Abstract
While previous studies have demonstrated significant eye problems in children with autism spectrum disorders (ASDs), refractive errors have not been extensively studied in the context of ASDs. We systematically reviewed twenty-eight articles to assess whether refractive errors are linked with ASDs, and to determine the prevalence of refractive errors in children with ASDs. We found no significant association between ASDs and myopia or hyperopia, but a significantly increased risk of astigmatism was observed in children with ASDs. Pooled results of single-arm studies revealed a 14.1% prevalence of myopia, a 9.8% prevalence of hyperopia, and a 16.5% prevalence of astigmatism in children with ASDs. Future studies should incorporate a prospective design with age-matched comparison groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Autism spectrum disorders (ASDs) encompass a variety of neurodevelopmental anomalies with overlapping signs and symptoms including persistent social and communication difficulties, and restricted, repetitive behavioral patterns (American Psychiatric Association, 2013). Over the past 30 years, the incidence of ASDs has increased by approximately 0.06% per year (Li et al., 2022), possibly due to a rise in awareness and understanding, leading to better diagnosis, and they are estimated to affect 0.3% of the global population (Solmi et al., 2022). ASDs have transitioned from rare childhood conditions with narrow diagnostic parameters to widely recognized, extensively researched, and heterogeneous lifelong conditions (Lord et al., 2018).
Several neurological anomalies present with ocular manifestations because the eyes and brain develop from the same embryonic tissue (London et al., 2013). As structural brain alterations contribute to the pathogenesis of ASDs (Ecker, 2017), some of the clinical manifestations of ASDs — such as visual sensory alterations — may be attributable to visual defects (Simmons et al., 2009). ASDs are usually detected between 3 and 7 years of age (Mandell et al., 2005), and visually impaired children develop social deficits and repetitive behaviour analogous to children with ASDs (Wrzesińska et al., 2017). ASDs are also associated with poor eye contact, possibly linked to neural function (Chevallier et al., 2012; Hirsch et al., 2022; Senju & Johnson, 2009).
Uncorrected refractive errors are the leading cause of visual impairment globally (Dandona & Dandona, 2001; Flaxman et al., 2017). Refractive errors manifest in various forms, including myopia (difficulty seeing far objects), hyperopia (difficulty seeing near objects), and astigmatism (failure to converge light rays on a single point), and can negatively impact social development (Shah et al., 2020). Hyperopic eyes tend to have a shorter axial length that is usually corrected using convex (plus) lens, while myopic eyes tend to have a longer axial length that is usually corrected by concave (minus) lens. Astigmatism arises when the rays of lights in different meridians converge at different points. In simple hyperopic or myopic astigmatism, one meridian is focused on the retina while the other is hyperopic or myopic respectively. In compound hyperopic or myopic astigmatism, both meridians are hyperopic or myopic, respectively, but to different degrees. Mixed astigmatism arises when one meridian is hyperopic and the other is myopic. Children are hyperopic at birth due to a shorter axial length, but the axial length increases progressively starting around 2 years of age leading to emmetropia; children prone to myopia have a longer axial length at birth (Subudhi & Agarwal, 2023). Hyperopia and astigmatism may be of several types and etiologies (Gurnani & Kaur, 2023; Majumdar & Tripathy, 2023). Since refractive errors are widespread in children of school-going age (Tajbakhsh et al., 2022), they may exacerbate communication challenges, hinder social interactions, and deteriorate academic performance in children with ASDs. While a recent study revealed association of several eye problems in ASDs (Perna et al., 2023), it did not focus on refractive errors, and a concrete correlation with refractive errors has yet to be established. In this systematic review and meta-analysis, we aim to address whether refractive errors are linked to ASDs in children and young adults. Our secondary objective is to assess the prevalence of refractive errors in children with ASDs by analyzing single-arm studies. Our review may help in a timely diagnosis of these refractive errors, thereby improving the quality of life of children with ASDs.
Methods
This review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021). We registered the protocol for this systematic review on the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42023433833).
Data Sources and Search Strategy
Two reviewers independently searched Medline (PubMed), Scopus, Science Direct, and Embase (Ovid) for relevant articles indexed from inception to May 2023. The searches were refreshed in October 2023 to identify any new articles of interest. No language restriction was applied. We combined the keywords “autism”, “autism spectrum disorder”, “refractive errors”, “myopia”, “hyperopia”, “astigmatism”, “child”, and “adolescent” with other synonyms. The detailed search strategy for each database can be found in Online Resource 1. We eliminated the duplicate studies using Endnote 20.2.1. Two reviewers independently assessed the articles based on titles and abstracts. The full texts of the remaining articles were retrieved, and articles matching our eligibility criteria were included. Disputes were resolved by a third reviewer.
Study Selection
The studies were considered eligible for our systematic review and meta-analysis if they (a) were original studies including case reports, case series, cross-sectional studies, or cohort studies; (b) included children and young adults (< 20 years of age) with a diagnosis of ASDs; (c) evaluated at least one of the following refractive errors: myopia, hyperopia, and astigmatism. A study was excluded if it (a) was not concerned with ASD, (b) did not measure refractive errors, (c) included no children or young adults, or (d) was in a language other than English. Additionally, letters, editorials, short communications, review articles, conference abstracts, non-human studies, and book chapters were also excluded.
Data Extraction
The primary outcomes of interest were the number of subjects with ASDs having either myopia, hyperopia, or astigmatism. The total number of cases of non-autistic children with myopia, hyperopia, and astigmatism was also recorded in studies with a comparison (C) group. Demographic data including location, age, and gender, the criteria used to assess ASDs and refractive errors, and associated neurodevelopmental comorbidities were also extracted. Specific data on race and ethnicity, socioeconomic status, and educational attainment levels were not recorded, as such data was not available in most studies. All data was extracted by two reviewers independently, and a third reviewer cross-checked it.
