Abstract
Purpose of Review
Epidemiologic evidence exists that many metals are associated with adverse neurobehavioral effects in young children, including lead (Pb), methylmercury (meHg), manganese (Mn), and arsenic (As) (Antunes dos Santos et al. J Trace Elem Med Biol. 38:99–107; Tolins et al. Ann Glob Health. 80(4):303–14; Vollet et al. Curr Environ Health Rep. 3(4):392–404; Bellinger Curr Opin Pediatr. 20(2):172–7). Importantly, chemical insult can vary depending on host factors and exposure circumstance. This systematic review summarizes the recent literature investigating modifying factors of the associations between metals and neurodevelopment, including immutable traits (sex or genetics) or exposure conditions (timing or co-exposures).
Recent Findings
Of the 53 studies included in this review, the number investigating the modification of exposure effects was as follows: 30 for sex, 21 for co-exposures, 12 for timing of exposure, and six for genetic modifiers. Sex-specific effects of metal-neurobehavioral associations were inconclusive for all metals, likely due to the heterogeneity of outcome domains assessed and the exposure time points measured. Seven studies evaluated both sex and exposure timing as modifying factors using deciduous teeth or other biomarkers with repeated measures to characterize metals exposure over time. Only five studies used statistical methods for mixtures to evaluate associations of more than two metals with neurobehavioral domains.
Summary
Despite the expansion of research on susceptibility to the neurodevelopmental effects of metals exposure, considerable gaps remain. This work remains critical, as characterizing susceptible subpopulations can aid in identifying biological mechanisms and is fundamental for the protection of public health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the USA, one in six children lives with a developmental disability and prevalence continues to rise [1]. While the etiology of cognitive and behavioral dysfunctions is complex, evidence supports the substantial involvement of environmental factors, even at low doses [2, 3]. Many metals and metalloids (hereafter collectively referred to as metals) are ubiquitous in the environment, commonly co-occur, and target the developing central nervous system [4]. Among the most prevalent environmental metals with neurotoxic potential are lead (Pb), methyl-mercury (meHg), arsenic (As), and manganese (Mn) [5,6,7,8]. Considerable epidemiologic evidence exists that these metals are associated with adverse neurobehavioral effects in young children, with more evidence for some (Pb, meHg) than for others (Mn, As) [3, 9,10,11]. Because neurodevelopment during the prenatal period and early life is a dynamic and complex process of critically timed events, chemical insult can not only have profound impacts but can also vary by immutable traits (sex or genetics) or exposure conditions (timing or co-exposures). Identifying susceptibility factors and other modifiers is important for understanding potential biological mechanisms and for the protection of vulnerable subpopulations.
This review surveys the recent literature on modifiers of the associations between metals and childhood neurobehavior. Mounting evidence suggests that the structure and functioning of many areas of the developing brain are sexually dimorphic [12], and some epidemiologic studies have reported sex-specific associations between individual metals and neurobehavioral outcomes [13, 14]. The differential expression of genes relating to metals’ toxicokinetics or toxicodynamics also has potential to alter the association between metals and neurobehavior [15, 16]. Co-exposure to other metals can produce synergistic or antagonistic effects [17••, 18]. Exposure timing also plays an important role, because the time at which the toxic insult interferes with the neurodevelopmental cascade can determine the extent of damage and the ensuing phenotype [19••, 20••, 21].
Methods
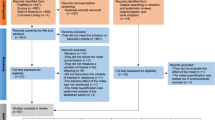
Our review encompasses epidemiologic studies that measured exposure to As, Hg, Mn, and/or Pb and evaluated cognitive and other behavioral outcomes in early childhood, up to a mean age of 8 years. We performed our search in PubMed using the following keywords: (“child,” “childhood,” “children,” “infant,” “pregnant,” “in utero,” “prenatal,” “postnatal,” “school,” “maternal exposure”) AND (“neurodevelopment,” “neurobehavior,” “cognition,” “behavior,” “intelligence,” “hyperactivity,” “ADHD,” “attention deficit,” “disruptive behavior disorders”, “executive function”) AND (“manganese” OR “arsenic” OR “lead” OR “mercury” OR “methylmercury”). The search was restricted to English language studies of humans and publication during the past 5 years (Nov 2014–Nov 2019). We included studies that reported results from cognitive (Table 1) or other behavioral (social, personality, affective, or behavioral control) (Table 2) outcomes and investigated modification by at least one of the following: sex, genetics, co-exposure to other metals (including through fish consumption or smoking status), or exposure timing. Studies that evaluated these factors in stratified models, as interactions, or using statistical models for mixtures (e.g., Bayesian kernel machine regression, weighted quantile sum regression-based methods, and principal component analysis or other clustering methods) were included. We excluded studies that adjusted for multiple metals as confounders only (i.e., did not examine interaction, stratification, or mixtures effects) and those that reported only autism spectrum disorder, motor outcomes, or unadjusted associations. The selection of studies can be viewed in Fig. 1, which follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart.
Lead and Neurodevelopment
The inverse association between Pb exposure and child neurological performance has been consistently demonstrated for IQ, for which no known safe level of exposure exists [8, 75]. Prior literature reporting on sex-specific effects of the association between Pb and neurobehavioral outcomes has been inconclusive [76]. In the past 5 years, Pb research has focused on blood levels < 5 μg/dL and has included novel biomarkers such as tooth dentine and placenta. Studies assessing Pb and childhood neurodevelopment have measured a variety of exposure windows and outcomes, making comparisons across studies challenging. Sex-specific findings were inconclusive across study outcomes, potentially due to the domain-specific nature of these sex-specific effects and the varying domains examined among studies.
