Abstract
Purpose of Review
The use of donor apoptotic cells is an emerging therapy for inducing transplantation tolerance. In this review, we will discuss current understanding of mechanisms of this approach, as well as crucial aspects necessary for successful translation of this approach to clinical transplantation.
Recent Findings
Transplantation tolerance by donor apoptotic cells is mediated by their homeostatic interaction with recipient phagocytes and subsequent expansion of suppressor cell populations as well as inhibition of effector T cells via deletion and anergy. To ensure their tolerogenicity, it is critical to procure non-stressed donor cells and to induce and arrest their apoptosis at the appropriate stage prior to their administration. Equally important is the monitoring of dynamics of recipient immunological status and its influences on tolerance efficacy and longevity. Emerging concepts and technologies may significantly streamline tolerogen manufacture and delivery of this approach and smooth its transition to clinical application.
Summary
Hijacking homeostatic clearance of donor apoptotic cells is a promising strategy for transplantation tolerance. Timing is now mature for concerted efforts for transitioning this strategy to clinical transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To achieve immunosuppression-free graft survival by inducing transplantation immune tolerance has been a long-sought goal of the transplant field. Clinical tolerance for human allogeneic kidney transplantation has now been achieved, using protocols incorporating donor stem cell transplantation that results in transient or permanent donor chimerism [1,2,3,4,5]. This approach, however, often requires highly toxic conditioning regimens to prepare the recipients for donor bone marrow transplant. In addition, long-term risks for graft versus host disease (GVHD) remain formidable.
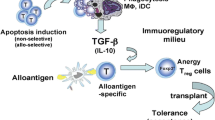
A conceptually different approach is to induce peripheral tolerance by providing donor antigens in an immunologically quiescent manner. One such approach is by using donor apoptotic cells. Billions of apoptotic cells are generated and cleared every day in the body in order to maintain its health and function [6••]. Significant progress has been made in understanding the highly complex cellular signaling network orchestrating such rapid, highly efficient and immunoquiescent clearance of apoptotic cells [7]. It is now generally agreed that apoptotic cell clearance suppresses inflammation in its local milieu [8]. Therefore, harnessing such immunosuppressive potential of apoptotic cell clearance for the therapeutic purpose of inducing transplantation tolerance is a rational approach and has been attempted by numerous groups [9].
As transplant antigens are of donor origin, i.e., donor major, or minor histocompatibility complexes (MHC or MiHC), or non-MHC linked non-self-antigens [10], providing apoptotic cells of donor origin would be necessary to deliver the entire spectrum of relevant donor antigens for inducing donor-specific transplant tolerance. Indeed, several approaches of infusion donor apoptotic cells in this context have been experimented. Most notably, UVB and γ-irradiation have been used as apoptotic stimuli to generate donor apoptotic splenocytes [11,12,13,14,15] followed by their infusion to the recipients. In rodent models of allogeneic cardiac, aortic and islet transplantation, such an intervention results in preventing acute allograft rejection in the complete absence of immunosuppression, and in some cases also in preventing chronic rejection [16, 17]. Our lab has serendipitously discovered that donor splenocytes simply treated with a chemical cross-linker called ethylene carbodiimide (ECDI) undergo rapid and efficient early apoptosis [18•]. When infused intravenously, they are readily phagocytized by recipients’ splenic antigen-presenting cells (APCs) [19] and induce robust donor-specific tolerance in murine models of allogeneic and xenogeneic transplantation [18•, 19,20,21,22,23,24]. This approach is currently being tested in non-human primate models of allogeneic and xenogeneic pancreatic islet transplantations with promising results (Hering, Miller and Luo, unpublished data). Independently, a recent phase I/IIa clinical trial has been published using a single infusion of donor early apoptotic mononuclear cells for prophylaxis of GVHD in 13 patients receiving allogeneic bone marrow transplantation [25••]. The study demonstrated the remarkable safety and potential efficacy of this approach in reducing acute GVHD [25••]. Collectively, these data highlight the potential use of donor apoptotic cells for inducing donor-specific tolerance for clinical transplantation. Table 1 summarizes published preclinical and clinical studies employing donor apoptotic cells for transplantation tolerance induction.
In the rest of this review, we will discuss critical variables pertaining to the efficacy of donor apoptotic cell-based tolerance therapies. We will further discuss emerging technologies, while exploiting the same concept of apoptotic cell clearance, may significantly simplify tolerogen manufacture and/or delivery.
Brief Overview of Mechanisms
APCs are the first point of encounter between the host and the infused apoptotic donor cells. APCs are critical regulators in maintaining homeostasis as well as in initiating innate and adaptive immune responses. Phagocytosis of apoptotic cells by APCs creates a local immunosuppressive milieu by promoting the expression of anti-inflammatory cytokines such as IL-10, TGF-β, indoleamine 2,3-dioxygenase, and nitric oxide while suppressing the production of inflammatory cytokines such as IL-1β, IL-6, IL-12, IL-23, TNF-α, and IFN-γ [13, 26]. Besides regulating the production of cytokines, phagocytosis of donor apoptotic cells also substantially influence the expression of cell-surface molecules on the phagocytes. Following uptake of apoptotic cells, dendritic cells express low levels of antigen presenting and co-stimulatory molecules such as MHC II, CD80, and CD86 and are refractory to further stimulation by activating signals such as LPS or TNF-α [13]. In addition, we have shown that splenic CD11c+ dendritic cells up-regulate expression of co-inhibitory molecules PD-L1 and PD-L2 upon uptaking donor apoptotic cells [19] and are subsequently involved in the deletion or anergy of alloreactive T cells [19, 20]. The role of CD4+CD25+Foxp3+ immunosuppressive regulatory T cells (Tregs) in mediating transplantation tolerance by apoptotic cells is also well documented. We and others have shown that Tregs expand in response to infusions of donor apoptotic cells, accumulate in allografts, and are obligatory for graft protection [14, 15, 19, 20, 27]. In addition to Tregs, another immunosuppressive population expanded by donor apoptotic cell infusions is myeloid-derived suppressor cells (MDSCs), including Gr1HI and Ly6CHI MDSCs [21, 23]. In models of cardiac transplantation [21, 23] and islet transplantation (Dangi, unpublished data), MDSC populations are observed to be obligatory for transplant tolerance induced by this approach.
