian J Physiol Pharmacol. 1991;35:249–54.
Taukoorah U, Mahomoodally MF. Crude Aloe vera gel shows antioxidant propensities and inhibits pancreatic lipase and glucose movement in vitro. Adv Pharmacol Sci. 2016;2016:3720850.
Zhang Y, Liu W, Liu D, Zhao T, Tian H. Efficacy of Aloe vera supplementation on prediabetes and early non-treated diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(7):E388.
Tanaka M, Misawa E, Ito Y, Habara N, Nomaqushi K, Yamada M, et al. Identification of five phytosterols from Aloe vera gel as anti-diabetic compounds. Biol Pharm Bull. 2006;29(7):1418–22.
Yongchaiyudha S, Rungpitarangsi V, Bunyapraphatsara N, Chokechaijaroenporn O. Antidiabetic activity of Aloe vera L. juice. I. Clinical trial in new cases of diabetes mellitus. Phytomedicine. 1996;3(3):241–3.
Can A, Akev N, Ozsoy N, Bolkent S, Arda BP, Yanardag R, et al. Effect of Aloe vera leaf gel and pulp extracts on the liver in type-II diabetic rat models. Biol Pharm Bull. 2004;27(5):694–8.
Wang GG, Lu XH, Li W, Zhao X, Zhang C. Protective effects of luteolin on diabetic nephropathy in STZ-induced diabetic rats. Evid Based Complement Alternat Med. 2011;2011:3231.
Itankar PR, Lokhande SJ, Verma PR, Arora SK, Sahu RA, Patil AT. Antidiabetic potential of unripe Carissa carandas Linn. fruit extract. J Ethnopharmacol. 2011;135:430–3.
El-Zein O, Kreydiyyeh SI. Pine bark extract inhibits glucose transport in enterocytes via mitogen-activated kinase and phosphoinositol 3-kinase. Nutrition. 2011;27:707–12.
Kulling SE, Rawel HM. Chokeberry (Aronia melanocarpa) – a review on the characteristic components and potential health effects. Planta Med. 2008;74(13):1625–34.
Dey L, Xie JT, Wang A, Wu J, Maleckar SA, Yuan CS. Anti-hyperglycemic effects of ginseng: comparison between root and berry. Phytomedicine. 2003;10(6–7):600–5.
Chuengsamarn SS, Rattanamongkolgul R, Luechapudiporn C, Phisalaphong SJ. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. 2012;35(11):2121–7.
Michael B, Krawinkel MD, Gudrun B, Keding MS. Bitter gourd (Momordica charantia): a dietary approach to hyperglycemia. Nutr Rev. 2006;64:331–7.
Fernando MR, Wickramasinghe N, Thabrew MI, Ariyananda PL, Karunanayake EH. Effect of Artocarpus heterophyllus and Asteracanthus longifolia on glucose tolerance in normal human subjects and in maturity-onset diabetic patients. J Ethnopharmacol. 1991;31:277–82.
Frati AC, Gordillo BE, Altamirano P, Ariza CR, Cortes-Franco R, Chavez-Negrete A. Acute hypoglycemic effect of Opuntia streptacantha Lemaire in NIDDM. Diabetes Care. 1990;13:455–6.
Ahmad N, Hassan MR, Halder H, Bennoor KS. Effect of Momordica charantia (Karolla) extracts on fasting and postprandial serum glucose levels in NIDDM patients. Bangladesh Med Res Counc Bull. 1999;25(1):11–3.
Al-Khazraji SM, Al-Shamaony LA, Twaij HA. Hypoglycaemic effect of Artemisia herba alba. Effect of different parts and influence of the solvent on hypoglycaemic activity. J Ethnopharmacol. 1993;40:163–6.
Patel JC, Dhirawani MK, Doshi JC. Karella in the treatment of diabetes mellitus. Indian J Med Sci. 1968;22(1):30–2. 48.
Baldwa VS, Bhandari CM, Pangaria A, Goyal RK. Clinical trial in patients with diabetes mellitus of an insulin-like compound obtained from plant source. Ups J Med Sci. 1977;82(1):39–41.