Cognitive Outcomes
Nine studies within our review parameters evaluated the effect of Pb and modifiers of metal co-exposure on cognitive outcomes among children [17••, 22,23,24,25, 26•, 27••, 28, 34••]. In a cohort study from Korea, late pregnancy maternal blood Pb (BPb) was associated with reduced cognition at 6 months among infants with lower prenatal iron intake (< 15.1 mg/day), compared with infants with sufficient prenatal iron intake (≥ 15.1 mg/day) [22]. In a cross-sectional study of 7–12-year-old Brazilian children, Pb neurotoxicity was enhanced by higher toenail Mn [23]. On the other hand, a cross-sectional study among 6–8-year-old Uruguayan children reported marginal evidence for an antagonistic interaction between hair Mn and BPb [24]. In a Spanish cohort, an adverse synergistic interaction between placental Pb and As was reported for general cognitive scores at 4–5 years old [26•]. Rodrigues et al. evaluated pairwise interactions of Pb with Mn and As in relation to cognitive scores among 20–40-month-olds in Bangladesh and reported a negative Pb-As interaction in Pabna, where Pb levels were lower (median < LOD of 3.3 μg/dL) [25]. Another study within the same districts reported joint adverse effects of a mixture of As, Pb, and Mn measured in cord blood on 2–3-year Bayley Scales of Infant Development (BSID) scores using Bayesian kernel machine regression (BKMR) [17••]. Pb was the most neurotoxic component of the mixture among children from one study site with higher BPb levels (mean 6.0 μg/dL), while Pb-BSID associations were largely null at the other study site, with lower Pb concentrations (mean 1.8 μg/dL). No interaction between Pb and other metals was observed [17••]. In a Mexico-based study also evaluating a mixture of metals, maternal BPb and cadmium (Cd) contributed the most to a reduction in executive function and rapid visual processing in 6–9-year-olds using weighted quantile sum regression (WQS)–based models [27••]. One study evaluating As, Cd, Mn, and Pb and BSID scores among 13–42-month-olds observed null results using latent class analysis [28].
Two other prospective cohort studies examined exposure at multiple time points in relation to Pb and cognition. In rural China, maternal BPb measured in late pregnancy was associated with decrements in auditory and visual sensory outcomes among 6-week-olds, whereas the association was weaker for BPb measured in early pregnancy and for cord BPb [29]. Among a Canadian cohort of 3–4-year-old children, no association was found between cognitive scores and prenatal maternal or concurrent child BPb concentrations [30].
Sexual dimorphism of the Pb-cognition association was explored in four studies. Two studies reported stronger adverse associations between prenatal BPb and IQ among boys, compared with girls, in Canadian preschoolers (3–4 years old) [30] and in preschool- to school-age children from the UK [31•]. In contrast, two studies suggested stronger adverse associations among girls compared with boys between Pb and executive function measured at 6–8 years [32, 33]. Maternal BPb was associated with poorer ability to plan/organize and worse performance on overall executive function related behavior among girls in the USA, although interaction terms provided little evidence of effect modification by sex [32]. Concurrent BPb levels were also marginally associated with poorer ability to inhibit inappropriate responses among first-grade Uruguayan girls [33].
Other Behavioral Outcomes
Within our review parameters, eight studies assessed the relationship between Pb and non-cognitive behavioral outcomes [19••, 32, 33, 34••, 65••, 66,67,68]. A prospective Korean study reported sex- and time-specific associations between early life BPb and total, internalizing and externalizing problems in 2–5-year-old children. Boys were more susceptible to prenatal Pb exposure, while girls were more susceptible to postnatal exposure [65••]. In contrast, a US study of 6–8-year-old children reported stronger associations between maternal BPb and behavioral difficulties among girls compared with boys [32]. Horton et al. evaluated dentine Pb and metals co-exposures and investigated potential windows of susceptibility in a Mexican birth cohort using reverse distributed lag models and lagged weighted quantile sum regression: Pb exposure measured at the 8- to 12-month time point was most strongly associated with increased anxiety and internalizing symptoms assessed at 8–11 years [19••]. A cross-sectional study of 7–12-year-old Brazilians found suggestive evidence of synergistic interaction between Mn and Pb with total internalizing and externalizing behavior problems [66]. Additionally, Arbuckle et al. reported stronger adverse associations between Pb and behavioral difficulties among 6–11-year-old children whose mothers smoked during pregnancy compared with those that did not [67].
When considering Pb-ADHD associations, two studies reported sex-specific findings. The association between early childhood BPb (< 4 years; 5–10 μg/dL vs. < 5 μg/dL) and 7–12-year-olds diagnosed with ADHD in a US cohort was stronger among boys than girls [68]. In contrast, childhood BPb was modestly associated with hyperactivity scores among girls but not boys in a Uruguayan cross-sectional study of first graders [36].