Based on the above understanding, a critical “checkpoint” of this strategy for ensuring tolerance efficacy is the initial encounter between host APCs and the infused apoptotic donor cells. We will now discuss important considerations for strict “quality control” of this encounter for the goal of achieving tolerance.
Critical Aspects for Preparing Tolerogenic Donor Apoptotic Cells
Successful manufacturing of tolerogenic donor apoptotic cells will likely require strict controls of the following parameters:
Control of Stage of Apoptosis
Early- and mid-stage apoptosis differs in the degree of translocation of the membrane phospholipid, phosphatidylserine (PS), although are both characterized by a yet intact plasma membrane. Late-stage apoptosis, on the other hand, is characterized by the loss of integrity of the plasma membrane and releasing of the intracellular content to its surrounding. Early- and mid-stage apoptosis send specific signals to phagocytes tasked with the cleanup. Series of synapses bridging such apoptotic cells and their interacting phagocytes result in suppression of inflammatory cytokines with simultaneous induction of anti-inflammatory cytokines [9, 28]. The ultimate effect is to allow removal of apoptotic cells by the phagocytes without rendering inflammation. In contrast, releasing of intracellular contents, such as genomic/mitochondrial DNA and heat shock proteins, during late-stage of apoptosis or further secondary necrosis engages receptors for damage-associated molecular patterns (DAMPs) and delivers a rather different set of signals. These signals are often inflammatory, leading to immune activation.
In vitro strategies for rendering donor cells apoptotic for transplantation tolerance include γ-irradiation [12], UVB irradiation [13], and chemical treatments such as with ethylene carbodiimide (ECDI) [20] or paraformaldehyde [29]. In using these approaches, efforts have been made to ensure that the treated donor cells enter an “early” apoptotic stage [12, 13, 18•], marked by annexin V+ but propidium iodide−, without progressing to “late” apoptotic or even secondary necrotic stage, marked by propidium iodide+. The need for this quality control will likely limit the “shelf-life” of apoptotic cell products. After being induced to undergo apoptosis, the cells will likely follow a kinetic process through the different stages of apoptosis, therefore exhibiting only a finite shelf-life during which they maintain their tolerogenic property. Another pragmatic consideration is the storability of donor cells prior to apoptosis induction, especially in settings of deceased donor transplantation. In this regard, we have reported that frozen and thawed donor cells may contain a large proportion of necrotic cells which compromise their suitability for manufacturing apoptotic cells and their ability for inducing transplantation tolerance [18•].
A long-standing concern of using donor apoptotic cells is the potential hazard of sensitization. This concern has been substantiated by studies showing that in vitro-generated, drug-conditioned donor-derived dendritic cells that demonstrate tolerogenic features in vitro could, in fact, be sensitizing in vivo [30, 31, 32•]. A common feature of these studies is that the infused donor-derived dendritic cells, while alive when injected (i.e., not induced to become apoptotic prior to injection), experienced a rather short life-span once injected before being quickly phagocytosed by recipient dendritic cells [32•]. However, the modality of their death prior to their ingestion has not been examined. In fact, depending on whether such donor-derived dendritic cells were generated by Flt-3 ligand or by GM-CSF, a dichotomous response of tolerance versus sensitization results upon their infusion to the recipients [30]. Evidence suggests that when the in vivo death of donor APCs is triggered by NK cell-mediated apoptosis involving caspases [33], tolerance ensues [34]. These findings underscore that strictly ensuring the pathways of ultimate demise may be necessary for the desired host immune responses. Therefore, for clinical translation, a quality control program for ensuring early apoptotic cells to encounter host APCs will likely be critical to the success of transplantation tolerance induction.
Control of Cell Stress
In addition to deliberate apoptosis-inducing treatments mentioned above, certain stress signals within the donor, including hypoxic, oxidative, and endoplasmic reticulum (ER) stresses, may also destine the retrieved donor cells to undergo apoptosis. One such example is cell death induced by stress from active microbial infections [35,36,37]. Such cell stress is closely linked to unfolded protein response signals downstream of toll-like receptors (TLRs) [38], therefore participating in inflammasome activation [39, 40] and supporting the production of pro-inflammatory cytokines [41] and consequent inflammatory rather than the homeostatic clearance of the dying cells [42]. Alternatively, cell stress may also trigger autophagy which can act to inhibit apoptosis via inhibition of apoptosis-associated caspase [43]. Therefore, employing “stressed” donor cells may not be able to induce tolerance and may even result in sensitization. In this regard, we have observed that cells retrieved from donors infected with murine cytomegalovirus (MCMV) were unable to induce transplant tolerance following their treatment with ECDI in contrast to cells from un-infected donors (Dangi, unpublished data). In addition to causing aberrant apoptosis, microbial infections of donor cells may further compromise tolerance efficacy [44] by directly transmitting pathogens to the recipient. Quality control for excluding “stressed” cells awaits identification of precise biomarkers of cell stress, and will likely also be crucial to the success of this tolerance approach, particularly in deceased donor transplantation.