Risk of Bias Assessment
The case reports, case series, and cross-sectional studies were assessed using the Joanna Brigg Institute’s (JBI) critical appraisal tools, while the Newcastle–Ottawa Scale (NOS) was applied to the cohort studies. Two reviewers independently assessed the quality of included studies. Discrepancies were resolved by a third reviewer. The studies were rated good (low risk of bias), fair (moderate risk of bias), and poor (high risk of bias) quality based on their scores.
Statistical Analysis
We calculated the risk ratios (RRs) in a DerSimonian-Laird random effects model to address heterogeneity between the studies (Higgins et al., 2003). We used Arcsine-transformed prevalence data for one-arm studies to stabilize variance as recommended (Barendregt et al., 2013; Munn et al., 2015). However, due to recently raised concerns regarding this approach (Lin & Xu, 2020), we presented our data with 95% confidence intervals in forest plots. Percentages were converted into raw data, and a correction factor of 0.5 was applied to zero values. The meta-analysis of two-arm studies was conducted via Review Manager 5.4 (Nordic Cochrane Center, Copenhagen, Denmark) and of one-arm studies via OpenMeta[Analyst] (Center of Evidence-Based Medicine, Rhode Island, USA) (Wallace et al., 2012). We assessed heterogeneity using Higgin’s I2 statistic, and an I2 value > 50% was considered significant (Higgins & Thompson, 2002). We used Comprehensive Meta Analyst (Biostat, Englewood, New Jersey, USA) to assess publication bias via funnel plots of standard error, and Egger’s regression test (Egger et al., 1997). We conducted sensitivity analyses using a leave-one-out approach, i.e., omitting one study at a time to investigate the impact of each study on the overall effect estimates. Sensitivity analyses were also conducted by excluding the poor-quality studies to explore potential causes of heterogeneity. We conducted subgroup analyses by study design and meta-regression with the mean age of participants. All studies for which raw data could be obtained or calculated were included in the meta-analysis. Case reports were excluded from the quantitative synthesis.
Results
Literature Search
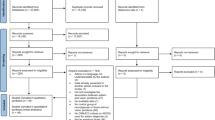
The initial search yielded 1346 papers, of which 248 were duplicates, leaving 1098 articles. The titles and abstracts of the remaining articles were assessed for eligibility, and 998 articles were excluded. The full texts of 3 papers could not be retrieved, and the full texts of 97 articles were subsequently reviewed. A secondary search yielded one additional relevant paper, and 28 papers were deemed eligible for our systematic review (ALGarzaie and Alsaqr, 2021; Amin et al., 2023; Anketell et al., 2016; Bhaskaran et al., 2018; Black et al., 2013; Cao et al., 2022a, 2022b; Chang et al., 2019; Dias et al., 2021; Ezegwui et al., 2014; Faron et al., 2021; Fryns et al., 1996; Gutiérrez et al., 2022; Ikeda et al., 2013; Kabatas et al., 2015; Kaur et al., 2016; Keith et al., 1972; Khanna et al., 2020; Lau et al., 2022; McCurry et al., 2013; Ozer et al., 2016; Pineles et al., 2010; Puri et al., 2015; Scharre & Creedon, 1992; Shen et al., 2011; Tsao et al., 2017; Tychsen et al., 2008; Wu et al., 2023; Zdonczyk et al., 2023). Figure 1 shows the PRISMA flow flowchart of the selection process.
Study Characteristics
Table 1 shows the characteristics of the included studies. The studies comprised eleven cohort studies, eleven cross-sectional studies, two case series, and four case reports published from 1972 to 2023. The studies varied in geographical distribution, with almost one-third of the studies originating from the USA (n = 9) (Black et al., 2013; Chang et al., 2019; Dias et al., 2021; Faron et al., 2021; Ikeda et al., 2013; Pineles et al., 2010; Scharre & Creedon, 1992; Tychsen et al., 2008; Zdonczyk et al., 2023). The population was predominantly male. The most used diagnostic criterion for ASD was the Diagnostic and Statistical Manual of Mental Disorders (DSM) (Chang et al., 2019; Ezegwui et al., 2014; Khanna et al., 2020; McCurry et al., 2013; Scharre & Creedon, 1992; Shen et al., 2011), followed by the International Classification of Diseases (ICD) (Anketell et al., 2016; Chang et al., 2019; Lau et al., 2022; Wu et al., 2023). Most studies considered refractive errors significant when necessitating spectacle prescription, but varying cutoff values were used. Only three studies compared the prevalence of refractive errors in children with ASDs with those without ASDs (ALGarzaie and Alsaqr, 2021; Anketell et al., 2016; Wu et al., 2023). The results of the included studies are depicted in Table 2.
Myopia
Of the twenty-eight included studies, nineteen reported raw data on myopia. Meta-analysis of three studies comparing ASD (1514/3710 participants) to C (14,797/35776 participants) groups yielded no significant association between myopia and ASD (RR 1.02, 95% CI 0.98 – 1.06; p = 0.46; I2 = 0%). Pooled estimates of sixteen studies without a comparison group showed a 14.1% (95% CI 0.082 – 0.213; I2 = 90.63%) incidence of myopia in the ASD group (165/1421 participants). The meta-analysis is depicted in Fig. 2.
No heterogeneity was present between the studies with a comparison group, while high heterogeneity was observed between the studies without a comparison group. Subgroup analysis by study design showed that the heterogeneity was lower for the cross-sectional studies (Fig. 2). Sensitivity analysis using the leave-one-out method revealed no substantial change in pooled effect estimates; RR 11.1% (95% CI 0.064 – 0.170) to RR 15.3% (95% CI 0.090 – 0.230). Sensitivity analysis omitting poor quality studies still presented considerable heterogeneity. Meta-regression with the mean age of participants showed no significant association (p = 0.240) with heterogeneity. We found no evidence of publication bias in the funnel plot (Egger’s regression test, p = 0.257).