Mercury and Neurodevelopment
Historically, the neurotoxic effects of high level meHg exposure on nervous system outcomes in children have been well described from unfortunate circumstances of mass poisoning events in the 1950s and 1970s in Japanese and Iraqi populations, respectively [77, 78]. The symptoms of prenatal meHg poisoning in these populations included speech difficulty, movement problems meeting criteria for diagnosis of cerebral palsy, primitive reflexes, seizures, and mental retardation [78]. In populations with moderate exposure to meHg from consumption of large predatory sea life, meHg biomarkers at birth have been associated with decrements in memory, attention, language, and visual-motor skills in childhood [79]. In recent years, results from epidemiologic research on low-level meHg exposure and children’s neurodevelopment remain mixed, likely due to the heterogeneity of fish and rice consumption patterns across populations [80, 81]. Fish consumption particularly complicates the associations between meHg and neurobehavioral outcomes because associated co-exposures may be beneficial (e.g., selenium, poly-unsaturated fatty acids (PUFAs) such as docosahexaenoic acid (DHA)) or deleterious (e.g., polychlorinated biphenyls (PCBs)) for neurodevelopment. Most recent studies have utilized prospective birth cohorts, in which meHg exposure was consistently associated with adverse neurobehavioral scores in Asian populations, but less consistently in European and North American populations. All studies used blood or hair total Hg as a surrogate for meHg exposure.
Cognitive Outcomes
Seven studies investigated effect measure modification of the association between prenatal Hg and cognition by co-exposures related to fish consumption, reporting beneficial [35,36,37,38,39], malign [42], or null [40] effects. One study in the Seychelles reported that gestational PUFA concentrations significantly modified the association of maternal hair Hg and 20-month BSID scores: the association was only adverse among children born from mothers with higher serum n-6:n-3 PUFA ratios (> 4.5), compared with children born from mothers with lower ratios (< 3.5), consistent with the proinflammatory properties of n-6 PUFAs [35]. In a study of 5-year-old South Koreans, increased maternal fish consumption enhanced inverse associations between third trimester maternal blood Hg and IQ [42]. In an Arctic population that regularly consumes beluga meat, cord blood Hg (CBHg) was associated with poorer 8–14-year-old IQ, but no interaction was found between Hg and other common pollutants (cord blood PCB-153 or Pb) or nutrients (cord blood selenium or DHA) from fish consumption [40]. In a US population, co-exposure to PCBs was evaluated when estimating the association between prenatal Hg and cognition in newborns: among infants with higher PCBs (> 61.6 ng/g lipid), maternal blood and CBHg were associated with less need for special handling, indicative of a calmer temperament. However, authors did not report whether this interaction persisted after adjustment for fish consumption [39]. In 4–5-year-old Spanish children, detectable placenta Hg was associated with worse general cognition only among boys [26•]. Interaction between Hg and Mn was significant, whereby the inverse association between placental Hg and verbal function was less pronounced among participants with higher placental Mn, suggesting protective Mn effects [26•]. None of these studies used statistical methods for mixtures.
Four studies evaluated exposure timing, either by evaluating multiple biomarkers that captured different exposure periods [41, 43] or by using the same biomarker across multiple time points [40, 44]. Kim et al. measured prenatal blood Hg at three time points (12–20 and 28–40 weeks gestation, birth) and assessed neurodevelopment at multiple ages (6, 12, 24 and 36 months) in South Korean children. Inverse associations with 6-month outcomes were strongest for maternal blood Hg collected during early pregnancy (12–20 weeks) [44]. Jacobson et al. measured hair and blood Hg at prenatal and childhood time points, but only CBHg was associated with poorer IQ assessed at 8–14 years of age [40].
Four studies investigated sex-specific effects of the Hg-cognition association [26•, 36, 45, 46]. One Asian study reported stronger inverse associations of prenatal Hg with newborn cognitive scores among boys [52], while another reported null findings for associations with 18-month BSID MDI scores [46]. A US study reported worse asymmetric reflex among newborn boys with increasing maternal blood Hg levels, as compared with positive findings among girls, although estimates were weak, especially after adjustment for fish consumption [39].
Cohort studies in the Seychelles [47, 48••], England [50] and near the Adriatic Sea [49, 51••] reported gene-environment interactions. In the Seychelles, inverse associations of prenatal Hg and 18–20-month BSID scores were reported for maternal hair, maternal blood, and CBHg among carriers of specific glutathione related gene variants [48••] as well as for maternal hair Hg among carriers of specific ATP-binding cassette transporters [47]. Snoj Tratnik et al. reported adverse associations between cord and maternal blood Hg and 18-month BSID scores, among children with at least one ApoE ε4 allele [49]. In an analysis combining three prospective cohorts from the Seychelles, Spain, Italy, and Greece, maternal hair Hg and CBHg were associated with higher 14–30-month BSID scores among carriers with high CYP3A activity alleles [51••].
Other Behavioral Outcomes
Only three studies investigated the association of meHg with non-cognitive behaviors, one reporting null findings [34••] and the others reporting adverse associations with anxiety [69, 70••]. Ng et al. evaluated genetics and sex as modifiers of the Hg-behavior association in Taiwanese 2-year-olds: among APOE ε4 carriers, stronger inverse associations between CBHg (> 12 μg/L) and behavioral outcomes were seen in boys, except between Hg and anxiety, where associations were strongest among female APOE ε4 carriers [69]. Patel et al. investigated sex and exposure timing (maternal blood at 16 and 26 weeks gestation and delivery, cord blood at birth) as modifiers in a US cohort of 2–8-year-olds: 16-week maternal Hg was most strongly associated with worse anxiety scores among boys compared with girls, while maternal Hg at delivery was most strongly associated with anxiety among girls [70••].