Control of Workload
Based on the above rationale, care should be taken to ensure that the workload of clearing apoptotic cells does not exceed the host’s capacity of clearance, because residual apoptotic cells may then be allowed to progress to late-stage apoptosis or even secondary necrosis, and consequently induce inflammation instead of tolerance. We have previously demonstrated that 4 × 108 cells/kg is the ideal dose of donor apoptotic cells for successful induction of tolerance in rodent models [18•]. However, the optimal dose for clinical application in human transplantation has not been defined. Ongoing experiments in non-human primates will hopefully address this question (Hering, Miller and Luo). Alternatively, we have observed that repetitive small doses of donor apoptotic cells are feasible and have additive efficacy for inducing graft protection [21]. This approach may be safer as the host’s capacity of homeostatic clearance is much less likely to be exceeded. Another consideration in this regard is the potential need for individual dose adjustment when the intended recipient carries certain diseases known to be associated with defects in apoptotic cell clearance, such as certain autoimmune diseases [45] or wide-spread atherosclerosis [46]. For such recipients, the use of donor apoptotic cells may need to be further scaled down or avoided altogether. A standardized assay using recipient-derived phagocytes for assessing clearance capacity of donor apoptotic cells will be highly desirable for determining the ideal dose of donor apoptotic cells to use in a given individual.
Recipient Conditioning and Monitoring
In applying pre-emptive donor apoptotic cells to transplant recipients for tolerance induction, several important recipient factors should be considered.
Prior Sensitization
It has been observed that the same apoptotic donor cell product, while tolerogenic in naïve hosts, is ineffective or even sensitizing in inflammatory hosts. This scenario was initially dissected in models of tolerance by donor specific transfusion (DST). Once transfused, the donor cells quickly become the target of recipient NK cells, are rendered apoptotic, and are ingested by recipient APCs. In humans, the sensitization state of the recipients determines whether DST is tolerizing or sensitizating [47]. In a carefully designed sensitized murine transplant model, Burns et al. show that pre-existing donor specific antibodies (DSAs) act as opsonins to the infused DST. Uptake of opsonized donor cells by APCs leads to their maturation, enhances their priming of alloreactive T cells, and ultimately prevents induction of transplantation tolerance [48•]. In this process, classical complement activation triggered by the preformed antibodies to donor antigens on the transfused donor cells and complement-dependent cytokine and chemokines secretion [49] likely play a role in further augmenting the adaptive immune response, consequently making the DST sensitizing rather than tolerizing.
Thus, a highly clinically relevant question is: how to effectively induce transplantation tolerance in sensitized recipients using apoptotic donor cell-based strategies? A useful framework to conceptualize a solution is to address two separate compartments in a sensitized host, i.e., (1) pre-formed anti-donor antibodies and (2) donor-specific memory cells.
Pre-existing anti-donor antibodies may be detrimental to tolerance induction by apoptotic donor cells, either by themselves as described above [48•] or in conjunction with allospecific memory B cells [50, 51]. Fortunately, therapeutic modalities to remove alloantibodies, at least transiently, are clinically readily available. A combination of plasmapheresis and IVIG is frequently employed in settings of antibody-mediated rejection to remove alloantibodies. For tolerance induction in sensitized recipients, it will be crucial to define the extent and duration for which pre-existing anti-donor antibodies should be removed. Our own data from studies of donor ECDI-SP in sensitized recipients suggests that DSAs, if present at a low level, may not interfere with tolerance efficacy. In fact, their production may be further suppressed by donor ECDI-SP treatment (Dangi, unpublished data). Further studies are needed to fully understand how antibody strength, subtypes, rebound, complement-fixing ability, and the nature of their interaction with donor apoptotic cells may differentially influence the outcome of tolerance by this approach.
The second barrier to tolerance induction in a sensitized recipient is the presence of donor-specific memory T cells. Allospecific memory T cells can be generated by prior rejection [52] or by infection through heterologous immunity [53]. Such memory T cells respond rapidly to repeat antigen stimulation, are less dependent on conventional costimulation, and are consequently more resistant to tolerance therapies such as by apoptotic donor cells [52]. Based on understanding of the biology of memory T cells, therapeutic strategies for controlling these cells include (1) targeting alternative costimulation pathways, such as OX40/OX40L [54] and CD27/CD70 [55], thought to be more commonly used by memory T cells for their activation and effector functions; (2) newer small molecules and biologics, such as 15-deoxyspergaulin analogue [56], sphingosine-1 phosphate receptor agonist [57], and anti-LFA-1 [58]; or (3) combinatorial therapies. These therapeutic interventions aiming to directly inhibit memory cells should now be tested in restoring tolerance efficacy by apoptotic donor cells in sensitized recipients. In our own studies of donor ECDI-SP, by utilizing T cell receptor transgenic T cells, we have found that a combinatorial therapy consisting of donor ECDI-SP, anti-CD40L, and rapamycin, but not individual therapies alone, is highly effective in eliminating alloantigen-specific memory T cells and promoting long-term allograft survival in sensitized recipients (Dangi, unpublished data). Our finding suggests that donor apoptotic cells, when combined with additional targeting strategies, may in fact be an effective modality for controlling memory T cells. Studies uncovering mechanisms of combinatorial therapies will likely be highly informative for designing effective tolerance strategies for sensitized recipients.
