Abstract
Purpose of Review
Head lice infestations remain an important public health problem, albeit poorly defined in most endemic countries. Reports of increasing resistance to first-line treatment have renewed scientific research into this neglected ectoparasitosis. Mapping and understanding resistance mechanisms are essential for the development of more effective treatments, as well as for prolonging the life of existing pediculicides. This review aims to synthetize recent data on the type, frequency, and distribution of genetic mutations associated with head lice resistance to chemical treatments.
Recent Findings
Head lice resistance is reported in all continents. The most studied mechanism of resistance is through Knockdown resistance (kdr) mutations, with biomarkers M815I, T917I, and L920F, most commonly reported. Other reported mechanisms such as cytochrome P450 enzyme inhibition, altered acetylcholinesterase binding, and GluCl mutations are still being investigated. Allele mutations linked to pyrethroid resistance differ between regions and countries, which highlights the need for monitoring resistance through region-specific genetic markers. In geographic areas where resistance is well-established, louse populations are predominantly homozygous. In regions where heterozygosity still prevails, selective pressure through the use of chemical pediculicides will cause resistance to increase.
Summary
Continuous surveillance of resistance is needed to monitor the frequency of resistance alleles, which is expected to increase as lice populations move into fixation. Further research should be conducted on new treatments with physical mechanisms of action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lice have shared a long history with their human hosts, and evidence of coevolution has been recorded as early as 8000 B.C. [1••, 2]. The origin and early development of lice is vastly unknown as there are few fossils, but some have been found in Brazil, Nepal, Peru, Greenland, and Mexico [3]. Humans are parasitized by two genera of sucking lice, derived from chewing lice. Human lice belong to the order Anoplura and family Pediculidae, which encompasses ecological types, human head lice (Pediculus humanus capitis) and human body lice (Pediculus humanus humanus) [2, 4•]. Body lice are dependent on clothing for attaching eggs, whereas head lice will use hair to attach their eggs [2]. The third type of lice that is known to infect humans, pubic lice (Pthirus pubis), belongs to the Pthiriadae family. The divergence between the families Pediculidae and Pthiriadae is estimated to be 3.32 MYA [2].
Due to human migration, Pediculus species can further be characterized by clade haplotypes, which can be identified genomically and by geographic location. The clade C divergence was the first to occur (2.0 MYA), and lice of this clade are currently seen in Africa, with a high density in Ethiopia, and Nepal [5]. Greater genetic diversity can be seen among African clade C lice, which mirrors the higher genomic diversity seen in humans in Africa, suggesting African origin for lice [6]. Clade B has been recorded in Central and North America, Europe, and Australia, but not Africa. Clade A lice have a global distribution, and most likely originated in Africa with its human host [2]. As international travel has become increasingly available, there is higher potential for interaction between clades, and genetic recombination has been recorded between clade A and clade C lice in Africa [2, 6]. Outbreeding in unrelated population is likely to continue [1••].
The head and body lice ecotype separation is much more recent and head lice are believed to be the source. This divergence came as a consequence of humans wearing clothing, which led to behavioral and eventual physiological adaptations in the lice [2]. Body lice are known to transmit infectious agents, Rickettsia prowazekii, Bartonella Quintana, Borrelia recurrentis, for typhus, trench fever, and relapsing fever, respectively [1••]. Head lice have not been recorded as a vector for disease, but may serve as a reservoir [7]. Recently, infectious agents for typhus and trench fever as well as Acinetobacter baumannii, involved in hospital acquired infections, have been found in human head lice [8, 9]. As human head and body lice are the same species, differentiating between the two is done solely by the location on the body part in which they are found.
The complete lifecycle of a head louse takes 15–30 days, and adults may survive up to a month. Adult head lice feed on blood from the scalp two to six times per day, and females will lay three to four eggs a day for the remainder of their lives [10]. Insects are transmitted most commonly through direct head-to-head contact, but also indirect transmission from sharing combs, hats, pillows, or other personal items can occur [11•, 12]. Risk factors for infection also include hair length, hair type, age, overcrowding, and socioeconomic status.
Those with longer hair are at higher risk of developing pediculosis [11•, 13,14,15]. Since in many countries, girls wear their hair longer than boys, girls are more likely to be infested [11•, 16]. It has also been reported that the thicker the hair, the more nits are found, which makes removal more difficult. Authors suggest that thinning or shortening this type of hair makes removal with a fine comb easier [13]. It is understood school children are more likely to have a head lice infestation than those who are older [11•, 14, 17, 18]. This may be because younger children are often in closer proximity to each other than those who are older, as they spend more time in crowded spaces such as schools and daycares. In addition, children with more siblings have a higher rate of infestation [14]. Socioeconomic status can also influence the rates of pediculosis exposure. In Jordan, it was reported that in families making less than the equivalent of $260 USD monthly, and those living in homes with less than three rooms, infestation rates were higher. Sanitation level is also influential on infestation rates, as rates are reportedly higher in those who wash their hair only once a week, although an inverse relationship was noted for bathing frequency each week [11•]. Firooziyan et al. [15] noted access to warm water for bathing as a risk factor for head lice infestation; however, this has not been reported by other researchers.