Arsenic and Neurodevelopment
The neurotoxic evidence for As exposure in children has been recently described by a meta-analysis of studies published between 2000 and 2012, which reported that a 50% increase in As exposure was associated with a reduction of 0.4 full-scale IQ points in children between 5 and 15 years of age [9]. Additionally, decrements were greater for verbal IQ than performance IQ [9]. However, less consistent evidence exists for low-level As concentrations (< 100 μg/L) in association with neurodevelopment [11]. Over the past 5 years, most studies on As and neurodevelopment have evaluated cognitive outcomes. While several studies have observed adverse effects of As exposure on child cognition, with some evidence of synergistic interactions with Pb, results have generally been inconsistent. Potential explanations include the different life stages examined, unmeasured confounding from fish or seafood consumption, and the various As exposure metrics used, which can reflect different As species. For example, while water As primarily reflects inorganic As (iAs), total blood and urinary As can reflect a combination of iAs, arsenobetaine (which is non-toxic and derived entirely from fish and seafood), and monomethyl (MMA) and dimethyl (DMA) arsenicals (derived from metabolized iAs or dietary sources, such as rice, fish, and seafood) [82, 83].
Cognitive Outcomes
Five studies meeting our review criteria examined the impacts of As and modifying metal co-exposures on child cognition [17••, 25, 26•, 27••, 28]. One small study clustered participants based on their concentrations of hair As, hair Cd, hair Mn, and BPb using latent class analysis and examined these clusters in relation to BSID scores at 13–42 months, but results were null [28]. Two larger studies were conducted in the same population in Bangladesh and evaluated BSID scores at 20–40 months. For both studies, As associations were most apparent in a subset of participants living in a region with high water As levels (range 4.4–130 μg/L) [17••, 25]. Rodrigues et al. used a cross-sectional study and traditional linear regression and reported inverse associations between water As and cognitive scores as well as a synergistic relationship with BPb, in which As neurotoxicity was enhanced at higher BPb concentrations. In sensitivity analyses, stronger associations were observed for water As measured in the prenatal period, as compared with 20–40 months of age. No significant associations were observed for water As at 1 month of age, possibly due to breastfeeding, as As levels in breast milk are low regardless of maternal consumption of As-contaminated drinking water [84]. Valeri et al. evaluated total As in cord blood as part of a mixture with Mn and Pb using BKMR [17••]. In contrast with the findings for water As [25], a suggestive protective relationship was observed between cord blood As and cognitive scores, possibly due to unmeasured confounding from seafood consumption [17••]. This unexpected finding was less pronounced at higher levels of Mn [17••]. Two studies examined the impacts of prenatal As exposure and co-exposures on cognitive outcomes in school-age children, and both observed adverse effects [26•, 27••]. One study was conducted in Mexico and measured total As in both second and third trimester maternal blood, which were evaluated as part of a larger mixture using a WQS approach (Cd, cesium, chromium, Pb, antimony) [27••]. Of the two time points evaluated, only third trimester As contributed to reduced executive function at 6–9 years of age [27••]. Consistent with this finding, a study in Spain observed an inverse association between placental As and executive function scores at 4–5 years of age. A significant interaction was also identified between As and Pb, indicating that this As-executive function association was more pronounced among children with high placental Pb [26•]. An inverse association between placental As and quantitative abilities was also reported [26•].
Six studies investigated sex-specific associations, although the majority reported no difference between boys and girls [26•, 52, 53••, 54•, 71]. In a cross-sectional study of 4–5-year-old children in Spain, speciated urinary As (iAs + MMA + DMA) was marginally associated with worse working memory for boys only [53••]. A cross-sectional study in Uruguay also evaluated associations between speciated urinary As (iAs + MMA + DMA) and cognitive outcomes at 5–8 years of age [54•]. Although they did not observe sex differences, urinary As was significantly associated with several subtests of the Woodcock-Muñoz Cognitive Battery within certain strata of dietary folate and urinary %MMA levels [54•]. However, the directions of the associations differed by subtest and were inconsistent within the folate and %MMA strata. A cross-sectional study of 7–8-year-old children in China also examined the relationship between total urinary As concentrations and cognitive outcomes by sex, but results were null, possibly due to unmeasured confounding from seafood consumption [55]. Finally, a cross-sectional study of 6–9-year-old Spanish children reported an inverse association between total urinary As and measures of attention [71]. Possible sex differences were examined, but none was observed.
Other Behavioral Outcomes
While limited, there is also some evidence that As may adversely impact non-cognitive behavioral outcomes. A cross-sectional study of 6–9-year-old Spanish children observed an inverse association between total urinary As and measures of attention, and there were no interactions with sex [71].
Manganese and Neurodevelopment
Manganese is an essential nutrient required for growth and neurodevelopment, but in excess is a potent neurotoxicant [85]. An ideal exposure range has not been identified, particularly for children, and it is unclear at what level Mn becomes toxic rather than beneficial [86,87,88]. In high excess, Mn neurotoxicity in adults is well described in occupational studies as manganism, a parkinsonian-like syndrome involving motor (kinetic tremor, bradykinesia, specific gait disturbances) and neuropsychological (diminished concentration, working memory, spatial orientation) symptoms [89,90,91]. However, less is known about how Mn affects the developing brain. Over the past 5 years, studies on modifying factors of the Mn-neurodevelopment association have been conducted around the world using drinking water and various matrices, including placenta and teeth, to estimate exposure. Results generally support adverse effects of Mn, with suggestive evidence for effect modification by sex and exposure timing. There is also evidence of interaction between Mn and other metals, although much remains to be understood given the heterogeneity in exposure metrics, route, levels, timing, and composition of the mixture.