Hyperopia
Of the twenty-eight included studies, twenty reported raw data on hyperopia. Of the three studies with a comparison group, ALGarzaie and Alsaqr (2021) had zero incidence of hyperopia in both groups, so it could not be included in the two-arm meta-analysis. Meta-analysis of two studies comparing ASD (436/3710 participants) to C (1893/35776 participants) groups yielded no significant association between hyperopia and ASD (RR 1.27, 95% CI 0.46 – 3.45; p = 0.65; I2 = 99%). The pooled results of seventeen studies without a comparison group revealed a 9.8% (95% CI 0.066 – 0.136; I2 = 73.93%) incidence of hyperopia in the ASD group (176/1437 participants). The meta-analysis is depicted in Fig. 3.
High heterogeneity was present between the studies with a comparison group, while moderate heterogeneity was observed between the studies without a comparison group. Subgroup analysis by study design failed to show much difference (Fig. 3). Sensitivity analysis using the leave-one-out method revealed no substantial change in pooled effect estimates; RR 8.8% (95% CI 0.060 – 0.122) to RR 10.8% (95% CI 0.075 – 0.146). Sensitivity analysis omitting poor quality studies still presented considerable heterogeneity. Meta-regression with the mean age of participants showed no significant association (p = 0.264) with heterogeneity. We found evidence of publication bias in the funnel plot (Egger’s regression test, p = 0.032).
Astigmatism
Of the twenty-eight included studies, nineteen reported raw data on astigmatism. Meta-analysis of three studies comparing ASD (825/3710 participants) to C (5468/35,776 participants) groups revealed that ASD significantly increases the risk of astigmatism (RR 1.63, 95% CI 1.26 – 2.12; p = 0.0002; I2 = 56%). Pooled results from the studies without a comparison group revealed a 16.5% (95% CI 0.108 – 0.232; I2 = 87.87%) incidence of refractive errors in the ASD group. The meta-analysis is depicted in Fig. 4.
Moderate heterogeneity was observed between the studies with a comparison group, and high heterogeneity was present between the studies without a comparison group. Subgroup analysis revealed no significant association of study design with heterogeneity (Fig. 4). Sensitivity analysis using the leave-one-out method revealed no substantial change in pooled effect estimates; RR 14.5% (95% CI 0.099 – 0.198) to RR 18.1% (95% CI 0.120 – 0.250). Sensitivity analysis omitting poor quality studies still presented considerable heterogeneity. Meta-regression with the mean age of participants showed no significant association (p = 0.134) with heterogeneity. We found no evidence of publication bias in the funnel plot (Egger’s regression test, p = 0.292).
Case Reports
Keith et al. (1972) presented a case of a child with autism, myopia, and abnormal facial features. Fryns et al. (1996) presented a case report of two twin sisters with Cohen syndrome, having autistic features and high-grade myopia. Tychsen et al. (2008) presented a series of twelve children undergoing surgery for refractive errors, with one child having autism and myopia. Pineles et al. (2010) presented a case report of three children with vitamin B12 optic neuropathy and autism, two of whom presented with myopia. Shen et al. (2011) reported a family with 16p11.2 deletion, having a child with autism and bilateral myopia. Cao et al. (2022b) reported a case of high myopia in a child with autism who developed neurotrophic keratopathy after laser retinopexy. Zdonczyk et al. (2023) presented a prospective case series including three children with autism spectrum disorder, with one case each of myopia, hyperopia, and astigmatism.
Risk of Bias Assessment
The JBI critical appraisal checklists rated the case reports 3 to 7 points: two good, two fair, and one poor quality; the case series were both rated 10: considered good quality; the cross-sectional studies were rated 3 to 6: two poor, four fair, and five good quality studies. The NOS scores for the cohort studies range from 3 to 5: one good and nine poor quality studies. The detailed quality assessment can be found in Online Resource 1.
Discussion
Our pooled analysis of twenty-eight studies suggests a significant association of astigmatism with ASDs, while associations with myopia and hyperopia remain unclear. These findings align with a previous study that suggested 22 to 44% of all children with autism also have refractive errors (Reynolds & Culican, 2023). Our results revealed a 16.5% prevalence of astigmatism, followed by a 14.1% prevalence of myopia and a 9.8% prevalence of hyperopia in children with ASD.
We could not establish a clear association between myopia and ASDs. Recent studies have observed a rise in the incidence of acquired myopia (Morgan et al., 2018). Furthermore, myopia shows variable prevalence in different ethnicities, geographical regions, and age groups (Foster & Jiang, 2014), and is affected by a multitude of environmental factors (Ramamurthy et al., 2015), which might explain the lack of a conclusive relationship.
We could not find evidence of an association between hyperopia and ASDs. Hyperopia is generally less prevalent than other refractive errors; however, young children are generally hyperopic (Jiang et al., 2019; Majumdar & Tripathy, 2023). Emmetropization occurs in the majority of hyperopic children in the first year of life, but some children may remain hyperopic (Mutti, 2007). A previous meta-analysis showed an 8.4% incidence of hyperopia in typically developing 6-year-olds that decreases with age (Castagno et al., 2014), which is comparable to our findings in children with ASDs.
The higher incidence of astigmatism in children with ASD is similar to its incidence in other neurodevelopmental disorders, such as Down’s syndrome (Little et al., 2009; Woodhouse et al., 1997), attention-deficit/hyperactivity disorder (ADHD) (Bellato et al., 2023; Reimelt et al., 2021), developmental delay (Nielsen et al., 2007), and hydrocephalus (Biglan, 1990; Patel et al., 2021). Astigmatism is generally more common in adults (Hashemi et al., 2014; Zhang et al., 2023); however, it remains a cause of concern in children (H. Cao et al., 2022a). In typically developing individuals, emmetropization occurs early in life (Abrahamsson et al., 1988). A failure in emmetropization might explain the higher incidence of astigmatism in younger populations with ASDs and other neurodevelopmental disorders (Doyle et al., 1998). Genetic and epigenetic components may be responsible in some cases: greater prevalence of astigmatism has been observed in Native American children (Harvey et al., 2010), and gene loci such as (2p16.3) downstream of the neurexin-1 gene and 2p13.3 in the VAX2 gene have been linked to astigmatism in Europeans (Li et al., 2015; Lopes et al., 2013). In our included studies, European Caucasian children were studied by Anketell et al. (2016). Other studies reported the country of origin, but specific racial/ethnicity data was not reported, nor were participants stratified by socioeconomic or educational levels.