In addition to individual risk factors mentioned above, environmental factors have been found to play a role in head lice infestation. Reports by Picollo et al. [19] suggest that temperature and humidity may have an influence on louse mortality; with deaths in untreated lice higher at high temperatures and low humidity, compared with low temperatures and high humidity.
Even though pediculosis is largely affecting children in remote and rural settings, the impact of these infestations is global [10]. Especially among a child population, infestation can lead to skin irritation, allergic reactions, and bacterial infections related to excessive scratching as well as sleep disturbances [9, 13]. There are also numerous descriptions of social and emotional distress as there are associations of stigma with infection, poor hygiene, and poverty [9, 16]. In high-income countries and affluent schools in low- and middle-income countries, “no nit” policies result in absenteeism and poor school performance [13]. In North America, a recorded 12–24 million school days missed can be attributed to head lice. Additionally, income may be lost by parents who must miss work in order to care for their children who are required to miss school, causing the exacerbation of the economic and psychological burden of infection [20]. Conversely, the government of Canada discourages “no nit” policies as head lice do not indicate lack of cleanliness nor are nits a disease risk [21]. In light of the different degrees of morbidity, high levels of stigma, and loss of productivity associated with lice infestations, the World Health Organization has recently included “scabies and other ectoparasites” in the priority list of neglected tropical diseases [22]. This decision will hopefully lead to higher level of interest in research and intervention of pediculosis.
Since antiquity, communities have used a variety of methods to control head lice infestation. At present, with pharmaceutical interventions only available to some sectors of the population—either due to availability of affordability issues—the use of natural or household products largely predominates in rural areas. Where resource constraints are not so dire, the use of commercial products is much more frequent, particularly in the form of shampoos containing pediculicide agents.
Permethrin, a synthetic pyrethroid, has been in use since 1986 as an over-the-counter topical agent [23]. It has been a top choice for treatment as it has few side effects and is easy to apply. Pyrethroids act in the same way as other insecticides such as dichlorodiphenyltrichloroethane (DDT). While DDT has been banned for use in many countries since the 1970s due to toxic effects on humans and the environment, it is still used in some places [24]. Other common insecticides include organophosphates (malathion) and carbamates (carbamyl). These compounds are not used often, as both have toxic effects, especially when used on children [12]. On the other hand, oral and topical ivermectin, which carry very low toxicity, if any, are an effective treatment; unfortunately, access to ivermectin is nearly non-existent in low-income, rural settings [10]. The use of ivermectin has been garnering considerable attention recently as it is effective to treat several parasitic nematodes and arthropods, including various soil-transmitted helminths, lice, and Sarcoptes scabiei [25]. In using a broad-spectrum antiparasitic such as ivermectin, many infections could be eliminated simultaneously, which would not only be cost effective but would help those receiving treatment for co-infection recover faster than treating each parasite separately. As ivermectin is capable of crossing the blood-brain barrier and blocking nerve transmission, children are at a higher risk of adverse reactions to the drug [23].
Drug Resistance
Due to the short lifecycle and constant intermingling between ecotypes, recombination and genetic exchange between lice can occur very quickly [2]. Genetic diversity—if favorable—can increase transmissibility of resistant lice, making the emergence of new mechanisms for resistance through recombination more likely. High levels of genetic exchange in combination with increased selection pressure and overuse/ misuse of insecticides led to the emergence of resistant species [1••, 9]. More recently, humans have become heavily reliant on pediculicides to deal with this parasitosis and as such, the emergence of resistance to certain drugs is inevitable. As current methods for treating pediculosis become less effective, new strategies to control infestations are needed [13, 26].
Pyrethroids, lindane, and malathion pesticides have all been associated with resistance and new methods for treatment such as benzyl alcohol and spinosad shampoos have recently been approved in the USA [23]. As a result of drug resistance or limited availability to pediculicides, some authors recommend more emphasis to be placed on the use of natural products [10, 12]. Among these, experiments with viscous substances that block air exchange in the nits and adult lice have been undertaken both in vivo and in vitro [23]. Other studies have demonstrated effectiveness of tea tree [27] and neem seed extract shampoo [28]. Petroleum jelly massaged onto the hair and left overnight has also been suggested, as the physical mechanism of action would be unlikely to result in emergence of resistance [23].