Cognitive Outcomes
Eleven studies evaluated metal co-exposures as modifiers of the Mn-cognition association, with most studies examining pairwise interactions of Mn with another metal [20••, 23,24,25, 26•, 56,57,58]. About half of these studies reported no modification of Mn associations [23, 25, 28, 34••, 57], while the other half reported some modification or joint effect [17••, 20••, 24, 26•, 56, 58]. In a prospective Bangladeshi study of a mixture of cord blood Mn, As, and Pb using BKMR, Mn was associated with lower 20–40-month BSID-III cognitive scores and contributed to a decline in scores with increasing levels (> 60th percentile) of the metal mixture [17••]. Another prospective study reported a beneficial association of placental Mn on cognition and executive function in 4–5-year-old Spanish children, which was stronger among children with detectable placental Hg even after adjustment for fish intake [26•]. Three other prospective studies used tooth dentine as a biomarker of early life exposure, all of which reported modification of Mn associations [20••, 56, 58]. Prenatal Mn was associated with lower 6- and 12-month BSID-II mental development index scores, but only among girls whose mothers had lower iron [56]. No Mn-Pb interactions were found, but in the same cohort, Mora et al. estimated beneficial prenatal Mn associations with cognition and memory at 7 years, which appeared harmful in the presence of higher BPb (≥ 0.8 μg/dL) [58]. This was consistent with findings from Mexico City, where prenatal dentine Mn was estimated to have beneficial effects on 6–16-year visuospatial abilities only at low dentine Pb levels [20••].
Twelve studies examined sex-specific associations of Mn with cognition. Six studies reported no or inconclusive evidence of sexual dimorphism [26•, 57, 59,60,61,62]. Most of these were cross-sectional analyses of school-age children, with only two prospective studies investigating prenatal exposure in relation to 24-month BSID-II score [60] and 4–5-year McCarthy Scales scores [26•]. Four studies with a range of participant ages from 12 months to 10.5 years reported adverse associations among girls and/or beneficial or null associations among boys [56, 58, 63, 64•]. In contrast, a study of 7–8-year-old Chinese children reported positive associations between urine Mn and IQ, which were stronger among girls [55], and in a prospective study in Mexico, early postnatal tooth Mn was associated with worse 6–16-year visuospatial abilities among boys only [20••].
Exposure timing was evaluated in six studies [20••, 25, 55, 56, 58, 64•]. Two studies of tooth dentine Mn both reported beneficial associations of prenatal Mn and harmful associations of postnatal Mn on cognition, memory, and visuospatial abilities in 6–16-year-old children [20••, 58]. This is somewhat consistent with a Costa Rican study, in which maternal hair Mn concentrations measured in the second half of pregnancy, compared with the first half, were more strongly associated with lower cognitive scores among girls [64•]. In Bangladesh, null associations were reported between 20- and 40-month cognitive scores and drinking water Mn measured both in gestation and early life [25]. In China, urine Mn in 7–8-year-old children, but not cord blood Mn, was associated with better IQ scores [55].
Other Behavioral Outcomes
Ten studies examined modifiers of the association between Mn and non-cognitive behaviors. Four studies evaluated co-exposures [19••, 34••, 58, 66]. Mostly adverse Mn associations and either synergistic or joint effects with Pb [34••, 66] or with Pb and Zn [19••] were reported. The exception was in a US prospective study of Mexican-American children, in which postnatal dentine Mn was associated with worse behaviors at 7 years, but associations were not modified by BPb, perhaps due to the low levels (median BPb 0.8 μg/dL) [58]. In a prospective cohort in Mexico City, co-exposure of dentine Mn with Pb and Zn at 12 months was associated with more anxiety in 8–11-year-olds [19••]. In a cross-sectional study of mother-infant pairs in Saudi Arabia, principal component analysis was used to find that the combination of breast milk Mn, Pb in maternal urine, and Mn and Se in maternal blood at delivery was correlated with lower parent-rated learning and behavior performance in 2–12-month infants [34••].
Five studies examined sex-specific Mn associations on behavioral outcomes [58, 59, 62, 64•, 73]. Two studies were from a cross-sectional evaluation of 7–12-year-old Brazilian children, in which hair Mn was adversely associated with girls’, but not boys’, inattention and externalizing behavior scores [73], whereas sex-specific associations were not seen with teachers’ ratings of hyperactivity [59]. The lack of sex-specific Mn associations with hyperactivity was also reported in a cross-sectional study of 6–13-year-old Canadian children exposed to Mn in drinking water [62] and in a prospective cohort of Mexican-American children in relation to early life Mn and 7-year behavioral scores [58].
Three studies evaluated exposure timing, two of which used tooth dentine as a metric of Mn exposure. In a Mexico City cohort, protective associations were reported for prenatal dentine Mn against externalizing behavior in 8–11-year-olds while postnatal Mn was associated with more anxiety and internalizing behaviors [19••]. In Mexican-American children in the USA, however, both prenatal and postnatal Mn measures were associated with worse 7-year internalizing and externalizing behaviors [58]. In Costa Rica, the negative association between maternal hair Mn and 12-month BSID social-emotional scores in boys was stronger in the second half of pregnancy than during the first half [64•].
Finally, two case-control studies on ADHD differed in their findings. In Swedish 5–17-year-olds, neither main effect of umbilical cord serum Mn nor interaction with Se was found in relation to ADHD [72]. In contrast, lower hair Mn levels were reported in 4–10-year-old ADHD cases, where girls had even lower levels than boys (− 25%) [74].