We found several comorbidities — ranging from genetic variations to neurodevelopmental disorders — which could affect the visual function in children with ASDs. Previous studies have also demonstrated that ASDs can present with several comorbidities, including genetic and chromosomal abnormalities (Bergbaum & Ogilvie, 2016; Genovese & Butler, 2020), anxiety, ADHD, and other psychiatric disorders (Hossain et al., 2020). Furthermore, visual impairments, including refractive errors, have also been observed in psychomotor disorders (Sobrado et al., 1999) and other neural disorders such as Fragile X syndrome and Prader-Willi syndrome (Van Splunder et al., 2003). Cerebral visual impairment is associated with vision defects (Fazzi et al., 2007) and is closely related to ASDs and intellectual disability (Chokron et al., 2020). ASDs show a multifactorial pattern of inheritance with complex interactions between genetic, environmental, and epigenetic factors (Genovese & Butler, 2020), and similar patterns have been observed for refractive errors (Harb & Wildsoet, 2019). All this evidence points to shared etiological factors contributing to ASDs and refractive errors.
Our study should be viewed in the context of some limitations. First, the generalizability of our findings might be limited due to the lack of a comparison group in most of the included studies. Second, our review did not include conference abstracts and unpublished studies, which might contribute to publication bias. Third, our studies encompassed a broad time period, and the diagnostic criteria for ASDs and refractive errors differ dramatically across the studies, potentially introducing heterogeneity. Lastly, all the included studies were observational, which might pose a risk of confounding bias.
We found no association between myopia and ASDs, but the prevalence of myopia and astigmatism is very similar in children with ASDs; optometrists should be mindful, and test for both whenever possible in children with ASDs. Drugs such as anticonvulsants are often prescribed to children with ASDs (Oswald & Sonenklar, 2007), which may cause transient refractive errors (Hadjikoutis et al., 2005). Acetazolamide has been reported to improve symptoms of ataxia in autistic patients (Martorell et al., 2022), but it is also a known risk factor for refractive errors (Garland et al., 1962; Hadjikoutis et al., 2005) Clinicians should exercise caution when prescribing such medications, and alternative drugs should be considered if possible. Children with ASDs are less likely to have access to eye care — in the USA, estimates from the National Survey of Children’s Health revealed that about 50% of children with ASDs have been evaluated by an eye care provider, which should ideally be increased to 100% (Swanson et al., 2020), and lower rates of vision screening have been observed in children with ASDs compared to those without ASDs, particularly in Black and younger children (Hoover et al., 2023). Regular eyecare is important to ensure that the visual impairment does not impact the development of children with ASDs.
There is a noticeable lack of studies comparing refractive errors in children with and without ASDs, with only three such included studies in our systematic review (ALGarzaie and Alsaqr, 2021; Anketell et al., 2016; Wu et al., 2023). Future research should incorporate robust prospective designs with age-matched comparison groups while adjusting for confounding factors and comorbid conditions such as ADHD. Furthermore, standardized metrics should be used for refractive errors and ASDs. As research on novel methods to slow or halt the progression of myopia continues (Agyekum et al., 2023), it would be prudent to look into its cost-effectiveness and develop cheaper and more widely applicable methods, as the families or caretakers of children with ASDs may be financially strained (Cidav et al., 2012). Lastly, future research should be expanded to include different demographics and ethnicities to get a broader picture.
To conclude, ASDs seem to be associated with a significantly higher risk of astigmatism, but no significant associations were observed for myopia and hyperopia. The retrospective nature of the included studies and lack of comparison groups are noteworthy limitations, and more robust studies should be designed in the future. Timely diagnosis and management may slow the progression of refractive errors in children with ASDs and lessen the impact on their everyday lives.
References
Abrahamsson, M., Fabian, G., & Sjostrand, J. (1988). Changes in astigmatism between the ages of 1 and 4 years: A longitudinal study. British Journal of Ophthalmology, 72(2), 145–149. https://doi.org/10.1136/bjo.72.2.145
Agyekum, S., Chan, P. P., Zhang, Y., Huo, Z., Yip, B. H. K., Ip, P., et al. (2023). Cost-effectiveness analysis of myopia management: A systematic review. Frontiers in Public Health, 11. https://doi.org/10.3389/fpubh.2023.1093836
ALGarzaie, M. A., & Alsaqr, A. M. (2021). A comparative study of corneal topography in children with autism spectrum disorder: a cross-sectional study. Vision, 5(1), 4. https://doi.org/10.3390/vision5010004
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5 (Vol. 5). Washington, DC: American Psychiatric Association.
Amin, N., Tariq, M., Arshad, M., & Cheema, M. A. (2023). Frequency of visual impairment in autistic children of autism school of Lahore, Pakistan. British Journal of Visual Impairment, 026461962211453. https://doi.org/10.1177/02646196221145367
Anketell, P. M., Saunders, K. J., Gallagher, S., Bailey, C., & Little, J.-A. (2016). Profile of refractive errors in European Caucasian children with autistic spectrum disorder; increased prevalence and magnitude of astigmatism. Ophthalmic and Physiological Optics, 36(4), 395–403. https://doi.org/10.1111/opo.12286
Barendregt, J. J., Doi, S. A., Lee, Y. Y., Norman, R. E., & Vos, T. (2013). Meta-analysis of prevalence. Journal of Epidemiology and Community Health, 67(11), 974–978. https://doi.org/10.1136/jech-2013-203104
Bellato, A., Perna, J., Ganapathy, P. S., Solmi, M., Zampieri, A., Cortese, S., & Faraone, S. V. (2023). Association between ADHD and vision problems. A systematic review and meta-analysis. Molecular Psychiatry, 28(1), 410–422. https://doi.org/10.1038/s41380-022-01699-0
Bergbaum, A., & Ogilvie, C. M. (2016). Autism and chromosome abnormalities-A review. Clinical Anatomy, 29(5), 620–627. https://doi.org/10.1002/ca.22719
Bhaskaran, S., Lawrence, L., Flora, J., & Perumalsamy, V. (2018). Functional and cognitive vision assessment in children with autism spectrum disorder. Journal of American Association for Pediatric Ophthalmology and Strabismus, 22(4), 304–308. https://doi.org/10.1016/j.jaapos.2018.03.010
Biglan, A. W. (1990). Ophthalmologic complications of meningomyelocele: A longitudinal study. Transactions of the American Ophthalmological Society, 88, 389–462. http://www.ncbi.nlm.nih.gov/pubmed/2095031. Accessed 4 September 2023.
