Abstract
Purpose of Review
Here, we review the recent literature on the use of neurofeedback in ADHD. We also discuss the progress and challenges in this field and offer future directions.
Recent Findings
There is promising and suggestive, but not conclusive, evidence suggesting that neurofeedback is an effective treatment for ADHD. Nonetheless, no firm conclusion about its clinical utility can be made because only a few neurofeedback trainings have been assessed.
Summary
Novel approaches to acquiring and analyzing brain data have expanded the possibilities of neurofeedback for understanding and treating ADHD. At the basic level, neurofeedback represents an exciting new approach to complement descriptive neuroimaging ADHD research by providing evidence of specific causal brain-(dys)function relationships. At the clinical level, it represents a promising non-invasive intervention to normalize or compensate for neuropsychological/behavioral dsyfunctions associated with ADHD. Further, well-controlled studies are needed to examine the feasibility and effectiveness of traditional and new potentially useful neurofeedback trainings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
ADHD: an Updated Overview
Attention deficit/hyperactivity disorder (ADHD) is one the most prevalent neurodevelopmental disorders worldwide with a prevalence ranging from 3 to 7% in children [1, 2] and from 2 to 5% in adults [3]. It is characterized by a persistent and age-inappropriate display of inattention, hyperactivity, or impulsiveness that causes significant functional impairment [4]. These symptoms are often accompanied by comorbid affective and cognitive disorders, including oppositional defiant disorder (ODD), anxiety and mood disorders, obsessive-compulsive disorder (OCD), autism spectrum disorder (ASD), and specific learning disabilities. Pure cases of ADHD are indeed the exception rather than the rule [5].
ADHD is currently diagnosed on the basis of clinical criteria (symptoms), since clinical biomarkers remain unavailable. The Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) and the International Classification of Disease, tenth edition (ICD-10) are the two systems that define the ADHD and the criteria for diagnosing it. Both provided similar lists of symptoms and share their intellectual underpinnings (e.g., a categorical approach of classifying and making a diagnosis), but they differ in some important aspects. For example, diagnosis of ADHD is four times more likely if DSM criteria are used than if ICD criteria are employed because the former are less restrictive [1, 6]. Such discrepancies reflect the limitations of classifying ADHD (and other neurodevelopmental and psychological disorders) using a categorical and syndromic approach [7]. Patients diagnosed with ADHD can vary in symptoms, severity, comorbidity, and outcome, as well as in their genetic, neurobiological, and neuropsychological profiles [5, 8, 9]. Moreover, boundaries between categorical entities and between wellness and illness are sometimes hard to establish using traditional diagnostic systems. New approaches are therefore needed for distinguishing individuals with ADHD from controls and for stratifying patients into homogenous subgroups, recognizing the complexity and continuous nature of the disorder, as well as using characteristics that are linked more closely to its neurobiological substrates than its clinical symptoms (i.e., intermediate phenotypes or endophenotypes).
Twin and adoption studies have provided convincing evidence that ADHD is strongly influenced by genetic factors [10]. Findings from diverse neuroimaging studies across distinct modalities, conducted with structural magnetic resonance imaging (sMRI), electroencephalography (EEG), magnetoencephalography (MEG), positron emission tomography (PET), single photon emission computed tomography (SPECT), and functional MRI (fMRI), converge in suggesting that large-scale changes in brain structure, functioning, and connectivity are associated with ADHD [11,12,13,14,15,16,17]. With the largest datasets to date (the ENIGMA cross-sectional sample), reduced volumes in subcortical regions (accumbens, putamen, caudate, amygdala, and hippocampus) and reduced cortical thickness in the fronto-temporal and cerebellar regions were found in individuals with ADHD compared with healthy controls [18, 19]. Functional brain studies have consistently observed hemodynamic and scalp electrical differences between patients and controls at rest and during performance of cognitive, motivational, and emotional tasks [11, 12, 20,21,22,23]. Results from these studies therefore emphasized the importance of other brain circuits beyond the prefronto-striatal network and other neuropsychological processes beyond inhibitory control in ADHD (including temporal, motivational, emotional, and preparatory-attentional functions). In light of such evidence, current models of ADHD accept the notion that different neurobiological and neuropsychological pathways could independently or in combination lead to similar ADHD phenotypes [24,25,26].
It should be noted that although structural and functional neuroimaging studies have provided invaluable insights into the global neuropathology of ADHD by means of comparing groups of patients and controls, they do not provide useful information regarding diagnosis, treatment, and course of the disorder at the individual level. Either these studies can establish causal relations between observed neural alterations and the disorder. Novel approaches to acquiring and analyzing neuroimaging data, such as supervised learning procedures, and to exploring brain functioning, such as adaptive (closed-loop) neurofeedback (in which neural activity is the independent variable), may help to bridge the gap from research to clinical practice, as well as from descriptive to causal/predictive neuroscience [27, 28••, 29].