Conclusions
As our interest in understanding susceptibility factors grows, research examining modifiers of the associations between Pb, meHg, As, and Mn and children’s neurobehavior is also expanding. Of the modifiers examined, sex was most commonly investigated. However, the evidence for sex-specific effects was mixed for all four metals, likely due in part to heterogeneity in the timing of exposure, neurobehavioral domains assessed, and ages of the participants at time of outcome assessment. Seven studies, however, evaluated both multiple exposure windows and sex-specific effects for Pb [65••], Hg [70••], or Mn [20••, 55, 56, 58, 64•]. More research examining multiple exposure time points together with sex-specific effects could help characterize sexually dimorphic relationships.
Although few studies evaluated exposure timing, potential windows of susceptibility were identified for Pb [19••, 29, 30, 65••], Hg [40, 44], As [25, 27••], and Mn [19••, 20••, 56, 58, 64•]. The majority of recent research also measured outcomes at only one time point and the time frame between exposure and outcome assessment was short. Yet effects of environmental exposures on neurodevelopment could span across childhood and early adulthood [21]. Thus, while more challenging and resource-intensive, studies measuring both exposures and outcomes at multiple time points are warranted.
Despite recent expansions to the literature on co-exposures that include studies using statistical mixtures methods to investigate joint exposure [17••, 19••, 27••, 28, 34••], research remains limited. Similarly, recent research on genetic modifiers, investigated in only five of the reviewed meHg studies [47, 48••, 49, 50, 51••], is sparse. Both of these areas represent ripe opportunities for future work.
In summary, our understanding of susceptibility to the neurodevelopmental effects of metals exposure is growing, but considerable gaps remain. Many studies conducted to date may not have been specifically designed to evaluate effect modification and may therefore lack statistical power. Larger prospective studies (n > 500) designed to address susceptibility factors may help unravel the complexity in metals-neurodevelopment associations. As we progress toward investigating the effects of the exposome, it is imperative that modifying factors be more fully examined. Characterizing susceptible subpopulations is critical for identifying biological mechanisms and is fundamental for the protection of public health.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Zablotsky B, Black LI, Maenner MJ, et al. Prevalence and trends of developmental disabilities among children in the United States: 2009–2017. Pediatrics. 2019;144(4). https://doi.org/10.1542/peds.2019-0811.
Landrigan PJ, Lambertini L, Birnbaum LS. A research strategy to discover the environmental causes of autism and neurodevelopmental disabilities. Environ Health Perspect. 2012;120(7):a258–60. https://doi.org/10.1289/ehp.1104285.
Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–8. https://doi.org/10.1016/S1474-4422(13)70278-3.
Claus Henn B, Coull BA, Wright RO. Chemical mixtures and children’s health. Curr Opin Pediatr. 2014;26(2):223–9. https://doi.org/10.1097/MOP.0000000000000067.
Antunes dos Santos A, Appel Hort M, Culbreth M, et al. Methylmercury and brain development: a review of recent literature. J Trace Elem Med Biol. 2016;38:99–107. https://doi.org/10.1016/j.jtemb.2016.03.001.
Tolins M, Ruchirawat M, Landrigan P. The developmental neurotoxicity of arsenic: cognitive and behavioral consequences of early life exposure. Ann Glob Health. 2014;80(4):303–14. https://doi.org/10.1016/j.aogh.2014.09.005.
Vollet K, Haynes EN, Dietrich KN. Manganese exposure and cognition across the lifespan: contemporary review and argument for biphasic dose-response health effects. Curr Environ Health Rep. 2016;3(4):392–404. https://doi.org/10.1007/s40572-016-0108-x.
Bellinger DC. Very low lead exposures and children’s neurodevelopment. Curr Opin Pediatr. 2008;20(2):172–7. https://doi.org/10.1097/MOP.0b013e3282f4f97b.
Rodríguez-Barranco M, Lacasaña M, Aguilar-Garduño C, B, et al. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. 2013;454-455:562–77. https://doi.org/10.1016/j.scitotenv.2013.03.047.
Sanders T, Liu Y, Buchner V, Tchounwou PB. Neurotoxic effects and biomarkers of lead exposure: a review. Rev Environ Health. 2009;24(1):15–45. https://doi.org/10.1515/REVEH.2009.24.1.15.
Tsuji JS, Garry MR, Perez V, Chang ET. Low-level arsenic exposure and developmental neurotoxicity in children: a systematic review and risk assessment. Toxicology. 2015;337:91–107. https://doi.org/10.1016/j.tox.2015.09.002.
Gillies G, Virdee K, McArthur S, Neuroscience JD. Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: a molecular, cellular and behavioral analysis. Elsevier. 2014 undefined. Accessed February 6, 2020. https://www.sciencedirect.com/science/article/pii/S0306452214004254
Bellinger DC. Prenatal exposures to environmental chemicals and children’s neurodevelopment: an update. Saf Health Work. 2013;4(1):1–11. https://doi.org/10.5491/SHAW.2013.4.1.1.
Coetzee DJ, McGovern PM, Rao R, Harnack LJ, Georgieff MK, Stepanov I. Measuring the impact of manganese exposure on children’s neurodevelopment: advances and research gaps in biomarker-based approaches. Environ Heal A Glob Access Sci Source. 2016;15(1). https://doi.org/10.1186/s12940-016-0174-4.