Black, K., McCarus, C., Collins, M. L. Z., & Jensen, A. (2013). Ocular manifestations of autism in ophthalmology. Strabismus, 21(2), 98–102. https://doi.org/10.3109/09273972.2013.786733
Cao, H., Cao, X., Cao, Z., Zhang, L., Han, Y., & Guo, C. (2022a). The prevalence and causes of pediatric uncorrected refractive error: Pooled data from population studies for global burden of disease (GBD) sub-regions. PLoS ONE, 17(7), e0268800. https://doi.org/10.1371/journal.pone.0268800
Cao, J., Zhu, A. Y., & Mireskandari, K. (2022b). Neurotrophic keratopathy following laser retinopexy for high myopia in an autistic child. Canadian Journal of Ophthalmology, 57(1), e24–e27. https://doi.org/10.1016/j.jcjo.2021.06.006
Castagno, V. D., Fassa, A. G., Carret, M. L. V., Vilela, M. A. P., & Meucci, R. D. (2014). Hyperopia: A meta-analysis of prevalence and a review of associated factors among school-aged children. BMC Ophthalmology, 14(1), 163. https://doi.org/10.1186/1471-2415-14-163
Chang, M. Y., Gandhi, N., & O’Hara, M. (2019). Ophthalmologic disorders and risk factors in children with autism spectrum disorder (ASD). Journal of American Association for Pediatric Ophthalmology and Strabismus, 23(4), e21–e22. https://doi.org/10.1016/j.jaapos.2019.08.071
Chevallier, C., Kohls, G., Troiani, V., Brodkin, E. S., & Schultz, R. T. (2012). The social motivation theory of autism. Trends in Cognitive Sciences, 16(4), 231–239. https://doi.org/10.1016/j.tics.2012.02.007
Chokron, S., Kovarski, K., Zalla, T., & Dutton, G. N. (2020). The inter-relationships between cerebral visual impairment, autism and intellectual disability. Neuroscience & Biobehavioral Reviews, 114, 201–210. https://doi.org/10.1016/j.neubiorev.2020.04.008
Cidav, Z., Marcus, S. C., & Mandell, D. S. (2012). Implications of childhood autism for parental employment and earnings. Pediatrics, 129(4), 617–623. https://doi.org/10.1542/peds.2011-2700
Dandona, R., & Dandona, L. (2001). Refractive error blindness. Bulletin of the World Health Organization, 79(3), 237–243. https://apps.who.int/iris/handle/10665/268285
Dias, C., Pfundt, R., Kleefstra, T., Shuurs-Hoeijmakers, J., Boon, E. M. J., van Hagen, J. M., et al. (2021). De novo variants in TCF7L2 are associated with a syndromic neurodevelopmental disorder. American Journal of Medical Genetics Part A, 185(8), 2384–2390. https://doi.org/10.1002/ajmg.a.62254
Doyle, S. J., Bullock, J., Gray, C., Spencer, A., & Cunningham, C. (1998). Emmetropisation, axial length, and corneal topography in teenagers with Down’s syndrome. British Journal of Ophthalmology, 82(7), 793–796. https://doi.org/10.1136/bjo.82.7.793
Ecker, C. (2017). The neuroanatomy of autism spectrum disorder: An overview of structural neuroimaging findings and their translatability to the clinical setting. Autism, 21(1), 18–28. https://doi.org/10.1177/1362361315627136
Egger, M., Smith, G. D., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315(7109), 629–634. https://doi.org/10.1136/bmj.315.7109.629
Ezegwui, I., Lawrence, L., Aghaji, A., Okoye, O., Okoye, O., Onwasigwe, E., & Ebigbo, P. (2014). Refractive errors in children with autism in a developing country. Nigerian Journal of Clinical Practice, 17(4), 467. https://doi.org/10.4103/1119-3077.134042
Faron, N., Hoekel, J., & Tychsen, L. (2021). Visual acuity, refractive error, and regression outcomes in 169 children with high myopia who were implanted with Ophtec-Artisan or Visian phakic IOLs. Journal of American Association for Pediatric Ophthalmology and Strabismus, 25(1), 27.e1-27.e8. https://doi.org/10.1016/j.jaapos.2020.09.011
Fazzi, E., Signorini, S. G., Bova, S. M., La Piana, R., Ondei, P., Bertone, C., et al. (2007). Spectrum of visual disorders in children with cerebral visual impairment. Journal of Child Neurology, 22(3), 294–301. https://doi.org/10.1177/08830738070220030801
Flaxman, S. R., Bourne, R. R. A., Resnikoff, S., Ackland, P., Braithwaite, T., Cicinelli, M. V., et al. (2017). Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. The Lancet Global Health, 5(12), e1221–e1234. https://doi.org/10.1016/S2214-109X(17)30393-5
Foster, P. J., & Jiang, Y. (2014). Epidemiology of Myopia. Eye, 28(2), 202–208. https://doi.org/10.1038/eye.2013.280
Fryns, J.-P., Legius, E., Devriendt, K., Meire, F., Standaert, L., Baten, E., & Berghe, H. (1996). Cohen syndrome: The clinical symptoms and stigmata at a young age. Clinical Genetics, 49(5), 237–241. https://doi.org/10.1111/j.1399-0004.1996.tb03780.x
Garland, M. A., Sholk, A., & Guenter, K. E. (1962). Acetazolamide-induced myopia. American Journal of Obstetrics and Gynecology, 84(1), 69–71. https://doi.org/10.1016/0002-9378(62)90674-9
Genovese, A., & Butler, M. G. (2020). Clinical assessment, genetics, and treatment approaches in autism spectrum disorder (ASD). International Journal of Molecular Sciences, 21(13), 4726. https://doi.org/10.3390/ijms21134726
Gurnani, B., & Kaur, K. (2023). Astigmatism. In StatPearls. StatPearls Publishing.