Medication and psychological interventions are the most studied treatments for ADHD. Clinical practice guidelines recommend either or both treatments depending on age, symptoms severity, and comorbidity (e.g., NICE ADHD guidelines). Various forms of psychological interventions are recommended for treatment of ADHD and associated problems. Systematic reviews and meta-analyses support the beneficial effects of behavioral treatments on a wide range of child and parent outcomes, but its efficacy on core ADHD symptoms remain uncertain [30, 31]. Behavioral interventions in which parents or teachers are taught to manipulate contingencies are the first-line treatment for preschool-age children with ADHD and, in combination with medication, the recommended treatment for school-age patients. For adolescents and adults with the disorder, cognitive-behavioral and meta-cognitive therapies (e.g., organizational, time management, and planning skills training) seem to provide significant benefits [32, 33]. Medication (stimulant or non-stimulant) has shown to be very effective in decreasing the core symptoms of ADHD in the short- and medium term [34, 35]. However, it has some limitations that should be considered: a relevant proportion of patients shows little or no symptom reduction, it is not recommended for preschool children with ADHD, adverse effects (although no usually severe) are common, long-term effectiveness remains to be determined, its efficacy for ADHD-related problems and meta-cognitive skills seems to be limited, and it does not consider the neurobiological and neuropsychological heterogeneity of the disorder.
The limitations of current treatments for ADHD are leading to explore the therapeutic potential of different types of neuromodulatory therapies including neurofeedback. Although neurofeedback has a long (and not completely successfully) history in ADHD, there has been a new focus on it given the recent advances in functional neuroimaging techniques and especially in methods for processing and analyzing brain signals. Real-time neurofeedback seems to be particularly well suited for ADHD, a disorder in which the neural substrates of self-regulatory and motivational functions have a delay in their maturation or are impaired [22, 36, 37], because it involves learning of self-regulation of brain activity and motivational components, including positive reinforcement and self-efficacy.
Real-Time Neurofeedback: a Brief Overview
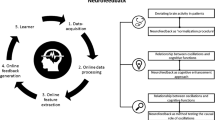
Neurofeedback is a neuromodulatory non-invasive intervention in which brain activity is measured and fed back to the individual in real-time to facilitate self-regulation of the putative neural activity underlying a particular behavior or mental function (including perceptual, cognitive, emotional, and motivational processes [28••]). The level of ongoing brain activity is typically outside voluntary control because we do not have conscious access to it. However, we can learn to gain control over a particular neural response if contingent feedback/reward is given each time the desired brain activity is achieved.
Neurofeedback involves the following main steps: (1) signal acquisition, (2) signal pre-processing, (3) selection and extraction of relevant features of the signal, and (4) computation and delivery of feedback/reward to the individual. Firstly, brain activity can be captured non-invasively by measuring different aspects of neural functioning, including the hemodynamic response after neural activation (neurons satisfy their increased metabolic demand by increased consumption of oxygen) and the electromagnetic response during neural activation (neurons generate electrical and magnetical fields through brief changes in the permeability of their cell membrane to particular ions). The magnetic or electric changes that reach the surface and can be measured by electroencephalography (EEG) or magnetoencephalography (MEG) are therefore direct reflections of neural functioning, whereas the hemodynamic response measured by functional magnetic resonance imaging (fMRI) are indirect reflections of neural functioning. Thus, the temporal dynamics of neural responses are better characterized using EEG/MEG than using fMRI. However, fMRI provides better spatial resolution than EEG/MEG, permitting more precise localization of mental functions and pathology. Moreover, fMRI can sample the activity of deep brain structures, such as the thalamus, basal ganglia, and amygdala, which cannot be measured using scalp-recorded EEG. Remarkably, although each functional technique has disadvantages (as well as advantages), growing evidence suggests that both type of signals (electromagnetic and hemodynamic) can be successfully used for real-time feedback [28••, 38].
The second step, signal pre-processing, involves (among others things) the detection and elimination of endogenous and exogenous artifacts, including blinks, eye movements, muscle movements, and other forms of noise. These artifacts should be correctly removed before the relevant features of the signal are extracted. However, this is not an easy task because artifacts can be difficult to distinguish from the neural activity. Indeed, sophisticated mathematical methods, such as independent components analysis (ICA), followed by visual inspection of the pre-processed signal are typically carried out to remove artifacts in conventional off-line analysis of brain signals. Therefore, this step represents an important methodological challenge for real-time data pre-processing.