Tolerance Longevity
While most studies have focused on tolerance induction, it is in fact the understanding of tolerance maintenance that will have a direct impact on our ability to ensure lasting tolerance once it is induced. While induction of tolerance by apoptotic cells has been shown to involve a multitude of mechanisms including regulation, deletion, and anergy, it appears that maintenance of tolerance relies on anergy more than any other mechanism [59, 60]. Our own studies using donor ECDI-SP support this notion and reveal that once tolerance is established, thorough depletion of CD25+ cells does not result in breaking of tolerance or precipitation of graft rejection. In contrast, blocking PD-1/PD-L1 interaction does lead to tolerance reversal in previously tolerized recipients [19] likely by reverting T cell anergic [59, 61, 62]. These findings suggest that tolerance by apoptotic donor cells could potentially be de-stabilized by signals capable of breaking T cell anergy. One such signal is infection. Using Listeria monocytogenes, Chong and Alegre et al. have shown that microbial infection results in TLR signaling and proinflammatory cytokine production, leading to loss of T cell anergy and acute rejection of previously accepted allografts in tolerant hosts [63]. They went on to show that transplantation tolerance abrogated in this manner can spontaneously restore phenotypically [64•]; however, the re-established tolerance exhibits an altered gene signature from that of the original tolerant state, alluding to a molecular compromise of the robustness of the restored tolerance [65]. Another highly clinically relevant infection in transplantation is CMV. Using donor ECDI-SP for tolerance induction and maintenance, we have observed that CMV infection abrogates tolerance induction as well as tolerance maintenance. At least one mechanism implicated in CMV-mediated tolerance impairment is their ability to modulate the differentiation and function of myeloid-derived suppressor cells via induction of type I interferon (Dangi, unpublished data). This leads to subsequent enhancement of host antigen presentation and T helper cell responses [66], as well as impairment of Treg numbers and function [66, 67]. As compelling data from independent groups have now converged on the detrimental effects of pathogens on tolerance longevity, there is now an urgent need to examine the individual mechanism of tolerance impairment by each clinically relevant pathogen and to design individualized therapeutic strategies aimed to preserve tolerance in settings of such infections.
Emerging Concepts and Technologies
In Vivo Apoptosis
An emerging concept for antigen-specific tolerance alternative to infusing ex vivo-generated apoptotic cells is to induce apoptosis in vivo. The feasibility of this approach was uncovered from studies delineating the tolerogenic mechanism of anti-CD3 monoclonal antibody. This antibody has long been used to treat autoimmune disorders and transplant rejection and has largely been thought to exert its effect through rapid depletion of T cells and generation of Tregs. However, it has been recently revealed that in fact this antibody induces immune tolerance by engaging phagocytes such as macrophages and immature dendritic cells to produce TGF-β in the process of ingesting and digesting apoptotic T cells [68]. This concept of harnessing the potential of in vivo apoptosis for the purpose of immune tolerance induction was further supported by a recent study demonstrating the ability of other in vivo apoptosis-inducing regimens to similarly achieve immune tolerance [69•]. In this work, the authors used a number of strategies to induce apoptosis of cells of hematopoietic origin, including systemic sub-lethal irradiation and depletion of B cells or CD8 T cells with specific monoclonal antibodies. In doing so, the resulting apoptotic cells trigger professional phagocytes to produce TGF-β, which in turn directs naïve CD4 T cells to differentiate into Foxp3+ Tregs. The antigen specificity of this approach is determined by the provision of antigenic peptides during the burst of TGF-β that confer antigen specificity to the in vivo-differentiated Foxp3+ Tregs. In murine models of autoimmunity, this approach has been shown to effectively establish antigen-specific immune tolerance to EAE and colitis. Effort should now be made to test the efficacy of this approach in alloimmune tolerance. Conceivably, donor specificity can be restricted by the transplanted organ itself which carries the full spectrum of relevant donor antigens, directing donor-specific Tregs to be induced from naïve CD4 T cells under the apoptosis-induced TGF-β milieu. Certainly, several questions will first need to be addressed in setting of alloimmune tolerance. These include (1) what cell populations will be the best to induce apoptosis; (2) what source of alloantigens will be the best for driving donor-specific Treg induction; and (3) what strategies specific to alloantigens will be necessary to maximize the robustness of this approach in transplant, particularly in light of the large allospecific T cell clone size in comparison to that present in autoimmunity. However, if successful strategies are identified, this approach could potentially eliminate many concerns of the ex vivo approach discussed above.
Nanoparticles for Tolerogenic Delivery of Donor Antigens
Instead of donor apoptotic cells, solubilized donor antigens in the form of donor cell lysate coupled to apoptotic recipient cells are able to induce transplant tolerance with equal efficacy [18•, 70]. This finding is of pragmatic importance, because it obviates the need for procuring large numbers of fresh donor cells for manufacturing apoptotic donor cell products, which can be logistically cumbersome at the time of deceased donor organ donation. Solubilized donor lysate can also be frozen for storage and later thawed for coupling when needed, providing additional flexibility to this approach. These findings prompted us to further test synthetic particles as an acellular carrier for delivering solubilized donor antigens for tolerance induction. Compared with cells, synthetic particles can be manufactured with more consistency and reproducibility. Solubilized donor antigens coupled to poly(lactide-co-glycolide) (PLG) particles significantly inhibit anti-donor responses, improve transplant graft survival, [71•] and prevent GVHD [72] and when combined with transient low dose rapamycin, induce permanent donor-specific tolerance [71•]. Synthetic carriers can further serve as a versatile platform for additional functionalization to enhance tolerogenic efficacy of the particles. For example, PLG particles modified with phosphatidylserine have been reported to be particularly efficient in promoting expansion of Tregs while suppressing activation of alloreactive T cells [73]. Nanoparticles can also be engineered for targeted delivery of anti-inflammatory biologics [74, 75]. An interesting recent study demonstrates that nanoparticles containing encapsulated antigens and rapamycin can be directly injected into local lymph nodes (LNs) [76•], resulting in local LN reorganization, systemic Treg expansion, and inhibition of autoimmunity in a mouse model of multiple sclerosis [76•]. These data collectively highlights the enormous potential of synthetic particles for delivering alloantigens for transplantation tolerance induction.