Gutiérrez, C., Santoni, J. L. M., Merino, P., & de Liaño, P. G. (2022). Ophthalmologic manifestations in autism spectrum disorder. Turkish Journal of Ophthalmology, 52(4), 246–251. https://doi.org/10.4274/tjo.galenos.2021.46588
Hadjikoutis, S., Morgan, J. E., Wild, J. M., & Smith, P. E. M. (2005). Ocular complications of neurological therapy. European Journal of Neurology, 12(7), 499–507. https://doi.org/10.1111/j.1468-1331.2005.01025.x
Harb, E. N., & Wildsoet, C. F. (2019). Origins of refractive errors: Environmental and genetic factors. Annual Review of Vision Science, 5(1), 47–72. https://doi.org/10.1146/annurev-vision-091718-015027
Harvey, E. M., Dobson, V., Clifford-Donaldson, C. E., Green, T. K., Messer, D. H., & Miller, J. M. (2010). Prevalence of astigmatism in native American infants and children. Optometry and Vision Science, 87(6), 400–405. https://doi.org/10.1097/OPX.0b013e3181d95b23
Hashemi, H., Rezvan, F., Yekta, A. A., Hashemi, M., Norouzirad, R., & Khabazkhoob, M. (2014). The prevalence of astigmatism and its determinants in a rural population of Iran: The “Nooravaran Salamat” mobile eye clinic experience. Middle East African Journal of Ophthalmology, 21(2), 175–181. https://doi.org/10.4103/0974-9233.129772
Higgins, J. P. T., & Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Statistics in Medicine, 21(11), 1539–1558. https://doi.org/10.1002/sim.1186
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., & Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560. https://doi.org/10.1136/BMJ.327.7414.557
Hirsch, J., Zhang, X., Noah, J. A., Dravida, S., Naples, A., Tiede, M., et al. (2022). Neural correlates of eye contact and social function in autism spectrum disorder. PLoS ONE, 17(11), e0265798. https://doi.org/10.1371/journal.pone.0265798
Hoover, K., Di Guglielmo, M. D., & Perry, B. (2023). Disparities in vision screening in primary care for young children with autism spectrum disorder. Pediatrics, 151(4), 2022059998. https://doi.org/10.1542/peds.2022-059998
Hossain, M. M., Khan, N., Sultana, A., Ma, P., McKyer, E. L. J., Ahmed, H. U., & Purohit, N. (2020). Prevalence of comorbid psychiatric disorders among people with autism spectrum disorder: An umbrella review of systematic reviews and meta-analyses. Psychiatry Research, 287, 112922. https://doi.org/10.1016/j.psychres.2020.112922
Ikeda, J., Davitt, B. V., Ultmann, M., Maxim, R., & Cruz, O. A. (2013). Brief report: Incidence of ophthalmologic disorders in children with autism. Journal of Autism and Developmental Disorders, 43(6), 1447–1451. https://doi.org/10.1007/s10803-012-1475-2
Jiang, X., Tarczy-Hornoch, K., Stram, D., Katz, J., Friedman, D. S., Tielsch, J. M., et al. (2019). Prevalence, characteristics, and risk factors of moderate or high hyperopia among multiethnic children 6 to 72 months of age. Ophthalmology, 126(7), 989–999. https://doi.org/10.1016/j.ophtha.2019.02.021
Kabatas, E. U., Ozer, P. A., Ertugrul, G. T., Kurtul, B. E., Bodur, S., & Alan, B. E. (2015). Initial ophthalmic findings in Turkish children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(8), 2578–2581. https://doi.org/10.1007/s10803-015-2428-3
Kaur, G., Thomas, S., Jindal, M., & Bhatti, S. M. (2016). Visual function and ocular status in children with disabilities in special schools of Northern India. Journal of clinical and diagnostic research : JCDR, 10(10), NC01–NC04. https://doi.org/10.7860/JCDR/2016/23615.8742
Keith, C. G., Dobbs, R. H., Shaw, D. G., & Cottrall, K. (1972). Abnormal facies, myopia, and short stature. Archives of Disease in Childhood, 47(255), 787–793. https://doi.org/10.1136/adc.47.255.787
Khanna, R. K., Kovarski, K., Arsene, S., Siwiaszczyk, M., Pisella, P.-J., Bonnet-Brilhault, F., et al. (2020). Ophthalmological findings in children with autism spectrum disorder. Graefe’s Archive for Clinical and Experimental Ophthalmology, 258(4), 909–916. https://doi.org/10.1007/s00417-019-04594-7
Lau, C. S. L., Tong, J. M. K., Tang, E. W. H., & Li, K. K. W. (2022). Ocular features and autism spectrum disorder: A 10-year retrospective review. Indian Pediatrics, 59(7), 581–582. https://doi.org/10.1007/s13312-022-2563-9
Li, Q., Wojciechowski, R., Simpson, C. L., Hysi, P. G., Verhoeven, V. J. M., Ikram, M. K., et al. (2015). Genome-wide association study for refractive astigmatism reveals genetic co-determination with spherical equivalent refractive error: The CREAM consortium. Human Genetics, 134(2), 131–146. https://doi.org/10.1007/s00439-014-1500-y
Li, Z., Yang, L., Chen, H., Fang, Y., Zhang, T., Yin, X., et al. (2022). Global, regional and national burden of autism spectrum disorder from 1990 to 2019: Results from the Global Burden of Disease Study 2019. Epidemiology and Psychiatric Sciences, 31, e33. https://doi.org/10.1017/S2045796022000178
Lin, L., & Xu, C. (2020). Arcsine-based transformations for meta-analysis of proportions: Pros, cons, and alternatives. Health Science Reports, 3(3), e178. https://doi.org/10.1002/hsr2.178