The third step, selection and extraction of relevant features of the signal, involves deciding which feature of the recorded brain signal will be selected to be trained. Brain signals are multidimensional, thereby providing many features to be examined even simultaneously. For instance, EEG comprises at least five dimensions including time, space, frequency, power (i.e., the strength or amplitude of frequency band-specific activity), and phase (the timing of the oscillation) [39]. EEG-based neurofeedback studies have almost exclusively focused on power dimension probably due to the fact that it is methodologically easier to compute than other dimensions (and clearly simpler than analyzing combined dimensions). In the so-called frequency band training, spontaneous power in different EEG frequency bands (e.g., gamma, beta, alfa, theta, or delta) has to be increased or decreased at rest. Frequency bands have been associated with different general brain states (from relaxed to aroused), mental functions, and pathologies. However, it remains to be determined what mental functions and behaviors are specifically associated with each EEG frequency band because multiple factors (endogenous or even exogenous) can contribute to its generation. Other EEG-related signal that can be modulated with neurofeedback is the slow cortical potential (SCP), which is described in detail in the following section. Similarly to EEG-based neurofeedback, most of fMRI neurofeedback studies have used unidimensional (univariate) approaches (e.g., processing and training the activity of circumscribed region by an a priori region-of-interest procedure), assuming that a mental function or behavior primarily relies on a single brain region. Although the picture is known to be more complex, results from these studies suggest that learned control of the local brain activity could lead to specific changes not only in neural activation but also in behavior and mental processes. For example, learned up-regulation of amygdala to positive memories via fMRI neurofeedback has been shown to reduce depressive symptoms in adults with major depressive disorder [40].
Although these univariate and a priori neurofeedback approaches have provided substantial information about the causal relationship between the brain and behavior and the clinical efficacy for the treatment of a number of disorders, the recent development of multivariate approaches (such as connectivity-based and multivariate pattern analyses) opens new perspectives for the utility of real-time neurofeedback both as a basic and a clinical tool.
In the fourth step, computation and delivery of feedback/reward, extracted relevant feature of the signal (e.g, activity level in a particular ROI/network or amplitude of the SCP) is presented to the individual through auditory and/or visual feedback (e.g., a thermometer whose temperature reflects increases and decreases of the signal). Typically, feedback is delivered as soon as data is acquired and pre-processed (practically in real-time in EEG neurofeedback; every 1–6 s to account for the hemodynamic delay in fMRI neurofeedback). Neurofeedback therefore uses (contingent) feedback as intrinsic reward to learn to gain control over the brain activity. Operant conditioning (instrumental learning) is thus considered as the main factor influencing neurofeedback learning. However, other general frameworks have been proposed to explain it, such as the dual process theory (for details see [28••]. In this theory, the learner would also play a role in learning (e.g, by searching for an effective mental strategy to achieve the desired brain activity level). In relation to this, it remains an open question if it is preferable and most effective to give or not explicit/specific instructions to learners on how to modulate their brain activity. Nonetheless, current evidence suggest that successful neurofeedback training is possible (and perhaps more effective) without providing explicit mental strategies to learners [41, 42]. It should be remarked, however, that neurofeedback training requires involvement and motivation and, therefore, an “active” role of the learner.
Neurofeedback in ADHD: Review of Current Evidence
Although neurofeedback has been largely studied in ADHD, there are relatively few studies using randomized controlled designs. On behalf of the European ADHD Guidelines Group (EAGG), Cortese and colleagues [43••] examined the effects of neurofeedback on core symptoms and neuropsychological deficits of ADHD through a meta-analysis of only randomized controlled trials (RCT). To our knowledge, this is the most comprehensive and recent meta-analysis of neurofeedback outcomes yet undertaken, including 13 independent RCT with a total sample size of more than 500 children and adolescents with the disorder. Overall, this meta-analysis showed significant effects of neurofeedback on ADHD symptoms, but only when they were rated by most proximal (often not blinded) assessors (parent ratings). Effects on symptomatology dropped to non-statistically significant levels when probably blinded outcomes were considered (teacher ratings), and when only trials with active or sham control conditions were selected. Similarly, no significant effects of neurofeedback on neuropsychological outcomes were found. Results from this meta-analysis would not support neurofeedback as an effective treatment for reducing core symptoms and neuropsychological deficits of ADHD. However, a general or firm conclusion about the potential utility of neurofeedback for treating ADHD should not be taken on the basis of these results for various reasons including the following.