Conclusions
The recent decade saw a major breakthrough in our ability to induce clinical transplantation tolerance via establishing bone marrow chimerism. However, the use of apoptotic donor cells may present an alternative and less toxic approach for tolerance induction. A great deal has been learned of the mechanisms and limitations of this tolerance approach. Aiming for clinical translation, it is of paramount importance to establish the following: (1) a standard for quality control of apoptosis and cell stress to ensure immunological quiescence when the cells are infused; (2) a standard for assessing recipient phagocytic competency; and (3) a standard for recipient immune monitoring that can accurately predict recipient sensitization, tolerance robustness and tolerance stability, and allow for tolerance personalization. Conceivably, the first clinical trial using donor apoptotic cells in organ transplant recipients will need to assess the risk of recipient sensitization by cells manufactured strictly according to the above standards. At the same time, emerging concepts (e.g., in vivo apoptosis) and technologies (e.g., nanocarriers) will immensely streamline the current process of donor apoptotic cell manufacturing and delivery and ultimately make its clinical translation readily achievable.
References
Papers of particular interest, published recently, are highlighted as: • Of importance •• Of major importance
Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358(4):362–8. https://doi.org/10.1056/NEJMoa074191.
Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4):353–61. https://doi.org/10.1056/NEJMoa071074.
Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4(124):124ra28. https://doi.org/10.1126/scitranslmed.3003509.
Leventhal JR, Mathew JM, Salomon DR, Kurian SM, Suthanthiran M, Tambur A, et al. Genomic biomarkers correlate with HLA-identical renal transplant tolerance. J Am Soc Nephrol. 2013;24(9):1376–85. https://doi.org/10.1681/ASN.2013010068.
Leventhal J, Abecassis M, Miller J, Gallon L, Tollerud D, Elliott MJ, et al. Tolerance induction in HLA disparate living donor kidney transplantation by donor stem cell infusion: durable chimerism predicts outcome. Transplantation. 2013;95(1):169–76. https://doi.org/10.1097/TP.0b013e3182782fc1.
•• Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol. 2015;16(9):907–17. https://doi.org/10.1038/ni.3253. This comprehensive review details recent advances in our understanding of the clearance of apoptotic bodies by professional and non-professional cell-types and signaling machinery involved in this process.
Elliott MR, Ravichandran KS. The dynamics of apoptotic cell clearance. Dev Cell. 2016;38(2):147–60. https://doi.org/10.1016/j.devcel.2016.06.029.
Henson PM. Dampening inflammation. Nat Immunol. 2005;6(12):1179–81. https://doi.org/10.1038/ni1205-1179.
Morelli AE, Larregina AT. Concise review: mechanisms behind apoptotic cell-based therapies against transplant rejection and graft versus host disease. Stem Cells. 2016;34(5):1142–50. https://doi.org/10.1002/stem.2326.
Oberbarnscheidt MH, Zeng Q, Li Q, Dai H, Williams AL, Shlomchik WD, et al. Non-self recognition by monocytes initiates allograft rejection. J Clin Invest. 2014;124(8):3579–89. https://doi.org/10.1172/JCI74370.
Bittencourt MC, Perruche S, Contassot E, Fresnay S, Baron MH, Angonin R, et al. Intravenous injection of apoptotic leukocytes enhances bone marrow engraftment across major histocompatibility barriers. Blood. 2001;98(1):224–30. https://doi.org/10.1182/blood.V98.1.224.
Sun E, Gao Y, Chen J, Roberts AI, Wang X, Chen Z, et al. Allograft tolerance induced by donor apoptotic lymphocytes requires phagocytosis in the recipient. Cell Death Differ. 2004;11(12):1258–64. https://doi.org/10.1038/sj.cdd.4401500.
Wang Z, Larregina AT, Shufesky WJ, Perone MJ, Montecalvo A, Zahorchak AF, et al. Use of the inhibitory effect of apoptotic cells on dendritic cells for graft survival via T-cell deletion and regulatory T cells. Am J Transplant. 2006;6(6):1297–311. https://doi.org/10.1111/j.1600-6143.2006.01308.x.
Mougel F, Bonnefoy F, Kury-Paulin S, Borot S, Perruche S, Kantelip B, et al. Intravenous infusion of donor apoptotic leukocytes before transplantation delays allogeneic islet graft rejection through regulatory T cells. Diabetes Metab. 2012;38(6):531–7. https://doi.org/10.1016/j.diabet.2012.08.008.
Wu C, Zhang Y, Jiang Y, Wang Q, Long Y, Wang C, et al. Apoptotic cell administration enhances pancreatic islet engraftment by induction of regulatory T cells and tolerogenic dendritic cells. Cell Mol Immunol. 2013;10(5):393–402. https://doi.org/10.1038/cmi.2013.16.
Perruche S, Kleinclauss F, Bittencourt Mde C, Paris D, Tiberghien P, Saas P. Intravenous infusion of apoptotic cells simultaneously with allogeneic hematopoietic grafts alters anti-donor humoral immune responses. Am J Transplant. 2004;4(8):1361–5. https://doi.org/10.1111/j.1600-6143.2004.00509.x.
Wang Z, Shufesky WJ, Montecalvo A, Divito SJ, Larregina AT, Morelli AE. In situ-targeting of dendritic cells with donor-derived apoptotic cells restrains indirect allorecognition and ameliorates allograft vasculopathy. PLoS One. 2009;4(3):e4940. https://doi.org/10.1371/journal.pone.0004940.