Rooney JPK, Woods NF, Martin MD, Woods JS. Genetic polymorphisms of GRIN2A and GRIN2B modify the neurobehavioral effects of low-level lead exposure in children. Environ Res. 2018;165:1–10. https://doi.org/10.1016/j.envres.2018.04.001.
Andreoli V, Sprovieri F. Genetic aspects of susceptibility to mercury toxicity: an overview. Int J Environ Res Public Health. 2017;14(1). https://doi.org/10.3390/ijerph14010093.
•• Valeri L, Mazumdar MM, Bobb JF, et al. The joint effect of prenatal exposure to metal mixtures on neurodevelopmental outcomes at 20–40 months of age: evidence from rural Bangladesh. Environ Health Perspect. 2017;125(6). https://doi.org/10.1289/EHP614. Associations of a mixture of Mn, Pb, and As were examined using Bayesian kernel machine regression, a statistical method for mixtures, in relation to cognition.
Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res. 2016;23(9):8244–59. https://doi.org/10.1007/s11356-016-6333-x.
•• Horton MK, Hsu L, Claus Henn B, et al. Dentine biomarkers of prenatal and early childhood exposure to manganese, zinc and lead and childhood behavior. Environ Int. 2018;121:148–58. https://doi.org/10.1016/j.envint.2018.08.045. Mn, Pb, and Zn were measured in a nearly continuous manner in tooth dentine, and associations with internalizing and externalizing behaviors were estimated with reversed distributed lag models.
•• Claus Henn B, Austin C, Coull BA, et al. Uncovering neurodevelopmental windows of susceptibility to manganese exposure using dentine microspatial analyses. Environ Res. 2018;161:588–98. https://doi.org/10.1016/j.envres.2017.12.003. Modification of Mn by sex, BPb, and exposure timing were evaluated in relation to visual-spatial scores, using tooth dentine as an exposure biomarker to estimate exposure timing in a nearly continuous manner from the prenatal period through childhood.
Rice D, Barone S Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 2000;108(January):511–533. https://doi.org/10.1289/ehp.00108s3511.
Shah-Kulkarni S, Ha M, Kim BM, et al. Neurodevelopment in early childhood affected by prenatal lead exposure and iron intake. Medicine. 2016;95(4). https://doi.org/10.1097/MD.0000000000002508.
Menezes-Filho JA, Carvalho CF, Rodrigues JLG, et al. Environmental co-exposure to lead and manganese and intellectual deficit in school-aged children. Int J Environ Res Public Health. 2018;15(11). https://doi.org/10.3390/ijerph15112418.
Frndak S, Barg G, Canfield RL, Quierolo EI, Mañay N, Kordas K. Latent subgroups of cognitive performance in lead- and manganese-exposed Uruguayan children: examining behavioral signatures. Neurotoxicology. 2019;73(April):188–98. https://doi.org/10.1016/j.neuro.2019.04.004.
Rodrigues EG, Bellinger DC, Valeri L, et al. Neurodevelopmental outcomes among 2- to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environ Heal A Glob Access Sci Source. 2016;15(1). https://doi.org/10.1186/s12940-016-0127-y.
• Freire C, Amaya E, Gil F, et al. Prenatal co-exposure to neurotoxic metals and neurodevelopment in preschool children: the Environment and Childhood (INMA) Project. Sci Total Environ. 2018;621:340–51. https://doi.org/10.1016/j.scitotenv.2017.11.273. Placenta was used as a biomarker of exposure to Mn, Pb, As, Mn, Hg, and Cd and examined in relation to cognition.
•• Levin-Schwartz Y, Gennings C, Schnaas L, et al. Time-varying associations between prenatal metal mixtures and rapid visual processing in children. Environ Health. 2019;18(1):92. https://doi.org/10.1186/s12940-019-0526-y. This study examined time-varying effects of a complex mixture of metals (As, Cd, Pb, cesium, chromium, antimony) during pregnancy in association with child neurobehavior, using the extensions of weighted quantile sum regression, a statistical method for mixtures.
Kordas K, Ardoino G, Coffman DL, et al. Patterns of exposure to multiple metals and associations with neurodevelopment of preschool children from Montevideo, Uruguay. J Environ Public Health. 2015;2015:1–9. https://doi.org/10.1155/2015/493471.
Silver MK, Li X, Liu Y, et al. Low-level prenatal lead exposure and infant sensory function. Environ Heal A Glob Access Sci Source. 2016;15(1). https://doi.org/10.1186/s12940-016-0148-6.
Desrochers-Couture M, Oulhote Y, Arbuckle TE, et al. Prenatal, concurrent, and sex-specific associations between blood lead concentrations and IQ in preschool Canadian children. Environ Int. 2018:1235–42. https://doi.org/10.1016/j.envint.2018.10.043.
• Taylor CM, Kordas K, Golding J, Emond AM. Effects of low-level prenatal lead exposure on child IQ at 4 and 8 years in a UK birth cohort study. Neurotoxicology. 2017;62:162–9. https://doi.org/10.1016/j.neuro.2017.07.003. This study measured prenatal Pb exposure and assessed sex differences in the association of Pb and child cognition at multiple points in childhood.
Fruh V, Rifas-Shiman SL, Amarasiriwardena C, et al. Prenatal lead exposure and childhood executive function and behavioral difficulties in project viva. Neurotoxicology. 2019;75:105–15. https://doi.org/10.1016/j.neuro.2019.09.006.