Firstly, although neurofeedback has been largely investigated in ADHD, most studies have almost exclusively focused on EEG signal. Indeed, all trials that met criteria for inclusion in this meta-analysis used EEG-based neurofeedback. Therefore, it remains to be determined whether other brain signals could be used for treating ADHD. In this sense, a recent proof-of-concept RCT in adolescents with ADHD has revealed promising results using fMRI-based neurofeedback [44••, 45•]. In this trial, adolescents with the disorder were trained to self-upregulate right inferior frontal gyrus (rIFG; experimental group) or left parahippocampal gyus (lPHG; active control group) activation. IFG is a cognitive control region crucially involved in response inhibition (and also in other functions) that has been found to be under-activated in individuals with ADHD. Of note, deficient response inhibition and abnormal functional activation in the IFC have been proposed as endophenotypes for ADHD [46, 47]. Moreover, IFC is one of the main targets of methylphenidate, the first-choice pharmacological treatment for individuals with the disorder. By contrast, PHG has not been related to ADHD. Both groups demonstrated an increase in the ability to upregulate the BOLD signal in their respective target regions across sessions, supporting that adolescents with ADHD can gain control over the BOLD signal through neurofeedback training. Both groups also showed significant reductions in ADHD symptoms immediately after the intervention and at 11-month follow-up, suggesting that neurofeedback has long-lasting clinical benefits. However, clinical improvements are not specific to the training of the targeted brain area (rIFG) because symptom severity also diminished in the active control group (PHG). This finding could be explained by non-specific factors that accompanied neurofeedback training including adherence to a training schedule, development of more general self-regulatory skills, an increase in positive reinforcement, or interaction with novel technology. It should be noted, however, that only the experimental group showed sustained effects in the trained region (rIFG) during a transfer session in which no feedback was provided. Moreover, activation in the trained region was negatively correlated to ADHD symptoms during this transfer session in the experimental (neurofeedback of rIFG) but not in the control (neurofeedback of lPHG) group. These results suggest that individuals of the experimental group were able to maintain the skill of up-regulating IFC activation in the absence of feedback, which represents the first evidence of successful transfer of trained strategies to real-life situations. Likewise, they indicate that this learning is associated with a reduction in ADHD symptoms. This well-conducted RCT thus provides preliminary evidence of the feasibility, safety, and efficacy of fMRI-based neurofeedback in ADHD.
Secondly, a frequency band training (specifically, the so-called beta/theta protocol or training) was used by most of the RCT included in the meta-analysis. It aims at decreasing and increasing the strength of spontaneous theta and beta band power, respectively, in order to achieve an optimal state of cortical arousal (i.e., tonic activation). This training is based upon the premise that individuals with ADHD have increased theta power and reduced beta power at rest when compared to control subjects, which has been interpreted as the consequence of an under-activated or under-aroused central nervous system. Indeed, an excessive theta/beta ratio was approved by Food and Drug Administration (FDA) as a diagnostic biomarker of ADHD. However, recent evidence indicates that theta/beta ratio cannot be considered a reliable diagnostic measure of ADHD [48, 49], since only a subgroup of patients with ADHD displayed this EEG pattern (e.g., an excessive theta activity). Therefore, this neurofeedback training would not be suitable for all patients with the disorder. Moreover, it should be also noted that the functional significance of spontaneous beta and theta rhythms remains to be established. For instance, theta activity has been associated with reduced attention after prolonged cognitive effort, but also with increased top-down cognitive functioning over short intervals [50]. In light of these data, it is not surprising that no significant effects on ADHD symptoms were found after beta/theta training according to probably blinded assessors [43••]. In the same line, a recent RCT that examined the effects of methylphenidate, beta/theta training, and physical activity on neuropsychological functioning (attention and inhibition: [51], response inhibition-related event-related potential (ERP) components [52] and spontaneous and task-related EEG frequency band power [53] showed significant greater effects of medication compared to the two other interventions. Effects of beta/theta training on neuropsychological and ERP measures were comparable to the physical activity training. Considering all these data, some researchers have recently suggested that it may be necessary to optimize and individualize frequency band neurofeedback training for ADHD [54].