• Wang S, Zhang X, Zhang L, Bryant J, Kheradmand T, Hering BJ, et al. Preemptive tolerogenic delivery of donor antigens for permanent allogeneic islet graft protection. Cell Transplant. 2015;24(6):1155–65. https://doi.org/10.3727/096368914X681027. This study defines several important parameters regarding the use of apoptotic cells for transplantation tolerance, including dose optimization, feasibility of using frozen cells, and compatibility with other immunosuppressive drugs.
Kheradmand T, Wang S, Bryant J, Tasch JJ, Lerret N, Pothoven KL, et al. Ethylenecarbodiimide-fixed donor splenocyte infusions differentially target direct and indirect pathways of allorecognition for induction of transplant tolerance. J Immunol. 2012;189(2):804–12. https://doi.org/10.4049/jimmunol.1103705.
Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105(38):14527–32. https://doi.org/10.1073/pnas.0805204105.
Chen G, Kheradmand T, Bryant J, Wang S, Tasch J, Wang JJ, et al. Intragraft CD11b(+) IDO(+) cells mediate cardiac allograft tolerance by ECDI-fixed donor splenocyte infusions. Am J Transplant. 2012;12(11):2920–9. https://doi.org/10.1111/j.1600-6143.2012.04203.x.
Wang S, Tasch J, Kheradmand T, Ulaszek J, Ely S, Zhang X, et al. Transient B-cell depletion combined with apoptotic donor splenocytes induces xeno-specific T- and B-cell tolerance to islet xenografts. Diabetes. 2013;62(9):3143–50. https://doi.org/10.2337/db12-1678.
Bryant J, Lerret NM, Wang JJ, Kang HK, Tasch J, Zhang Z, et al. Preemptive donor apoptotic cell infusions induce IFN-gamma-producing myeloid-derived suppressor cells for cardiac allograft protection. J Immunol. 2014;192(12):6092–101. https://doi.org/10.4049/jimmunol.1302771.
Kang HK, Wang S, Dangi A, Zhang X, Singh A, Zhang L, et al. Differential role of B cells and IL-17 versus IFN-gamma during early and late rejection of pig islet xenografts in mice. Transplantation. 2016; https://doi.org/10.1097/TP.0000000000001489.
•• Mevorach D, Zuckerman T, Reiner I, Shimoni A, Samuel S, Nagler A, et al. Single infusion of donor mononuclear early apoptotic cells as prophylaxis for graft-versus-host disease in myeloablative HLA-matched allogeneic bone marrow transplantation: a phase I/IIa clinical trial. Biol Blood Marrow Transplant. 2014;20(1):58–65. https://doi.org/10.1016/j.bbmt.2013.10.010. This clinical trial highlights the safety and potential efficacy of the use of donor apoptotic cells in preventing acute GVHD after allogeneic bone marrow transplantation.
Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390(6658):350–1. https://doi.org/10.1038/37022.
Bonnefoy F, Masson E, Perruche S, Marandin A, Borg C, Radlovic A, et al. Sirolimus enhances the effect of apoptotic cell infusion on hematopoietic engraftment and tolerance induction. Leukemia. 2008;22(7):1430–4. https://doi.org/10.1038/sj.leu.2405061.
Morelli AE, Larregina AT. Apoptotic cell-based therapies against transplant rejection: role of recipient’s dendritic cells. Apoptosis. 2010;15(9):1083–97. https://doi.org/10.1007/s10495-010-0469-9.
Yamaguchi J, Kanematsu T, Shiku H, Nakayama E. Long-term survival of orthotopic Lewis liver grafts in Wistar Furth rats. Elimination or inactivation of effector CTL and altered antigenicity as possible reasons for tolerance. Transplantation. 1994;57(3):412–8.
Yamano T, Watanabe S, Hasegawa H, Suzuki T, Abe R, Tahara H, et al. Ex vivo-expanded DCs induce donor-specific central and peripheral tolerance and prolong the acceptance of donor skin grafts. Blood. 2011;117(9):2640–8. https://doi.org/10.1182/blood-2010-07-293860.
de Kort H, Crul C, van der Wal AM, Schlagwein N, Stax AM, Bruijn JA, et al. Accelerated antibody-mediated graft loss of rodent pancreatic islets after pretreatment with dexamethasone-treated immature donor dendritic cells. Transplantation. 2012;94(9):903–10. https://doi.org/10.1097/TP.0b013e31826acd01.
• Smyth LA, Ratnasothy K, Moreau A, Alcock S, Sagoo P, Meader L, et al. Tolerogenic donor-derived dendritic cells risk sensitization in vivo owing to processing and presentation by recipient APCs. J Immunol. 2013;190(9):4848–60. https://doi.org/10.4049/jimmunol.1200870. This study highlights the potential risk of recipient sensitization when using donor tolerogenic dendritic cells for tolerance induction. The data presented in this study suggest that a precise quality control method will be necessary while using donor cells for tolerance induction.
Warren HS, Smyth MJ. NK cells and apoptosis. Immunol Cell Biol. 1999;77(1):64–75. https://doi.org/10.1046/j.1440-1711.1999.00790.x.
Yu G, Xu X, MD V, Kilpatrick ED, Li XC. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J Exp Med. 2006;203(8):1851–8. https://doi.org/10.1084/jem.20060603.
He B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 2006;13(3):393–403. https://doi.org/10.1038/sj.cdd.4401833.
Smith JA. A new paradigm: innate immune sensing of viruses via the unfolded protein response. Front Microbiol. 2014;5:222. https://doi.org/10.3389/fmicb.2014.00222.