Al-Habori M, Raman A, Lawrence MJ, Skett P. In vitro effect of fenugreek extracts on intestinal sodium-dependent glucose uptake and hepatic glycogen phosphorylase A. Int J Expt Diabetes Res. 2001;2(2):91–9.
Gupta A, Gupta R, Lal B. Effect of Trigonella foenum-graecum (fenugreek) seeds on glycemic control and insulin resistance in type 2 diabetes mellitus: a double blind placebo controlled study. J Assoc Physicians India. 2001;49:1057–61.
Patel MM, Mishra S. A kinetic study for in-vitro intestinal uptake of monosaccharide across rat everted gut sacs in the presence of some antidiabetic medicinal plants. Internet J Altern Med. 2009;7(1):1–7.
Madar Z, Abel R, Samish S, Arad J. Glucose-lowering effect of fenugreek in non-insulin dependent diabetics. Eur J Clin Nutr. 1988;42(1):51–4.
Moorthy R, Prabhu KM, Murthy PS. Anti-hyperglycemic compound (GII) from fenugreek (Trigonella foenum-graecum Linn.) seeds, its purification and effect in diabetes mellitus. Indian J Exp Biol. 2010;48(11):1111–8.
Sharma RD, Raghuram TC, Rao NS. Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur J Clin Nutr. 1990;44(4):301–6.
Kohli K, Singh R. A clinical trial of jambu (Eugenia jambolana) in non-insulin dependent diabetes mellitus. J Res Ayurveda Siddha. 1993;13:89–97.
Srivastava Y, Bhatt H, Gupta O, Gupta P. Hypoglycemia induced by Syzygium cumini Linn. seeds in diabetes mellitus. Asian Med J. 1983;26:489–92.
Sauvaire Y, Petit P, Broca C, Manteghetti M, Baissac Y, Fernandez-Alvarez J, et al. 4-Hydroxyisoleucine: a novel amino acid potentiator of insulin secretion. Diabetes. 1998;47(2):206–10.
Teixeira CC, Fuchs FD, Weinert LS, Esteves MDJ. The efficacy of folk medicines in the management of type 2 diabetes mellitus: results of a randomized controlled trial of Syzygium cumini (L.) Skeels. J Clin Pharm Ther. 2006;31(1):1–5.
Deguchi YK, Osada KU, Kimura H, Oshikawa MY, Kudo T, Yasui H, et al. Effects of extract of guava leaves on the development of diabetes in the db/db mouse and on the postprandial blood glucose of human subjects. Nippon Nogei Kagaku Kaishi. 1998;72:923–31.
Wu Z, Luo JZ, Luo L. American ginseng modulates pancreatic beta cell activities. Chin Med. 2007;2:11–0.
Krawinkel MB, Keding GB. Bitter gourd (Momordica charantia): a dietary approach to hyperglycemia. Nutr Rev. 2006;64:331–7.
Fuangchan A, Sonthisombat P, Seubnukarn T, Chanouan R, Chotchaisuwat P, Sirigulsatien V, et al. Hypoglycemic effect of bitter melon compared with metformin in newly diagnosed type 2 diabetes patients. J Ethnopharmacol. 2011;134(2):422–8.
Ghosh D, Konishi T. Anthocyanins and anthocyanin-rich extracts: role in diabetes and eye function. Asia Pac J Clin Nutr. 2007;16(2):200–8.
Martineau LC, Couture A, Spoor D, Benhaddou-Andaloussi A, Harris C, Meddah B, et al. Anti-diabetic properties of the Canadian low bush blueberry Vaccinium angustifolium Ait. Phytomedicine. 2006;13(9–10):612–23.
Liu J, Gao F, Ji B, Wang R, Yang J, Liu H, et al. Anthocyanins-rich extract of wild Chinese blueberry protects glucolipotoxicity-induced INS832/13 β-cell against dysfunction and death. J Food Sci Technol. 2015;52(5):3022–9.
Saha RK, Nesa A, Nahar K, Akter M. Anti-diabetic activities of the fruit Aegle mamelos. J Mol Biomark Diagn. 7:272.
Sahib AS. Anti-diabetic and antioxidant effect of cinnamon in poorly controlled type-2 diabetic Iraqi patients: a randomized, placebo-controlled clinical trial. J Intercult Ethnopharmacol. 2016;5(2):108–13.