Little, J.-A., Woodhouse, J. M., & Saunders, K. J. (2009). Corneal power and astigmatism in Down Syndrome. Optometry and Vision Science, 86(6), 748–754. https://doi.org/10.1097/OPX.0b013e3181a59d5d
London, A., Benhar, I., & Schwartz, M. (2013). The retina as a window to the brain—From eye research to CNS disorders. Nature Reviews Neurology, 9(1), 44–53. https://doi.org/10.1038/nrneurol.2012.227
Lopes, M. C., Hysi, P. G., Verhoeven, V. J. M., Macgregor, S., Hewitt, A. W., Montgomery, G. W., et al. (2013). Identification of a candidate gene for astigmatism. Investigative Opthalmology & Visual Science, 54(2), 1260. https://doi.org/10.1167/iovs.12-10463
Lord, C., Elsabbagh, M., Baird, G., & Veenstra-Vanderweele, J. (2018). Autism spectrum disorder. The Lancet, 392(10146), 508–520. https://doi.org/10.1016/S0140-6736(18)31129-2
Majumdar, S., & Tripathy, K. (2023). Hyperopia. Encyclopedia of the Eye (pp. 257–262). StatPearls Publishing. https://doi.org/10.1016/B978-0-12-374203-2.00244-X
Mandell, D. S., Novak, M. M., & Zubritsky, C. D. (2005). Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics, 116(6), 1480–1486. https://doi.org/10.1542/peds.2005-0185
Martorell, L., Macaya, A., Pérez-Dueñas, B., & Ortigoza-Escobar, J. D. (2022). Acetazolamide improves episodic ataxia in a patient with non-verbal autism and paroxysmal dyskinesia due to PRRT2 biallelic variants. Movement Disorders Clinical Practice, 9(7), 979–982. https://doi.org/10.1002/mdc3.13528
McCurry, T. C., Lawrence, L. M., Wilson, M. E., & Mayo, L. (2013). The plusoptiX S08 photoscreener as a vision screening tool for children with autism. Journal of American Association for Pediatric Ophthalmology and Strabismus, 17(4), 374–377. https://doi.org/10.1016/j.jaapos.2013.05.006
Morgan, I. G., French, A. N., Ashby, R. S., Guo, X., Ding, X., He, M., & Rose, K. A. (2018). The epidemics of myopia: Aetiology and prevention. Progress in Retinal and Eye Research, 62, 134–149. https://doi.org/10.1016/j.preteyeres.2017.09.004
Munn, Z., Moola, S., Lisy, K., Riitano, D., & Tufanaru, C. (2015). Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. International Journal of Evidence-Based Healthcare, 13(3), 147–153. https://doi.org/10.1097/XEB.0000000000000054
Mutti, D. O. (2007). To emmetropize or not to emmetropize? The question for hyperopic development. Optometry and Vision Science, 84(2), 97–102.
Nielsen, L. S., Skov, L., & Jensen, H. (2007). Visual dysfunctions and ocular disorders in children with developmental delay. II. Aspects of refractive errors, strabismus and contrast sensitivity. Acta Ophthalmologica Scandinavica, 85(4), 419–426. https://doi.org/10.1111/j.1600-0420.2007.00881.x
Oswald, D. P., & Sonenklar, N. A. (2007). Medication use among children with autism spectrum disorders. Journal of Child and Adolescent Psychopharmacology, 17(3), 348–355. https://doi.org/10.1089/cap.2006.17303
Ozer, P. A., Kabatas, E. U., Bicer, B. K., Bodur, S., & Kurtul, B. E. (2016). Does correction of strabismus improve quality of life in children with autism spectrum disorder: Results of a parent survey by ophthalmologists. Seminars in Ophthalmology, 33(2), 1–6. https://doi.org/10.1080/08820538.2016.1182559
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372, n71. https://doi.org/10.1136/bmj.n71
Patel, A., Abou-Al-Shaar, H., Chiang, M. C., Algattas, H. N., McDowell, M. M., Stone, J. G., et al. (2021). Neuroophthalmological manifestations of congenital aqueductal stenosis. Journal of Neurosurgery: Pediatrics, 28(3), 320–325. https://doi.org/10.3171/2021.2.PEDS20824
Perna, J., Bellato, A., Ganapathy, P. S., Solmi, M., Zampieri, A., Faraone, S. V., & Cortese, S. (2023). Association between autism spectrum disorder (ASD) and vision problems. A systematic review and meta-analysis. Molecular Psychiatry, 9, 1–13. https://doi.org/10.1038/s41380-023-02143-7
Pineles, S. L., Avery, R. A., & Liu, G. T. (2010). Vitamin B12 optic neuropathy in Autism. Pediatrics, 126(4), e967–e970. https://doi.org/10.1542/peds.2009-2975
Puri, S., Bhattarai, D., Adhikari, P., Shrestha, J. B., & Paudel, N. (2015). Burden of ocular and visual disorders among pupils in special schools in Nepal. Archives of Disease in Childhood, 100(9), 834–837. https://doi.org/10.1136/archdischild-2014-308131
Ramamurthy, D., Lin Chua, S. Y., & Saw, S. (2015). A review of environmental risk factors for myopia during early life, childhood and adolescence. Clinical and Experimental Optometry, 98(6), 497–506. https://doi.org/10.1111/cxo.12346
Reimelt, C., Wolff, N., Hölling, H., Mogwitz, S., Ehrlich, S., & Roessner, V. (2021). The underestimated role of refractive error (hyperopia, myopia, and astigmatism) and strabismus in children with ADHD. Journal of Attention Disorders, 25(2), 235–244. https://doi.org/10.1177/1087054718808599
Reynolds, M., & Culican, S. M. (2023). Visual autism. Children, 10(4), 606. https://doi.org/10.3390/children10040606
Scharre, J. E., & Creedon, M. P. (1992). Assessment of visual function in autistic children. Optometry and Vision Science, 69(6), 433–439. https://doi.org/10.1097/00006324-199206000-00004