This review aims to synthesize the available literature regarding genetic markers of resistance of P. humanus capitis to pediculicides, to identify the most common biomarkers of resistance, and the geographic location of their occurrence as well as the laboratory methods utilized to identify them. Based on this synthesis, implications associated with the development of resistant species are discussed and new methods for head lice control are summarized.
Methods
A search was conducted in NCBI PubMed using the search terms Pediculus humanus capitis, Pediculus resistance mechanisms, pyrethroid resistance, Pediculus clades, Pediculus capitis divergence, risk factors, and Pediculus capitis. Search parameters restricted articles to English language with no exclusions by year of publication. Articles were sorted by best match and their abstracts read carefully. Only those relevant to the objectives were fully analyzed. Additionally, reference lists on the selected articles were checked to obtain further sources. ArcGIS Pro 2.4.3 software by Esri was used to map geographical locations of resistance reports.
Resistance Mechanisms
Kdr Resistance Mechanism
Pyrethroid drugs bind to voltage-sensitive sodium channels (VSSC) in the nervous system and cause prolonged opening of the channels. Rapid and uncontrolled sodium influx leads to nerve depolarization and hyperpolarization which eventually causes muscle paralysis and death [29]. Knockdown resistance (kdr) is the most common mechanism of resistance to insecticides and is the result of target site insensitivity [30•]. Three-point mutations (M815I, T917I, and L920F) in the VGSC α-subunit gene can be identified in resistant lice and have been used as markers for kdr [9, 15, 29, 31••].
Clark [31••] reported M815I and L920F mutations reduced sensitivity to permethrin by 2–3-fold when expressed alone, but when the T917I mutation was present, whether in combination or alone, permethrin sensitivity was abolished. Thus, the T917I mutation must have a significant role in permethrin resistance and can be used as a genetic marker for resistance in the USA. Kdr is shown to be a recessive trait, as low levels of resistance are seen with heterozygous specimens [29].
GST-Based Mechanism
Research by Hemingway et al. [32] proposed a glutathione S-transferase (GST)–based resistance mechanism against DDT. As there was no recorded resistance associated with pyrethroids, a cross-resistance mechanism was very unlikely. Additionally, higher levels of monooxygenases were associated with resistance lice. An increase in GST and cytochrome P450 activity was associated with higher ability to metabolize and detoxify DDT through a dehydrochlorination mechanism, with no enhancement of esterase activity reported. Similar findings were noted by Picollo et al. [30•] as well as by Barrios et al. [33] suggesting that monooxygenases and esterases were responsible for the observed resistance. However, Barrios et al. [33] reported that GST had no critical role in pyrethroid detoxification and the increase in activity could be related to the increase in esterase and oxidase activity, noting kdr to be the main factor for resistance.
Cytochrome P450 Activity and Resistance
Insect’s cytochrome P450 enzymes are largely responsible for detoxification and increased enzymatic activity and have been associated with resistant species. In combination with high level of GST activity, metabolic processes involving P450 enzymes were associated with kdr resistance processes. Metabolic resistance involves heightened ability to degrade and detoxify insecticides [34]. Pyrethroid-resistant mosquitoes have been recorded to have higher levels of P450 activity in Africa [24]. Piperonyl butoxide (PBO) has been reported as a P450 enzyme inhibitor, which heightened effectiveness of pyrethroids when used in combination. The association of P450 with resistance indicates the role of enhanced oxidative metabolism by resistant organisms [26].
Altered Acetylcholinesterase Binding Site
Malathion-resistance has been reported in the UK, which is believed to be attributed to an altered acetylcholinesterase binding site [35]. As malathion actively binds to acetylcholinesterase irreversibly, function is inhibited, leading to paralysis and death. Although resistance is reported to act through altering acetylcholinesterase action, no mechanisms have been reported in head lice [4•].
GluCl Mutations
Ivermectin has a different mechanism of action from most common pediculicide classes and provides opportunities to counter resistance that already exists. The drug targets glutamate-gate chloride (GluCl) channels, which are only present in invertebrates, making it safer for human use [8]. As the GluCl channel is incredibly important for nervous system function, modifications of the channel will cause paralysis and death [25]. Allele mutations S46P, A251V, and H272R were detected in ivermectin-resistant lice with the most prevalent mutation being A251V. However, mutation frequencies were low overall which suggests selection pressure could be acting on these genes [8].