Esra S, Sievenpiper JL, Vladimir D, Adrian IC, Ha V, Viranda HJ, et al. The effect of ginseng (the genus Panax) on glycemic control: a systematic review and meta-analysis of randomized controlled clinical trials. PLoS One. 2014;9(9):e107391.
Kim HO, Park M, Han J. Effects of fermented red ginseng supplementation on blood glucose and insulin resistance in type 2 diabetic patients. J Korean Soc Food Sci Nutr. 2011;40:696–703.
Kim JH, Kang MJ, Choi HN, Jeong SM, Lee YM, Kim JI. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr Res Pract. 2011;5:107–11.
Shin SK, Kwon JH, Jeong YJ, Jeon SM, Choi JY, Choi MS. Supplementation of cheonggukjang and red ginseng cheonggukjang can improve plasma lipid profile and fasting blood glucose concentration in subjects with impaired fasting glucose. J Med Food. 2011;14:108–13.
Mucalo I, Rahelic D, Jovanovski E, Bozikov V, Romic Z, et al. Effect of American ginseng (Panax quinquefolius L.) on glycemic control in type 2 diabetes. Coll Antropol. 2012;36:1435–40.
Chang CLT, Lin Y, Bartolome AP, Chen YC, Chiu SC, Yang WC. Herbal therapies for type 2 diabetes mellitus: chemistry, biology, and potential application of selected plants and compounds. Evid Based Complement Alternat Med. 2013;2013:378657.
Saeed MK, Shahzadi I, Ahmad I, Ahmad R, Shahzad K, Ashraf M, et al. Nutritional analysis and antioxidant activity of bitter gourd (Momordica charantia) from Pakistan. Pharmacol Online. 2010;1:252–60.
Chang CI, Chen CR, Liao YW, Cheng HL, Chen YC, Chou CH. Cucurbitane-type triterpenoids from Momordica charantia. J Nat Prod. 2006;71:1327–30.
Sekar DS, Sivagnanam K, Subramanian S. Antidiabetic activity of Momordica charantia seeds on streptozotocin induced diabetic rats. Pharmazie. 2005;60(5):383–7.
Dans AML, Villarruz MVC, Jimeno CA, Javelosa MAU, Chua J, Bautista R, et al. The effect of Momordica charantia capsule preparation on glycemic control in type 2 diabetes mellitus needs further studies. J Clin Epidemiol. 2007;60(6):554–9.
Uebanso T, Arai H, Taketani Y, Fukaya M, Yamamoto H, Mizuno A, et al. Extracts of Momordica charantia suppress postprandial hyperglycemia in rats. J Nutr Sci Vitaminol (Tokyo). 2007;53(6):482–8.
Cummings E, Hundal HS, Wackerhage H, Hope M, Belle M, Adeghate E, et al. Momordica charantia fruit juice stimulates glucose and amino acid uptakes in L6 myotubes. Mol Cell Biochem. 2004;261(1–2):99–104.
Tongia A, Tongia SK, Dave M. Phytochemical determination and extraction of Momordica charantia fruit and its hypoglycemic potentiation of oral hypoglycemic drugs in diabetes mellitus (NIDDM). Indian J Physiol Pharmacol. Apr 2004;48(2):241–4.
Barrie SA, Jonathan ND, Wright MD, Pizzorno ND. Effects of garlic oil on platelet aggregation, serum lipids and blood pressure in humans. J Orthomol Med. 1987;2:15–21.
Aaron C. Garlic & life. N Am Rev. 1996;281:14–24.
Colín-González AL, Santana RA, Silva-Islas CA, Chánez-Cárdenas ME, Santamaría A, Maldonado PD. The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxidative Med Cell Longev. 2012;2012:907162.
Bayan L, Koulivand PH, Gorji A. Garlic: a review of potential therapeutic effects. Avicenna J Phytomed. 2014;4:1–14.
Atkin M, Laight D, Cummings MH. The effects of garlic extract upon endothelial function, vascular inflammation, oxidative stress and insulin resistance in adults with type 2 diabetes at high cardiovascular risk. A pilot double blind randomized placebo controlled trial. J Diabetes Complicat. 2016;30(4):723–7.