The efficacy of SCP neurofeedback training in ADHD has not been assessed by meta-analysis due to the insufficient number of RCT [43••]. It should be noted, however, that preliminary evidence is promising. SCP is the slow end of the field potential. Synaptic activity at apical dendrites of pyramidal cortical neurons is thought to be the main factor contributing to its generation. SCP is not an oscillation (such as frequency bands) because it does not contain rhythmic activity [55]. It can be better defined as a slow electrical fluctuation (lasting from several hundreds of milliseconds to several seconds). Negative shifts are thought to reflect synchronized depolarization in large networks of neurons (i.e., increased cortical excitability), which are typically observed during motor and cognitive (e.g., attentional) preparation. By contrast, positive shifts represent reduced cortical excitability during low (motor, cognitive, or motivational) preparatory demand conditions. Training of SCP therefore addresses the phasic regulation of cortical excitability through the learned modulation of these shifts. Interestingly, the contingent negative variation (CNV), an ERP component typically categorized as a SCP, has been shown to be reduced in ADHD [56,57,58]. This finding (observed at the group level) suggests the presence of preparation deficits that are independent of executive dysfunctions in at least a significant proportion of patients with the disorder. Notably, a recent longitudinal studies indicate that preparatory deficits (including an attenuated CNV) are reliable markers of ADHD persistence/remission, improving concurrently with ADHD symptoms [56,57,58]. Therefore, they represent excellent targets for the development of neurofeedback (as well as cognitive) interventions. It should be also noted that a large and well-conducted RCT showed an increase of the CNV associated with SCP training, which was observed in the experimental (neurofeedback training) but not in the control (attention skills training) group [59, 60].
Recently, a large multicenter RCT examined the efficacy of SCP neurofeedback in comparison to electromyographic (EMG) biofeedback using similar training setups for controlling un-specific factors (e.g., time schedule and amount of reinforcement or interaction with the therapists [61••]). A greater reduction of ADHD symptoms was observed after SCP than after EMG training, but only using probably non-blinded ratings (parent-rated outcome). Successful self-regulation of SCP was only found in the experimental group (which would support the specificity of this neurofeedback training), but this trained skill was not observed during transfer trials without contingent feedback. These results would limit the stability and generalizability of the learned self-regulation of SCP into everyday life situations. Further evidence is therefore requested to investigate which factors contribute to the transfer of the learned control of SCP.
Thus, at present, no firm conclusion about the clinical utility of neurofeedback can be made because only the efficacy of a specific neurofeedback training (classical theta/beta protocol) has been evaluated by a sufficient number of RCT. Further large-scale RCT are necessary to assess and confirm the feasibility and effectiveness of other potentially useful neurofeedback trainings such as the EEG-based neurofeedback of SCP or the fMRI-based neurofeedback of IFC.
Neurofeedback in ADHD: Challenges and Future Directions
Although the clinical use of neurofeedback in ADHD has been investigated for decades [62], relatively little progress has been made in understanding its mechanisms of action, testing the full spectrum of its efficacy (e.g., functional impairment, comorbidity, and other secondary outcomes in addition to core symptoms), assessing the learning process-curves during training, testing the specificity, generalizability and persistence of the learned regulation, or in developing new effective trainings (protocols). These important issues are only beginning to be addressed. Progress in this field has been limited (among others) by time-consuming data processing and analysis that impeded on-line handling of large data sets. However, in the last years, recent advances in functional neuroimaging techniques and especially in methods for processing and analyzing brain signals in real-time have greatly expanded the possibilities of neurofeedback in ADHD, both as a basic and a clinical tool. Such advances will probably speed up the rate of progress in this field.
ADHD is a clinically and neurobiologically heterogeneous disorder. Individuals with ADHD can vary in symptoms, severity, comorbidity, as well as in their genetic, neurobiological and neuropsychological characteristics. Indeed, multiple neuropsychological deficits and underlying etiological factors have been associated with the disorder [9, 26]. Thus, it is unlikely that neurofeedback training of a single brain signal will be helpful for all patients with the disorder. In view of this heterogeneity, a useful approach might be to group (and train) patients with ADHD according to neurocognitive criteria (on the basis of neuroendophenotypes). In this sense, right IFC/response inhibition phenotype represents a particularly good candidate to be trained in many (but not all) individuals with the disorder (since not all patients show deficits in response inhibition). Others, for example, could benefit more if the target of the neurofeedback training is cognitive preparation-related neural activity. Of course, further investigations remain necessary to investigate the validity of these ideas. On the other hand, given the complexity of mental functions (and behaviors), multivariate approaches (model-driven and especially data-driven) are better suited than univariate approaches. These multivariate data-driven approaches open a new set of possibilities for real-time neurofeedback.
For neurofeedback RCT to be scientifically credible, they require large sample sizes, blinded assessments, and the inclusion of an appropriate control condition for overcoming any potential confounds. Sham-controlled designs are the gold-standard across most clinical research domains but they might be inappropriate to examine the clinical efficacy of neurofeedback in ADHD. Sham feedback (e.g., subjects receive feedback from the brain of another individual) can be detected by participants, impairing their motivation, perception of self-efficacy, and adherence to treatment. Moreover, in contrast to real neurofeedback, sham neurofeedback does not promote learning, a core component of this intervention. Thus, active control conditions such as providing feedback from a different neural substrate not related to the disorder that can be regulated by the individual might be preferable to sham conditions. This condition would therefore involve learning of self-regulation and control for non-specific effects. Placebo effects associated with the use of neuroimaging technology can be assessed by comparing real neurofeedback with (no brain-related) active groups such as computerized or biofeedback training.