Cross-resistance
Pyrethroids and DDT share a common mechanism of action, so cross-resistance has been identified [33]. Barrios et al. [33] suggested the cross-resistance developed as a result of kdr. In Argentina, cross-resistance has been recorded between permethrin and deltamethrin, as well as sumithrin, which had not been used as a pediculicide before; however, no resistance was reported to carbaryl in the late 1990s [19, 35]. As lice are still sensitive to carbaryl, resistance to malathion might not be associated with an altered acetylcholinesterase binding site. This suggests head lice are not resistant to other organophosphates.
Geographic Distribution of Resistance
A study by Toloza et al. [9] in Argentina describes a much higher level of homozygous resistant lice, compared with heterozygous, suggesting that in the studied population, pyrethroid resistance is well established and on its way to fixation, where only the resistant allele will remain. DDT cross-resistance has also been reported. Kdr and monooxygenase mechanisms are the most likely explanation for pyrethroid resistance in Israel [32]. As of 1999 in the UK, resistance was reported to both permethrin and malathion but not carbaryl. As carbaryl is only available by prescription, the limited use could be a reason why resistance has not been recorded [35]. Recently, a T917I mutation associated with kdr has been reported in Mexico in all head lice sampled [18]. Additionally, kdr has been identified in Chile, but T917I was the only mutant gene recorded [36]. Eremeeva et al. [37] reported that in rural Georgia, US kdr biomarkers for the T917I mutation were found in high frequency (0.99). In Senegal, allele mutations A251V and S46P are associated with GluCl gene alterations supporting ivermectin resistance [8]. Data from Iran indicated resistance related alleles, R292V, L930M, L932M, acted through kdr mechanisms with majority of haplotypes recorded RS [15]. Clark [31••] reported on kdr allele frequency relating to permethrin resistance, and zygosity in 14 countries with highest levels of resistance (100% RR) reported in the UK, Uruguay, and Australia. Argentina (89.5% RR), Israel (87.5% RR), Denmark (83.3% RR), the USA (75.1% RR), and Brazil (62.5% RR) followed with levels of resistance > 50% recorded. Conversely, only homozygous sensitive lice were found in Australia, Thailand, South Korea, and Ecuador (100% SS). Samples from Egypt (85% SS) and Czechia (formerly Czech Republic) (67% SS) were mostly homozygous sensitive but some heterozygotes were reported [31••].
In Canada, although no reports were found on homozygote vs. heterozygote allele frequencies, single R and S allele frequencies were determined in Ontario, Quebec, and British Columbia. All cities in British Columbia and Quebec, as well as 9/12 cities sampled in Ontario, had a Resistance Allele Frequency (RAF) of 100%. The other 3 cities in Ontario had RAF values of 80%, 90.9%, and 92.3%, for Toronto, Oakville, and Sudbury, respectively. Additionally, the resistant allele frequency of the Canadian head louse overall was 97.1%, while the susceptible allele frequency was 2.9% [38].
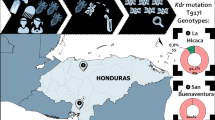
As a summary, the most commonly reported biomarkers of resistance are shown in Table 1, whereas the geographic distribution of resistant alleles is depicted in Fig. 1.
Geographic distribution of resistant lice populations. Red color indicates that reports of resistance in the highlighted country are available. Allele frequencies are displayed for the countries in which data are available; if data on allele frequencies for the same country are available from different sources, the most recent data available were used. Figure was created based on data presented in Table 1 using arcGIS software
As biomarkers are region specific, there was interest in knowing whether they are clade specific as well, however little data on resistance specified clades. An analysis of a worldwide sample showed no correlation between nuclear genetic clusters of clade A and B lice [1••]. As there was limited data linking sampled lice to specific clades, no inferences could be made surrounding susceptibility to resistance or the development of specific mechanisms to a certain clade. Current microsatellite nuclear markers provided support that lice in Honduras and Canada belong to a single genetic cluster, serving as evidence of global inbreeding. This could have resulted from population bottleneck trends induced by selection pressure and will serve as an additional selection for the survival of resistant homozygotes [38]. Alternatively, it is likely similar pediculicide-resistant phenotypes evolved from independent mutations in separate populations, as variability is seen in kdr haplotypes by geographic area [1••]. As there is evidence of resistance globally (Fig. 1), the need for regional testing in order to monitor resistant and susceptible strains is increasing and should be completed regularly [1••, 13, 31••].