Valadao AL, Aguiar RS, de Arruda LB. Interplay between inflammation and cellular stress triggered by Flaviviridae viruses. Front Microbiol. 2016;7:1233. https://doi.org/10.3389/fmicb.2016.01233.
Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11(5):411–8. https://doi.org/10.1038/ni.1857.
Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488(7413):670–4. https://doi.org/10.1038/nature11290.
Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492(7427):123–7. https://doi.org/10.1038/nature11588.
Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833(12):3460–70. https://doi.org/10.1016/j.bbamcr.2013.06.028.
Knudsen S, Schardt A, Buhl T, Boeckmann L, Schon MP, Neumann C, et al. Enhanced T-cell activation by immature dendritic cells loaded with HSP70-expressing heat-killed melanoma cells. Exp Dermatol. 2010;19(2):108–16. https://doi.org/10.1111/j.1600-0625.2009.00962.x.
Song S, Tan J, Miao Y, Li M, Zhang Q. Crosstalk of autophagy and apoptosis: involvement of the dual role of autophagy under ER stress. J Cell Physiol. 2017; https://doi.org/10.1002/jcp.25785.
Chong AS, Alegre ML. The impact of infection and tissue damage in solid-organ transplantation. Nature Reviews Immunol. 2012;12(6):459–71. https://doi.org/10.1038/nri3215.
Fond AM, Ravichandran KS. Clearance of dying cells by phagocytes: mechanisms and implications for disease pathogenesis. Adv Exp Med Biol. 2016;930:25–49. https://doi.org/10.1007/978-3-319-39406-0_2.
Thorp EB. Mechanisms of failed apoptotic cell clearance by phagocyte subsets in cardiovascular disease. Apoptosis. 2010;15(9):1124–36. https://doi.org/10.1007/s10495-010-0516-6.
Eikmans M, Waanders MM, Roelen DL, van Miert PP, Anholts JD, de Fijter HW, et al. Differential effect of pretransplant blood transfusions on immune effector and regulatory compartments in HLA-sensitized and nonsensitized recipients. Transplantation. 2010;90(11):1192–9. https://doi.org/10.1097/TP.0b013e3181fa943d.
• Burns AM, Chong AS. Alloantibodies prevent the induction of transplantation tolerance by enhancing alloreactive T cell priming. J Immunol. 2011;186(1):214–221. doi:10.4049/jimmunol.1001172. This study highlights the ability of alloantibodies to function as opsonins to prevent transplantation tolerance by donor-specific transfusion in sensitized recipients.
Saethre M, Schneider MK, Lambris JD, Magotti P, Haraldsen G, Seebach JD, et al. Cytokine secretion depends on Galalpha(1,3)Gal expression in a pig-to-human whole blood model. J Immunol. 2008;180(9):6346–53. https://doi.org/10.4049/jimmunol.180.9.6346.
Burns AM, Ma L, Li Y, Yin D, Shen J, Xu J, et al. Memory alloreactive B cells and alloantibodies prevent anti-CD154-mediated allograft acceptance. J Immunol. 2009;182(3):1314–24. https://doi.org/10.4049/jimmunol.182.3.1314.
Chong AS, Sciammas R. Memory B cells in transplantation. Transplantation. 2015;99(1):21–8. https://doi.org/10.1097/TP.0000000000000545.
Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2(6):501–9. https://doi.org/10.1034/j.1600-6143.2002.20603.x.
Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111(12):1887–95. https://doi.org/10.1172/JCI17477.
Vu MD, Clarkson MR, Yagita H, Turka LA, Sayegh MH, Li XC. Critical, but conditional, role of OX40 in memory T cell-mediated rejection. J Immunol. 2006;176(3):1394–401. https://doi.org/10.4049/jimmunol.176.3.1394.
Yamaura K, Boenisch O, Watanabe T, Ueno T, Vanguri V, Yang J, et al. Differential requirement of CD27 costimulatory signaling for naive versus alloantigen-primed effector/memory CD8+ T cells. Am J Transplant. 2010;10(5):1210–20. https://doi.org/10.1111/j.1600-6143.2010.03089.x.
Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010;90(12):1312–20. https://doi.org/10.1097/TP.0b013e3181fed001.
Zhang Q, Chen Y, Fairchild RL, Heeger PS, Valujskikh A. Lymphoid sequestration of alloreactive memory CD4 T cells promotes cardiac allograft survival. J Immunol. 2006;176(2):770–7. https://doi.org/10.4049/jimmunol.176.2.770.
Ramsey H, Pilat N, Hock K, Klaus C, Unger L, Schwarz C, et al. Anti-LFA-1 or rapamycin overcome costimulation blockade-resistant rejection in sensitized bone marrow recipients. Transpl Int. 2013;26(2):206–18. https://doi.org/10.1111/tri.12021.
Miller ML, Daniels MD, Wang T, Wang Y, Xu J, Yin D, et al. Tracking of TCR-Tg T cells reveals multiple mechanisms maintain cardiac transplant tolerance in mice. American J Transplant. 2016;16(10):2854–64. https://doi.org/10.1111/ajt.13814.
Besancon A, Baas M, Goncalves T, Valette F, Waldmann H, Chatenoud L, et al. The induction and maintenance of transplant tolerance engages both regulatory and anergic CD4+ T cells. Front Immunol. 2017;8:218. https://doi.org/10.3389/fimmu.2017.00218.
Parekh VV, Lalani S, Kim S, Halder R, Azuma M, Yagita H, et al. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J Immunol. 2009;182(5):2816–26. https://doi.org/10.4049/jimmunol.0803648.