Conclusions
The take-home message of the present review can be summarized as follows. To date, there is suggestive but not conclusive evidence that neurofeedback is an effective treatment for ADHD. However, recent advances in functional neuroimaging techniques as well as in methods for processing and analyzing brain signals in real-time (such as MPA-based activity classification) have greatly expanded the possibilities of neurofeedback for understanding and treating ADHD. At the basic level, real-time neurofeedback represents an exciting new approach to complement traditional (descriptive) neuroimaging research on ADHD by providing evidence of specific causal relationship between (altered) brain activity and mental functions implicated in the disorder (response inhibition, working memory, cognitive preparation, and emotional regulation, among others). At the therapeutic level, real-time neurofeedback represents a promising non-invasive intervention to normalize or compensate for specific neuropsychological-behavioral dsyfunctions associated with ADHD. In this vein, the combination of closed-loop (adaptative) neurofeedback with multivariate approaches could maximize training efficacy and transferability by customizing the intervention to each individual and each moment (e.g., by detecting moment-to-moment), the neural pattern that reflects lapses of attention [27]. Notably, medication and psychological interventions should be considered as the first-choice treatment for ADHD until evidence from randomized well-controlled trials strongly supports the effectiveness of each neurofeedback training.
Change history
12 August 2017
An erratum to this article has been published.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatr. 2007;164(6):942–8.
Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol. 2014;43(2):434–42.
Fayyad J, De Graaf R, Kessler R, Alonso J, Angermeyer M, Demyttenaere K, et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 2007;190(5):402–9.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub; 2013.
Larson K, Russ SA, Kahn RS, Halfon N. Patterns of comorbidity, functioning, and service use for US children with ADHD, Pediatrics; 2011: peds. 2010–0165.
Rohde LA, Szobot C, Polanczyk G, Schmitz M, Martins S, Tramontina S. Attention-deficit/hyperactivity disorder in a diverse culture: do research and clinical findings support the notion of a cultural construct for the disorder? Biol Psychiatry. 2005;57(11):1436–41.
Owen MJ. New approaches to psychiatric diagnostic classification. Neuron. 2014;84(3):564–71.
Klein M, Onnink M, van Donkelaar M, Wolfers T, Harich B, Shi Y et al. Brain imaging genetics in ADHD and beyond—mapping pathways from gene to disorder at different levels of complexity. Neurosci Biobehav Rev. 2017;80:115–155.
Sjöwall D, Roth L, Lindqvist S, Thorell LB. Multiple deficits in ADHD: executive dysfunction, delay aversion, reaction time variability, and emotional deficits. J Child Psychol Psychiatry. 2013;54(6):619–27.
Schachar R. Genetics of attention deficit hyperactivity disorder (ADHD): recent updates and future prospects. Curr Dev Disord Rep. 2014;1(1):41–9.
Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiat. 2013;70(2):185–98.
Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatr. 2012;169(10):1038–55.
Johnstone SJ, Barry RJ, Clarke AR. Ten years on: a follow-up review of ERP research in attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2013;124(4):644–57.
Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatr. 2011;168(11):1154–63.
Fernández-Jaén A, López-Martín S, Albert J, Fernández-Mayoralas DM, Fernández-Perrone AL, Tapia DQ, et al. Cortical thinning of temporal pole and orbitofrontal cortex in medication-naive children and adolescents with ADHD. Psychiatry Res Neuroimaging. 2014;224(1):8–13.
Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin Neurophysiol. 2003;114(2):171–83.
Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp. 2010;31(6):904–16.
Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LS, et al. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry. 2017;4(4):310–9.
Shaw P, Hoogman M, Bratlen J, Onnink M, Shumskaya E, Mennes M, et al. 99-a large scale study of cortical and cerebellar morphology in ADHD across the life span: an ENIGMA-ADHD collaboration. Biol Psychiatry. 2017;81(10):S41–S2.
López-Martín S, Albert J, Fernández-Jaén A, Carretié L. Emotional distraction in boys with ADHD: neural and behavioral correlates. Brain Cogn. 2013;83(1):10–20.
López-Martín S, Albert J, Fernández-Jaén A, Carretié L. Emotional response inhibition in children with attention-deficit/hyperactivity disorder: neural and behavioural data. Psychol Med. 2015;45(10):2057–71.