Laboratory Methods for Identifying Resistance
PCR
Polymerase chain reaction (PCR) procedures can be used for DNA amplification of genes. Theoretically, only small amounts of targeted DNA regions are required to run procedures, making it appropriate for genetic analysis of lice [39]. PCR procedures were used for amplification of DNA fragments for kdr and GluCl polymorphism identification worldwide [5, 9, 18, 20, 37, 40]. As the procedure is straightforward to use and materials are relatively ubiquitous in laboratories, PCR is a good tool for resistance gene analysis.
QS
Quantitative sequencing (QS) has been used as a population genotyping method to determine frequencies of mutations in head lice populations and can be used as a preliminary monitoring tool for frequencies of resistance higher than 7.4% [20]. QS use has been recorded in the USA but is not yet a widespread method of monitoring resistant alleles [20, 29].
rtPASA
The real-time PCR amplification of Specific Allele protocol was developed to determine mutation frequencies at levels lower than what can be detected by QS [20]. Clark [31••] reported use of rtPASA to detect kdr allele frequencies in lice as low as 1.13%.
SISAR
Serial invasive signal amplification reaction (SISAR) is used to detect single nucleotide polymorphisms (SNPs). This protocol is very effective at detecting VGSC mutations that are associated with permethrin resistance in individual lice [20]. SISAR use was reported in Canada and the USA, to determine resistance kdr allele frequency in a sample of collected lice [29, 38]. The use of SISAR provides information on allele zygosity, which is helpful in understanding dynamics surrounding resistance, especially during early stages when the resistant allele is heterozygous [20]. Heterozygote deficiencies were reported in the global sample of lice tested by Ascunce et al. [1••] when genotype proportions were significantly different that Hardy-Weinberg expectations. Resistance is believed to be strongly established as there is a lack of heterozygous kdr alleles being recovered [1••, 9, 20, 26].
Attempts to Combat Resistance: New Methods of Pediculosis Treatment
Pediculicides should be used cautiously in regions where resistance is high. At the same time, alternative treatments that act by a different mechanism of action can be used or developed in order to extend the lifespan of existing pediculicides [20]. Marcoux et al. [38] suggest pediculicide treatments that act by a physical mode of action, such as altering the exoskeleton, and provide a promising method for treatment with no associations to resistance. A non-traditional method for eliminating pediculosis involves a physical mechanism of action, reported by Barnett et al. [17], that disrupts the wax layer important for protecting the cuticle of the louse exoskeleton. This wax layer that covers the exoskeleton is essential to maintain hydration, and when the wax is disrupted, water loss becomes uncontrollable and will lead to death [21]. The new proposed method involves using an isopropyl myristate/cyclomethicone D5 (IPM/D5) topical solution. As this topical solution acts by epicuticular hydrocarbon extraction, resistance is not expected to develop against isopropyl myristate. In the study, an 82% cure rate was noted after two 10-min treatments, 1 week apart. IPM/D5 could be considered an alternative to traditional pediculicides and is currently marketed as Full Marks™ in Europe, Australia, and Russia, Resultz® in Canada, Takeda in Belgium, and Lapidot in Israel. The Government of Ontario, Committee to evaluate drugs supports isopropyl myristate as being both safer and more effective compared with other drugs available in Ontario. Additionally, the drug is reported to be available under the Ontario Drug Benefit Formulary [41].
Rassami and Soonwera [12] suggest a non-toxic method of dealing with the increasing resistance to insecticides for pediculosis in Thailand. Native Thai plant extracts from T. indica, Av. Bilimbi, and Ac. Concinna contained in shampoo were shown to be more toxic than carbaryl shampoo, with T. indica being the most effective. These plants are common and have been used for thousands of years in traditional medicine practices in Thailand. As these plant extracts are natural, they are biodegradable and exhibit very low levels of toxicity. However, further analysis should be completed on levels of toxicity before herbal shampoos are used as pediculicides [12].
The use of synergists to inhibit enzymes needed for drug inactivation and detoxification may be used to improve control of resistant strains of lice. Addition of PBO to insecticide treatment slows biotransformation processes associated with cytochrome P450, improving effectiveness of the drugs [4•]. The multifunction oxidase inhibitor PBO was effective at enhancing permethrin toxicity in a sample from Argentina. It was able to enhance the effects of permethrin in all resistant colonies and the combined treatment led to a partial reversion of resistant populations. The lack of synergism with d-phenothrin resistance by PBO suggests a non-oxidative mechanism and altered site of action is also present in resistant lice [30•]. Research by Hemingway et al. [32] suggested monooxygenases and esterases can influence resistance through increased metabolism. However, treatment with PBO did not produce synergistic effects on phenothrin. Similarly, Bailey and Prociv [13] recorded increasing the dosage of pediculicides or the use of synergists does not overcome resistance. As results involving the use of synergists to already present pediculicides to increase effectiveness are inconsistent, more research should be conducted.