Padiya R, Banerjee SK. Garlic as an anti-diabetic agent: recent progress and patent reviews. Recent Pat Food Nutr Agric. 2013;5(2):105–27.
Huang DW, Chang WC, Wu JS, Shih RW, Shen SC. Gallic acid ameliorates hyperglycemia and improves hepatic carbohydrate metabolism in rats fed a high-fructose diet. Nutr Res. 2016;36(2):150–60.
Chourey S, Narsinghani T, Soni LK. Effect of Allium sativum on the pharmacokinetic of metformin in rat plasma: a herb-drug interaction study. Scholars Research Library Der Pharma Chemica. 2011;3(2):287–91.
Ashraf R, Khan RA, Ashraf I. Garlic (Allium sativum) supplementation with standard antidiabetic agent provides better diabetic control in type 2 diabetes patients. Pak J Pharm Sci. 2011;24(4):565–70.
Ashraf R, Aamir K, Shaikh AR, Ahmed T. Effects of garlic on dyslipidemia in patients with type 2 diabetes mellitus. J Ayub Med Coll Abbottabad. 2005;17(3):60–4.
Sharma RD, Raghuram TC, Rao NS. Effect of fenugreek seeds on blood glucose and serum lipids in type 1 diabetes. Eur J Clin Nutr. 1990;44(4):301–6.
Valette G, Sauvaire Y, Baccou JC, Ribes G. Hypocholesterolaemic effect of fenugreek seeds in dogs. Atherosclerosis. 1984;50:105–11.
Shani J, Goldschimied A, Joseph B, Ah Aronson Z, Sulman FG. Hypoglycemic effect of Trigonella foenum graecum and Lupinus termis (Leguminosae) and their major alkaloids in alloxan-induced diabetic and normal rats. Arch Int Pharmacodyn Ther. 1974;210:27–37.
Amin R, Abdul Ghani AS, Suleiman MS. Effect of fenugreek and lupine seeds on the development of experimental diabetes in rats. Planta Med. 1988;54:286–90.
Hannan JMA, Rokeya B, Faruque O, Nahar N, Moshiuzzaman M, Azad Khan AK, et al. Effect of soluble dietary fibre fraction of Trigonella foenum graecum on glycemic, insulinemic, lipidemic and platelet aggregation status of type 2 diabetic model rats. J Ethnopharmacol. 2003;88:73–7.
Neelakantan N, Narayanan M, de Souza RJ, van Dam RM. Effect of fenugreek (Trigonella foenum-graecum L.) intake on glycemia: a meta-analysis of clinical trials. Nutr J. 2014;18:13–7.
Mohammad Y, Mohammad I. Clinical evaluation of antidiabetic activity of Trigonella seeds and Aegle marmelos leaves. World Appl Sci J. 2009;7(10):1231–4.
Khan A, Bryden NA, Polansky MM, Anderson RA. Insulin potentiating factor and chromium content of selected foods and spices. Biol Trace Elem Res. 1990;24:183–8.
Anderson RA, Broadhurst CL, Polansky MM, Schmidt WF, Khan A, Flanagan VP, et al. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J Agric Food Chem. 2004 Jan 14;52(1):65–70.
Adisakwattana S, Lerdsuwankij O, Poputtachai U, Minipun A, Suparpprom C. Inhibitory activity of cinnamon bark species and their combination effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Plant Foods Hum Nutr. 2011 Jun;66(2):143–8.
Hlebowicz J, Hlebowicz A, Lindstedt S, Björgell O, Höglund P, Holst JJ, et al. Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. Am J Clin Nutr. 2009;89(3):815–21.
Zhu R, Liu H, Chenyue Liu, Wang L, Rufeng Ma R, Chen B, Li L, Niu J, Fu M, Zhang D, Gao S. Cinnamaldehyde in diabetes: A review of pharmacology, pharmacokinetics and safety. Pharmacol Res. 2017;122:78–89.
Kirkham S, Akilen R, Sharma S, Tsiami A. The potential of cinnamon to reduce blood glucose levels in patients with type 2 diabetes and insulin resistance. Diabetes Obes Metab. 2009;11(12):1100–13.
Khan R, Khan Z, Shah SH. Cinnamon may reduce glucose, lipid and cholesterol level in type 2 diabetic individuals. Pak J Nutr. 2010;9(5):430–3.