Plichta MM, Scheres A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci Biobehav Rev. 2014;38:125–34.
Posner J, Park C, Wang Z. Connecting the dots: a review of resting connectivity MRI studies in attention-deficit/hyperactivity disorder. Neuropsychol Rev. 2014;24(1):3–15.
Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci. 2006;10(3):117–23.
Sonuga-Barke E, Bitsakou P, Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(4):345–55.
Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci. 2012;16(1):17–26.
Cohen JD, Lee RF, Norman KA, Turk-Browne NB. Closed-loop training of attention with real-time brain imaging. Nat Neurosci. 2015;18(3):470–5.
•• Sitaram R, Ros T, Stoeckel L, Haller S, Scharnowski F, Lewis-Peacock J et al. Closed-loop brain training: the science of neurofeedback. Nat Rev Neurosci. 2017;18:86–100. An excellent and detailed review examing the mechanisms underlying neurofeedback as well as its basic and clinical applications.
Pourahmadi M, Noorbaloochi S. Multivariate time series analysis of neuroscience data: some challenges and opportunities. Curr Opin Neurobiol. 2016;37:12–5.
Daley D, Van der Oord S, Ferrin M, Danckaerts M, Doepfner M, Cortese S, et al. Behavioral interventions in attention-deficit/hyperactivity disorder: a meta-analysis of randomized controlled trials across multiple outcome domains. J Am Acad Child Adolesc Psychiatry. 2014;53(8):835–47. e5
Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatr. 2013;170(3):275–89.
Sprich SE, Safren SA, Finkelstein D, Remmert JE, Hammerness P. A randomized controlled trial of cognitive behavioral therapy for ADHD in medication-treated adolescents. J Child Psychol Psychiatry. 2016;57(11):1218–26.
Solanto MV, Marks DJ, Wasserstein J, Mitchell K, Abikoff H, Alvir JMJ, et al. Efficacy of meta-cognitive therapy for adult ADHD. Am J Psychiatr. 2010;167(8):958–68.
Cunill R, Castells X, Tobias A, Capellà D. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology. 2016;233(2):187–97.
Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry. 2010;19(4):353–64.
Volkow ND, Wang G-J, Newcorn JH, Kollins SH, Wigal TL, Telang F, et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry. 2011;16(11):1147–54.
Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch J, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci. 2007;104(49):19649–54.
Zotev V, Phillips R, Yuan H, Misaki M, Bodurka J. Self-regulation of human brain activity using simultaneous real-time fMRI and EEG neurofeedback. NeuroImage. 2014;85:985–95.
Cohen MX. Analyzing neural time series data: theory and practice. MIT Press; 2014.
Young KD, Siegle GJ, Zotev V, Phillips R, Misaki M, Yuan H et al. Randomized clinical trial of real-time fMRI amygdala neurofeedback for major depressive disorder: effects on symptoms and autobiographical memory recall. Am J Psychiatr. 2017: appi. ajp. 2017.16060637.
Bray S, Shimojo S, O'Doherty JP. Direct instrumental conditioning of neural activity using functional magnetic resonance imaging-derived reward feedback. J Neurosci. 2007;27(28):7498–507.
Shibata K, Watanabe T, Sasaki Y, Kawato M. Perceptual learning incepted by decoded fMRI neurofeedback without stimulus presentation. Science. 2011;334(6061):1413–5.
•• Cortese S, Ferrin M, Brandeis D, Holtmann M, Aggensteiner P, Daley D, et al. Neurofeedback for attention-deficit/hyperactivity disorder: meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry. 2016;55(6):444–55. To our knowledge, this is the most comprehensive and recent meta-analysis of neurofeedback outcomes yet made, including 13 independent RCT with a total sample size of more than 500 children and adolescents with ADHD. Of note, all trials that met criteria for inclusion in this meta-analysis used EEG-based neurofeedback and, primarily, frequency band training.
•• Alegria AA, Wulff M, Brinson H, Barker GJ, Norman LJ, Brandeis D et al. Real-time fMRI neurofeedback in adolescents with attention deficit hyperactivity disorder. Hum Brain Mapp. 2017;38(6):3190–3209 The first proof-of-concept fMRI-based neurofeedback randomized controlled trial in adolescents with ADHD. This single-blinded study compared the clinical efficacy and the effects of neurofeedback training of the inferior frontal gyrus (experimental group; n=18) and of the middel parahippocampal gyrus (control group; n=13). The results obtained are promising and support further research in this field.
Zilverstand A, Sorger B, Slaats-Willemse D, Kan CC, Goebel R, Buitelaar JK. fMRI neurofeedback training for increasing anterior cingulate cortex activation in adult attention deficit hyperactivity disorder. An exploratory randomized, single-blinded study. PLoS One. 2017;12(1):e0170795.
Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1285–92.
McAuley T, Crosbie J, Charach A, Schachar R. The persistence of cognitive deficits in remitted and unremitted ADHD: a case for the state-independence of response inhibition. J Child Psychol Psychiatry. 2014;55(3):292–300.
Arns M, Conners CK, Kraemer HC. A decade of EEG theta/beta ratio research in ADHD: a meta-analysis. J Atten Disord. 2013;17(5):374–83.
Saad JF, Kohn MR, Clarke S, Lagopoulos J, Hermens DF. Is the theta/beta EEG marker for ADHD inherently flawed? J Atten Disord. 2015:1087054715578270.
Clayton MS, Yeung N, Kadosh RC. The roles of cortical oscillations in sustained attention. Trends Cogn Sci. 2015;19(4):188–95.
Geladé K, Bink M, Janssen TW, van Mourik R, Maras A, Oosterlaan J. An RCT into the effects of neurofeedback on neurocognitive functioning compared to stimulant medication and physical activity in children with ADHD. Eur Child Adolesc Psychiatry. 2016:1–12.
Janssen TWP, Bink M, Geladé K, van Mourik R, Maras A, Oosterlaan J. A randomized controlled trial investigating the effects of neurofeedback, methylphenidate, and physical activity on event-related potentials in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2016;26(4):344–53.
Janssen TW, Bink M, Geladé K, Mourik R, Maras A, Oosterlaan J. A randomized controlled trial into the effects of neurofeedback, methylphenidate, and physical activity on EEG power spectra in children with ADHD. J Child Psychol Psychiatry. 2016;57(5), 633-644
Bluschke A, Roessner V, Beste C. Editorial perspective: how to optimise frequency band neurofeedback for ADHD. J Child Psychol Psychiatry. 2016;57(4):457–61.
He BJ, Raichle ME. The fMRI signal, slow cortical potential and consciousness. Trends Cogn Sci. 2009;13(7):302–9.
Clerkin SM, Schulz KP, Berwid OG, Fan J, Newcorn JH, Tang CY, et al. Thalamo-cortical activation and connectivity during response preparation in adults with persistent and remitted ADHD. Am J Psychiatr. 2013;170(9):1011–9.
Cheung CH, Rijsdijk F, McLoughlin G, Brandeis D, Banaschewski T, Asherson P, et al. Cognitive and neurophysiological markers of ADHD persistence and remission. Br J Psychiatry. 2016;208(6):548–55.
Doehnert M, Brandeis D, Schneider G, Drechsler R, Steinhausen HC. A neurophysiological marker of impaired preparation in an 11-year follow-up study of attention-deficit/hyperactivity disorder (ADHD). J Child Psychol Psychiatry. 2013;54(3):260–70.
Wangler S, Gevensleben H, Albrecht B, Studer P, Rothenberger A, Moll GH, et al. Neurofeedback in children with ADHD: specific event-related potential findings of a randomized controlled trial. Clin Neurophysiol. 2011;122(5):942–50.
Heinrich H, Gevensleben H, Freisleder FJ, Moll GH, Rothenberger A. Training of slow cortical potentials in attention-deficit/hyperactivity disorder: evidence for positive behavioral and neurophysiological effects. Biol Psychiatry. 2004;55(7):772–5.
•• Strehl U, Aggensteiner P, Wachtlin D, Brandeis D, Albrecht B, Arana M et al. Neurofeedback of slow cortical potentials in children with attention-deficit/hyperactivity disorder: A multicenter randomized trial controlling for unspecific effects. Front Hum Neurosci. 2017; 11–135. This large-scale and multicenteder randomized controlled trial examined the efficacy of neurofeedback of slow cortical potentials (SCP) in ADHD. A semi-active control condition (feedback of EMG) was used.
Arns M, Heinrich H, Strehl U. Evaluation of neurofeedback in ADHD: the long and winding road. Biol Psychol. 2014;95:108–15.
Acknowledgements
This work was supported by the grant H2015/HUM-3327 from the Comunidad de Madrid.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
The original version of this article was revised: a modification has been made to the name of the third author. The correct name of the third author can also be found in the erratum for this article.
This article is part of the Topical Collection on ADHD
Rights and permissions
About this article
Cite this article
Albert, J., Sánchez-Carmona, A.J., Fernández-Jaén, A. et al. Neurofeedback for ADHD: a Critical Review and Suggested Future Directions. Curr Dev Disord Rep 4, 86–93 (2017). https://doi.org/10.1007/s40474-017-0117-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40474-017-0117-y