As nits are surrounded by a protective membrane, many pediculicides are unable to target the embryos, and as a result, treatment is usually repeated after 7–10 days [13, 23]. Mougabure et al. [34] proposed that when insecticides are used on nits, the eggs can be exposed to insecticides and if they are to reach the embryo, selection pressure may lead to the development of new resistant mechanisms. These could be in addition to any inherited resistance mechanisms and further evidence is needed to evaluate the spread of resistance through fast-evolving mechanisms. It is still largely unknown whether resistance evolved once and then spread through recombination and genetic exchange or if it arose independently as a consequence of artificial selection and selection pressure [9]. Frequent monitoring of resistant alleles and a further understanding of resistance mechanisms including detoxification methods would provide grounds for development of new compounds that break resistant trends [20].
Conclusions
Pediculus humanus capitis is an important public health concern that affects children worldwide [11•]. The global presence of head lice, especially those that are treatment resistant, creates complications for eradication; and as they are currently regarded as reservoirs, a change in virulence could transform these ectoparasites into vectors. The World Health Organization (WHO) refers to pediculosis as being prevalent around the world, especially in areas where there is overcrowding [42]. As transmission of pediculosis relies greatly on population density, the increasing global population provides additional challenges with regard to treatment.
The information presented here provides further evidence that resistance is on the rise and will be an ongoing complication. Recently, the WHO officially recognized scabies and other ectoparasites including lice, as neglected tropical diseases, and as a result is working to have ivermectin added to the model list of Essential Medicines when the list is updated [42].
As genetic mutations can happen quickly in such small organisms, constant monitoring and updating of resistance biomarkers are essential for determining best course of treatment. The understanding of which genetic markers are linked to drug resistance in lice is necessary for quick detection and monitoring of resistance, especially when analyzed from specific geographic areas. A further understanding of mechanisms for resistance is essential in finding new methods to treat pediculosis, especially for developing treatment methods where the development of resistance is less likely. As pediculosis is a global issue, any further research in eliminating these serious infestations will help to control a neglected ectoparasite.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Ascunce MS, Toups MA, Kassu G, Fane J, Scholl K, Reed DL. Nuclear genetic diversity in human lice (Pediculus humanus) reveals continental differences and high inbreeding among worldwide populations. PLoS One. 2013;8(2):e57619. https://doi.org/10.1371/journal.pone.0057619This article provides insightful discussion on head lice selection pressure for resistance development.
Boutellis A, Abi-Rached L, Raoult D. The origin and distribution of human lice in the world. Infect Genet Evol. 2014;23(2014):209–17. https://doi.org/10.1016/j.meegid.2014.01.017.
Araujo A, Ferreira LF, Guidon N, Maues da Serra Freire N, Reinhard KJ, Dittmar K. Ten thousand years of head lice infection. Parasitol Today. 2000;16(7):269.
• Durand R, Bouvresse S, Berdjane Z, Izri A, Chosidow O, Clark JM. Insecticide resistance in head lice: clinical, parasitological and genetic aspects. Clin Microbiol Infect. 2012;18:338–44. https://doi.org/10.1111/j.1469-0691.2012.03806.xA comprehensive background on various aspects of head lice resistance.
Light JE, Allen JM, Long LM, Carter TE, Barrow L, Suren G, et al. Geographic distributions and origins of human head lice (pediculus humanus capitis) based on mitochondrial data. J Parasitol. 2008;94(6):1275–81. https://doi.org/10.1645/ge-1618.1.
Veracx A, Boutellis A, Raoult D. Genetic recombination events between sympatric clade A and clade C lice in Africa. J Med Entomol. 2013;50(5):1165–8. https://doi.org/10.1603/me13028.
Tomita T, Yaguchi N, Mihara M, Takahashi M, Agui N, Kasai S. Molecular analysis of a para sodium channel gene from pyrethroid-resistant head lice, Pediculus humanus capitis (Anoplura: Pediculidae). J Med Entomol. 2003;40(4):468–74.
Amanzougaghene N, Fenollar F, Diatta G, Sokhna C, Raoult D, Mediannikov O. Mutations in GluCl associated with field ivermectin-resistant head lice from Senegal. Int J Antimicrob Agents. 2018;52(5):593–8. https://doi.org/10.1016/j.ijantimicag.2018.07.005.
Toloza AC, Lucia A, Zerba E, Mashuh H, Picollo MI. Eucalyptus essential oil toxicity against permethrin-resistant Pediculus humanus capitis (Phthiraptera: Pediculidae). Parasitol Res. 2010;106:409–14.