Pham AQ, Kourlas H, Pham DQ. Cinnamon supplementation in patients with type 2 diabetes mellitus. Pharmacotherapy. 2007;27(4):595–9.
Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth DO, et al. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. Eur J Clin Investig. 2006;36(5):340–4.
Suppapitiporn S, Kanpaksi N, Suppapitiporn S. The effect of Cinnamon cassia powder in type 2 diabetes mellitus. J Med Assoc Thail. 2006;89(3):S200–5.
Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26(12):3215–8.
Lu F, BoLi YL. Okara dietary fiber and hypoglycemic effect of okara foods. Bioact Carbohydr Diet Fibre. 2013;2(2):126–32.
Martin FW. Okra, potential multiple purpose crop for the temperate zones and tropics. Econ Bot. 1982;36(3):340–5.
Sabitha V, Ramachandran S, Naveen KR, Panneerselvam K. Antidiabetic and antihyperlipidemic potential of Abelmoschus esculentus (L.) Moench. in streptozotocin-induced diabetic rats. J Pharm Bioallied Sci. 2013;3(3):397–402.
Shridhar PBS, Rao SM, Byregowda ML, Satyanarayana, Purushotham KM. Antidiabetic effect of Gymnema sylvestre in streptozotocin induced diabetes in rats. Braz J Vet Pathol. 2015;8(2):36–45.
Shanmugasundaram ER, Rajeswari G, Baskaran K, Rajesh Kumar BR, Radha Shanmugasundaran K, Kizar AB. Use of Gymnema sylvestre leaf extract in the control of blood glucose in insulin-dependent diabetes mellitus. J Ethnopharmacol. 1990;30:281–94.
Al-Romaiyan A, King AJ, Persaud SJ, Jones PM. A novel extract of Gymnema sylvestre improves glucose tolerance in vivo and stimulates insulin secretion and synthesis in vitro. Phytother Res. 2013;27:1006–11.
Al-Romaiyan AB, Liu H, Asare-Anane CR, Maity SK, Chatterjee N, Koley TB, et al. A novel Gymnema sylvestre extract stimulates insulin secretion from human islets in vivo and in vitro. Phytother Res. 2010;24:1370–6.
Cooper TH, Clark JG, Guzinski JA. In: Ho CT, Osawa T, Rosen T, editors. Food phytochemicals for cancer prevention II: teas, spices, and herbs, vol. 23. Washington DC: American Chemical Society; 1994. p. 231–6.
Olatunde A, Joel EB, Tijjani H, Obidola SM, Luka CD. Anti-diabetic activity of aqueous extract of Curcuma longa (Linn) rhizome in normal and alloxan-induced diabetic rats. Researcher. 2014;6(7):58–65.
Pandey KB, Rizvi SI. Role of red grape polyphenols as antidiabetic agents. Integr Med Res. 2014;3:119–25.
Pietta P, Minoggio M, Bramati L. Plant polyphenols: structure, occurrence and bioactivity. Stud Nat Prod Chem. 2003;28:257–312.
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47.
Adlercreutz H. Lignans and human health. Crit Rev Clin Lab Sci. 2007;44:483–525.
Coskun O, Kanter M, Korkmaz A, Oter Quercetin S. A flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol Res. 2005;51:117–23.
Yanqing Z, Sato H, Kiharu I. Anti-diabetic effects of a kaempferol glycoside-rich fraction from unripe soybean (edamame, Glycine max L. Merrill. ‘Jindai’) leaves on KK-Ay mice. Biosci Biotechnol Biochem. 2011;75(9):1677–84.
Zang Y, Zhang L, Igarashi K, Yu C. The anti-obesity and anti-diabetic effects of kaempferol glycosides from unripe soybean leaves in high-fat-diet mice. Food Funct. 2015;6(3):834–41.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Clinical Pharmacology
Rights and permissions
About this article
Cite this article
Zafar, A., Alruwaili, N.K., Panda, D.S. et al. Potential of Natural Bioactive Compounds in Management of Diabetes: Review of Preclinical and Clinical Evidence. Curr Pharmacol Rep 7, 107–122 (2021). https://doi.org/10.1007/s40495-021-00255-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-021-00255-8