Baas M, Besancon A, Goncalves T, Valette F, Yagita H, Sawitzki B, et al. TGFbeta-dependent expression of PD-1 and PD-L1 controls CD8(+) T cell anergy in transplant tolerance. elife. 2016;5:e08133. https://doi.org/10.7554/eLife.08133.
Wang T, Ahmed EB, Chen L, Xu J, Tao J, Wang CR, et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am J Transplant. 2010;10(7):1524–33. https://doi.org/10.1111/j.1600-6143.2010.03066.x.
• Miller ML, Daniels MD, Wang T, Chen J, Young J, Xu J, et al. Spontaneous restoration of transplantation tolerance after acute rejection. Nat Commun. 2015;6:7566. https://doi.org/10.1038/ncomms8566. This study demonstrates that after abrogation of transplantation tolerance by infection, donor-specific tolerant state can re-emerge, allowing spontaneous acceptance of a donor-matched second transplant. The study demonstrates a setting in which memory of allograft tolerance exists during tolerance maintenance.
Young JS, Daniels MD, Miller ML, Wang T, Zhong R, Yin D, et al. Erosion of transplantation tolerance after infection. Am J Transplant. 2017;17(1):81–90. https://doi.org/10.1111/ajt.13910.
Pace L, Vitale S, Dettori B, Palombi C, La Sorsa V, Belardelli F, et al. APC activation by IFN-alpha decreases regulatory T cell and enhances Th cell functions. J Immunol. 2010;184(11):5969–79. https://doi.org/10.4049/jimmunol.0900526.
Raker V, Steinbrink K. Research in practice: the impact of interferon-alpha therapy on immune tolerance. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology: JDDG. 2014;12(4):315–9. https://doi.org/10.1111/ddg.12297.
Perruche S, Zhang P, Liu Y, Saas P, Bluestone JA, Chen W. CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells. Nat Med. 2008;14(5):528–35. https://doi.org/10.1038/nm1749.
• Kasagi S, Zhang P, Che L, Abbatiello B, Maruyama T, Nakatsukasa H, et al. In vivo-generated antigen-specific regulatory T cells treat autoimmunity without compromising antibacterial immune response. Science Translat Med. 2014;6(241):241ra78. https://doi.org/10.1126/scitranslmed.3008895. This study presents an attractive approach for inducing antigen-specific tolerance via in vivo cell-death in a disease model of autoimmunity. This study paves a way for inducing transplantation tolerance in which several variables pertaining the donor and the ex vivo-manufactured donor apoptotic cells can be overcome.
Martin AJ, McCarthy D, Waltenbaugh C, Goings G, Luo X, Miller SD. Ethylenecarbodiimide-treated splenocytes carrying male CD4 epitopes confer histocompatibility Y chromosome antigen transplant protection by inhibiting CD154 upregulation. J Immunol. 2010;185(6):3326–36. https://doi.org/10.4049/jimmunol.1000802.
• Bryant J, Hlavaty KA, Zhang X, Yap WT, Zhang L, Shea LD, et al. Nanoparticle delivery of donor antigens for transplant tolerance in allogeneic islet transplantation. Biomaterials. 2014;35(31):8887–94. https://doi.org/10.1016/j.biomaterials.2014.06.044. This report presents that nanoparticles can be utilized as carriers for the delivery of alloantigens in lieu of intact donor cells. This approach when combined with the immunosuppressive drug rapamycin induces robust transplantation tolerance.
Hlavaty KA, McCarthy DP, Saito E, Yap WT, Miller SD, Shea LD. Tolerance induction using nanoparticles bearing HY peptides in bone marrow transplantation. Biomaterials. 2015;76:1–10. https://doi.org/10.1016/j.biomaterials.2015.10.041.
Roberts RA, Eitas TK, Byrne JD, Johnson BM, Short PJ, McKinnon KP, et al. Towards programming immune tolerance through geometric manipulation of phosphatidylserine. Biomaterials. 2015;72:1–10. https://doi.org/10.1016/j.biomaterials.2015.08.040.
Tang L, Azzi J, Kwon M, Mounayar M, Tong R, Yin Q, et al. Immunosuppressive activity of size-controlled PEG-PLGA nanoparticles containing encapsulated cyclosporine A. J Transp Secur. 2012;2012:896141. https://doi.org/10.1155/2012/896141.
Solhjou Z, Uehara M, Bahmani B, Maarouf OH, Ichimura T, Brooks CR, et al. Novel application of localized nanodelivery of anti-interleukin-6 protects organ transplant from ischemia-reperfusion injuries. Am J Transplant. 2017; https://doi.org/10.1111/ajt.14266.
• Tostanoski LH, Chiu YC, Gammon JM, Simon T, Andorko JI, Bromberg JS, et al. Reprogramming the local lymph node microenvironment promotes tolerance that is systemic and antigen specific. Cell Rep. 2016;16(11):2940–52. https://doi.org/10.1016/j.celrep.2016.08.033. This is an elegant study which highlights that local delivery of autoantigens to lymph nodes via nanocarriers is highly efficacious in inducing systemic tolerance in the EAE model. The efficacy of this approach now needs to be evaluated in settings of transplantation.
Acknowledgements
This work was supported by grants from the National Institutes of Health P01 AI112522 (A.D.), U01 AI102463 (X.L.), and R01 EB009910 (X.L.).
Author information
Authors and Affiliations
Contributions
A.D. and X.L. conceptualized and wrote the manuscript. X.L. edited and finalized the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Immunology
Rights and permissions
About this article
Cite this article
Dangi, A., Luo, X. Harnessing Apoptotic Cells for Transplantation Tolerance: Current Status and Future Perspectives. Curr Transpl Rep 4, 270–279 (2017). https://doi.org/10.1007/s40472-017-0167-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-017-0167-4