Coscione S, Kositz C, Marks M. Head lice: an under-recognized tropical problem. Am J Trop Med. 2017;97(6):1636–7. https://doi.org/10.4269/ajtmh.17-0656.
• Mohammed AB. Head lice infestation in schoolchildren and related factors in Mafraq governorate. Jordan Int J Dermatol. 2012;51:168–72 An in-depth analysis of risk factors associated with head lice infestations.
Rassami W, Soonwera M. In vitro pediculicidal activity of herbal shampoo base on Thai local plants against head louse (Pediculus humanus capitis de Geer). Parasitol Res. 2013;112(4):1411–6. https://doi.org/10.1007/s00436-013-3292-8.
Bailey A, Prociv P. Persistent head lice following multiple treatments: evidence for insecticide resistance in Pediculus humanus capitis. Australas J Dermatol. 2000;41(4):250–4.
Birkemoe T, Lindstedt HH, Ottesen P, Soleng A, Næss Ø, Rukke BA. Head lice predictors and infestation dynamics among primary school children in Norway. Fam Pract. 2016;33(1):23–9. https://doi.org/10.1093/fampra/cmv081.
Firooziyan S, Sadaghianifar A, Taghilou B, Galavani H, Ghaffari E, Gholizadeh S. Identification of novel voltage-gated sodium channel mutations in human head and body lice (Phthiraptera: Pediculidae). J Med Entomol. 2017;54(5):1337–43. https://doi.org/10.1093/jme/tjx107.
Jamani S, Rodríguez C, Rueda MM, Matamoros G, Canales M, Bearman G, et al. Head lice infestations in rural Honduras: the need for an integrated approach to control neglected tropical diseases. Int J Dermatol. 2019;58(5):548–56. https://doi.org/10.1111/ijd.14331.
Barnett E, Palma KG, Clayton B, Ballard T. Effectiveness of isopropyl myristate/cyclomethicone D5 solution of removing cuticular hydrocarbons from human head lice (Pediculus humanus capitis). BMC Dermatol. 2012;12:1–5. https://doi.org/10.1186/1471-5945-12-15.
Ponce-Garcia G, Villanueva-Segura K, Trujillo-Rodriguez G, Rodriguez-Sanchez IP, Lopez-Monroy B, Flores AE. First detection of the Kdr mutation T929I in head lice (Phthiraptera: Pediculidae) in schoolchildren of the metropolitan area of Nuevo Leon and Yucatan, Mexico J Med Entomol 2017;54(4):1025–1030. doi:https://doi.org/10.1093/jme/tjx045.
Picollo MI, Vassena CV, Casadio AA, Massimo J, Zerba EN. Laboratory studies of susceptibility and resistance to insecticides in Pediculus capitis (Anoplura; Pediculidae). J Med Entomol. 1998;35(5):814–7.
Clark JM. Determination, mechanism and monitoring of knockdown resistance in permethrin-resistant human head lice, Pediculus humanus capitis. J Asia-Pacific Entomol. 2009;12(1):1–7. https://doi.org/10.1016/j.aspen.2008.10.007.
Cummings C, Finlay CJ, MacDonald EN. Head lice infestations: a clinical update. Paediatr Child Health. 2018;23(1):e18–24.
World Health Organization. Report of the tenth meeting of the WHO Strategic and Technical Advisory Group for neglected tropical diseases. Geneva: WHO2017.
Verma P, Namdeo C. Treatment of pediculosis capitis. Indian J Dermatol. 2015;60(3):238–47. https://doi.org/10.4103/0019-5154.156339.
David JP, Ismail HM, Chandor-Proust A, Paine MJI. Role of cytochrome P450s in insecticide resistance: impact on the control of mosquito-borne diseases and use of insecticides on earth. Philos Trans R Soc B Biol Sci. 2013;368(1612). https://doi.org/10.1098/rstb.2012.0429.
Lynagh T, Lynch JW. Ivermectin binding sites in human and invertebrate Cys-loop receptors. Trends Pharmacol Sci. 2012;33(8):432–41. https://doi.org/10.1016/j.tips.2012.05.002.
Bouvresse S, Berdjane Z, Durand R, Bouscaillou J, Izri A, Chosidow O. Permethrin and malathion resistance in head lice: results of ex vivo and molecular assays. J Am Acad Dermatol. 2012;67(6):1143–50. https://doi.org/10.1016/j.jaad.2012.04.011.
Gonzalez Audino P, Vassena C, Zerba E, Picollo M. Effectiveness of lotions based on essential oils from aromatic plants against permethrin resistant Pediculus humanus capitis. Arch Dermatol Res. 2007;299(8):389–92. https://doi.org/10.1007/s00403-007-0772-7.