Senju, A., & Johnson, M. H. (2009). Atypical eye contact in autism: Models, mechanisms and development. Neuroscience & Biobehavioral Reviews, 33(8), 1204–1214. https://doi.org/10.1016/j.neubiorev.2009.06.001
Shah, K., Frank, C. R., & Ehrlich, J. R. (2020). The association between vision impairment and social participation in community-dwelling adults: A systematic review. Eye, 34(2), 290–298. https://doi.org/10.1038/s41433-019-0712-8
Shen, Y., Chen, X., Wang, L., Guo, J., Shen, J., An, Y., et al. (2011). Intra-family phenotypic heterogeneity of 16p11.2 deletion carriers in a three-generation Chinese family. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 156(2), 225–232. https://doi.org/10.1002/ajmg.b.31147
Simmons, D. R., Robertson, A. E., McKay, L. S., Toal, E., McAleer, P., & Pollick, F. E. (2009). Vision in autism spectrum disorders. Vision Research, 49(22), 2705–2739. https://doi.org/10.1016/j.visres.2009.08.005
Sobrado, P., Suárez, J., García-Sánchez, F. A., & Usón, E. (1999). Refractive errors in children with cerebral palsy, psychomotor retardation, and other non-cerebral palsy neuromotor disabilities. Developmental Medicine & Child Neurology, 41(6), S0012162299000869. https://doi.org/10.1017/S0012162299000869
Solmi, M., Song, M., Yon, D. K., Lee, S. W., Fombonne, E., Kim, M. S., et al. (2022). Incidence, prevalence, and global burden of autism spectrum disorder from 1990 to 2019 across 204 countries. Molecular Psychiatry, 27(10), 4172–4180. https://doi.org/10.1038/s41380-022-01630-7
Subudhi, P., & Agarwal, P. (2023). Myopia. Encyclopedia of the Eye (pp. 98–105). StatPearls Publishing. https://doi.org/10.1016/B978-0-12-374203-2.00241-4
Swanson, M. W., Lee, S. D., Frazier, M. G., Bade, A., & Coulter, R. A. (2020). Vision screening among children with autism spectrum disorder. Optometry and Vision Science, 97(11), 917–928. https://doi.org/10.1097/opx.0000000000001593
Tajbakhsh, Z., Talebnejad, M. R., Khalili, M. R., Masoumpour, M. S., Mahdaviazad, H., Mohammadi, E., et al. (2022). The prevalence of refractive error in schoolchildren. Clinical and Experimental Optometry, 105(8), 860–864. https://doi.org/10.1080/08164622.2021.2003687
Tsao, W.-S., Hsieh, H.-P., Chuang, Y.-T., & Sheu, M.-M. (2017). Ophthalmologic abnormalities among students with cognitive impairment in eastern Taiwan: The special group with undetected visual impairment. Journal of the Formosan Medical Association, 116(5), 345–350. https://doi.org/10.1016/j.jfma.2016.06.013
Tychsen, L., Hoekel, J., Ghasia, F., & Yoon-Huang, G. (2008). Phakic intraocular lens correction of high ametropia in children with neurobehavioral disorders. Journal of American Association for Pediatric Ophthalmology and Strabismus, 12(3), 282–289. https://doi.org/10.1016/j.jaapos.2007.12.001
Van Splunder, J., Stilma, J. S., & Evenhuis, H. M. (2003). Visual performance in specific syndromes associated with intellectual disability. European Journal of Ophthalmology, 13(6), 566–574. https://doi.org/10.1177/112067210301300610
Wallace, B. C., Dahabreh, I. J., Trikalinos, T. A., Lau, J., Trow, P., & Schmid, C. H. (2012). Closing the gap between methodologists and end-users: R as a computational back-end. Journal of Statistical Software, 49(5), 1–15. https://doi.org/10.18637/jss.v049.i05
Woodhouse, J. M., Pakeman, V. H., Cregg, M., Saunders, K. J., Parker, M., Fraser, W. I., et al. (1997). Refractive errors in young children with Down syndrome. Optometry and Vision Science : Official Publication of the American Academy of Optometry, 74(10), 844–851. https://doi.org/10.1097/00006324-199710000-00023
Wrzesińska, M., Kapias, J., Nowakowska-Domagała, K., & Kocur, J. (2017). Visual impairment and traits of autism in children. Psychiatria Polska, 51(2), 349–358. https://doi.org/10.12740/PP/OnlineFirst/61352
Wu, C., Tsai, T., Chen, W., Tsai, H., & Chien, Y. (2023). Ophthalmologic diagnoses in youths with autism spectrum disorder: Prevalence and clinical correlates. Autism Research. https://doi.org/10.1002/aur.3019
Zdonczyk, A., Tychsen, L., Constantino, J. N., Culican, S. M., Badawi, A. A., & Reynolds, M. (2023). Impact of ocular conditions and improvements after refractive surgery in quality of life for children with neurodevelopmental disorders. American Journal of Ophthalmology, 247, 9–17. https://doi.org/10.1016/j.ajo.2022.10.022
Zhang, J., Wu, Y., Sharma, B., Gupta, R., Jawla, S., & Bullimore, M. A. (2023). Epidemiology and burden of astigmatism: A systematic literature review. Optometry and Vision Science, 100(3), 218–231. https://doi.org/10.1097/OPX.0000000000001998
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This study was registered with PROSPERO (CRD42023433833).
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nadeem, Z.A., Akram, U., Khalid, T.B. et al. Refractive Errors Linked to Autism Spectrum Disorders in the Pediatric Population and Young Adults: A Systematic Review and Meta-Analysis. Rev J Autism Dev Disord (2024). https://doi.org/10.1007/s40489-024-00468-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40489-024-00468-9