Mehlhorn H, Abdel-Ghaffar F, Al-Rasheid KAS, Schmidt J, Semmler M. Ovicidal effects of a neem seed extract preparation on eggs of body and head lice. Parasitol Res. 2011;109(5):1299–302. https://doi.org/10.1007/s00436-011-2374-8.
Gellatly KJ, Krim S, Palenchar DJ, Shepherd K, Yoon KS, Rhodes CJ, et al. Expansion of the knockdown resistance frequency map for human head lice (phthiraptera: Pediculidae) in the United States using quantitative sequencing. J Med Entomol. 2016;53(3):653–9. https://doi.org/10.1093/jme/tjw023.
• Picollo MI, Vassena CV, Cueto GAM, Vernetti M, Zerba EN. Resistance to insecticides and effect of synergists on permethrin toxicity in Pediculus capitis (Anoplura: Pediculidae) from Buenos Aires. J Med Entomol. 2000;37(5):721–5. https://doi.org/10.1603/0022-2585-37.5.721This study provides evidence of head lice cross-resistance to pyrethroid insecticides.
•• Clark JM. Permethrin resistance due to knockdown gene mutations is prevalent in human head louse populations. Open Dermatol J. 2010;4(2010):63–8 A seminal study using SISAR technology for individual genotyping of head lice and determining kdr allele frequency in specimens from 14 countries.
Hemingway J, Miller J, Mumcuoglu Y. Pyrethroid resistance mechanisms in the head louse Pediculus capitis from Israel: implications for control. Med Vet Entomol. 1999;13:89–96.
Barrios S, Zerba E, Picollo MI, Audino PG. Activity of increased specific and non-specific esterases and glutathione transferases associated with resistance to permethrin in pediculus humanus capitis (phthiraptera: Pediculidae) from Argentina. Parasitol Res. 2010;106(2):415–21. https://doi.org/10.1007/s00436-009-1677-5.
Mougabure Cueto G, Zerba EN, Picollo MI. Evidence of pyrethroid resistance in eggs of Pediculus humanus capitis (phthiraptera: Pediculidae) from Argentina. J Med Entomol. 2008;45(4):693–7. https://doi.org/10.1603/0022-2585(2008)45[693:eoprie]2.0.co;2.
Downs AMR, Stafford KA, Harvey I, Coles GC. Evidence for double resistance to permethrin and malathion in head lice. Br J Dermatol. 1999;141(508):508–11.
Roca-Acevedo G, del Solar Kupfer CP, Dressel Roa P, Toloza AC. First determination of pyrethroid knockdown resistance alleles in human head lice (Phthiraptera: Pediculidae) from Chile. J Med Entomol. 2019. https://doi.org/10.1093/jme/tjz101.
Eremeeva ME, Capps D, Winful EB, Warang SS, Braswell SE, Tokarevich NK, Bonilla DL, Durden LA Molecular markers of pesticide resistance and pathogens in human head lice (Phthiraptera: Pediculidae) from Rural Georgia, USA J Med Entomol 2017;54(4):1067–1072. doi:https://doi.org/10.1093/jme/tjx039.
Marcoux D, Palma KG, Kaul N, Hodgdon H, Van Geest A, Previte DJ, et al. Pyrethroid pediculicide resistance of head lice in Canada evaluated by serial invasive signal amplification reaction. J Cutan Med Surg. 2010;14(3):115–8. https://doi.org/10.2310/7750.2010.09032.
Fulton TL, Stiller M. Anicent DNA: Methods and protocols. Methods Mol Biol 2012;42(3):81–85. doi:https://doi.org/10.1007/978-1-61779-516-9.
Amanzougaghene N, Fenollar F, Sangaré AK, Sissoko MS, Doumbo OK, Raoult D, et al. Detection of bacterial pathogens including potential new species in human head lice from Mali. PLoS One. 2017;12(9):e0184621.
Committee to Evaluate Drugs. Isoproyl myristate: recommendations and reasons: Ontario Ministry of Health and Long term Care April 2010.
World Health Organization. Neglected tropical diseases: scabies and other ectoparasites. WHO. 2019. https://www.who.int/neglected_diseases/diseases/scabies/en/. Accessed January 11, 2020 2020.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hot Topics in Tropical Medicine
Rights and permissions
About this article
Cite this article
Fox, K., Larkin, K. & Sanchez, A. Global Trends in Genetic Markers of Pediculus humanus capitis Resistance Mechanisms. Curr Trop Med Rep 7, 65–73 (2020). https://doi.org/10.1007/s40475-020-00204-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40475-020-00204-3