Barg G, Daleiro M, Queirolo EI, et al. Association of low lead levels with behavioral problems and executive function deficits in schoolers from Montevideo, Uruguay. Int J Environ Res Public Health. 2018;15(12). https://doi.org/10.3390/ijerph15122735.
•• Al-Saleh I, Al-Mohawes S, Al-Rouqi R, Elkhatib R. Selenium status in lactating mothers-infants and its potential protective role against the neurotoxicity of methylmercury, lead, manganese, and DDT. Environ Res. 2019;176(March):108562. https://doi.org/10.1016/j.envres.2019.108562. Multiple biomarkers for Mn, Hg, and other metals were evaluated using principal component analysis in relation to cognitive and other behavioral outcomes.
Strain JJ, Yeates AJ, Van Wijngaarden E, et al. Prenatal exposure to methyl mercury from fish consumption and polyunsaturated fatty acids: associations with child development at 20 mo of age in an observational study in the Republic of Seychelles. Am J Clin Nutr. 2015;101(3):530–7. https://doi.org/10.3945/ajcn.114.100503.
Golding J, Hibbeln JR, Gregory SM, Iles-Caven Y, Emond A, Taylor CM. Maternal prenatal blood mercury is not adversely associated with offspring IQ at 8 years provided the mother eats fish: a British prebirth cohort study. Int J Hyg Environ Health. 2017;220(7):1161–7. https://doi.org/10.1016/j.ijheh.2017.07.004.
Hibbeln J, Gregory S, Iles-Caven Y, Taylor CM, Emond A, Golding J. Total mercury exposure in early pregnancy has no adverse association with scholastic ability of the offspring particularly if the mother eats fish. Environ Int. 2018;116:108–15. https://doi.org/10.1016/j.envint.2018.03.024.
Llop S, Ballester F, Murcia M, et al. Prenatal exposure to mercury and neuropsychological development in young children: the role of fish consumption. Int J Epidemiol. 2017;46(3):827–38. https://doi.org/10.1093/ije/dyw259.
Xu Y, Khoury JC, Sucharew H, Dietrich K, Yolton K. Low-level gestational exposure to mercury and maternal fish consumption: associations with neurobehavior in early infancy. Neurotoxicol Teratol. 2016;54:61–7. https://doi.org/10.1016/j.ntt.2016.02.002.
Jacobson JL, Muckle G, Ayotte P, Dewailly É, Jacobson SW. Relation of prenatal methylmercury exposure from environmental sources to childhood IQ. Environ Health Perspect. 2015;123(8):827–33. https://doi.org/10.1289/ehp.1408554.
Marques RC, Bernardi JVE, Abreu L, Dórea JG. Neurodevelopment outcomes in children exposed to organic mercury from multiple sources in a tin-ore mine environment in Brazil. Arch Environ Contam Toxicol. 2015;68(3):432–41. https://doi.org/10.1007/s00244-014-0103-x.
Jeong KS, Park H, Ha E, et al. High maternal blood mercury level is associated with low verbal IQ in children. J Korean Med Sci. 2017;32(7):1097–104. https://doi.org/10.3346/jkms.2017.32.7.1097.
Barbone F, Rosolen V, Mariuz M, et al. Prenatal mercury exposure and child neurodevelopment outcomes at 18 months: results from the Mediterranean PHIME cohort. Int J Hyg Environ Health. 2019;222(1):9–21. https://doi.org/10.1016/j.ijheh.2018.07.011.
Kim Y, Ha EH, Park H, et al. Prenatal mercury exposure, fish intake and neurocognitive development during first three years of life: prospective cohort mothers and Children’s environmental health (MOCEH) study. Sci Total Environ. 2018;615:1192–8. https://doi.org/10.1016/j.scitotenv.2017.10.014.
Wang J, Wu W, Li H, et al. Relation of prenatal low-level mercury exposure with early child neurobehavioral development and exploration of the effects of sex and DHA on it. Environ Int. 2019;126:14–23. https://doi.org/10.1016/j.envint.2019.02.012.
Tatsuta N, Nakai K, Sakamoto M, Murata K, Satoh H. Methylmercury exposure and developmental outcomes in Tohoku study of child development at 18 months of age. Toxics. 2018;6(3):49. https://doi.org/10.3390/toxics6030049.
Engström K, Love TM, Watson GE, et al. Polymorphisms in ATP-binding cassette transporters associated with maternal methylmercury disposition and infant neurodevelopment in mother-infant pairs in the Seychelles child development study. Environ Int. 2016;94:224–9. https://doi.org/10.1016/j.envint.2016.05.027.
•• Wahlberg K, Love TM, Pineda D, et al. Maternal polymorphisms in glutathione-related genes are associated with maternal mercury concentrations and early child neurodevelopment in a population with a fish-rich diet. Environ Int. 2018;115:142–9. https://doi.org/10.1016/j.envint.2018.03.015. This article investigated genetic polymorphisms that may modify the association between multiple prenatal meHg biomarkers and Bayley Scales of Infant Development in a large prospective birth cohort.
Snoj Tratnik J, Falnoga I, Trdin A, et al. Prenatal mercury exposure, neurodevelopment and apolipoprotein E genetic polymorphism. Environ Res. 2017;152:375–85. https://doi.org/10.1016/j.envres.2016.08.035.
Julvez J, Davey Smith G, Ring S, Grandjean P. A birth cohort study on the genetic modification of the association of prenatal methylmercury with child cognitive development. Am J Epidemiol. 2019;188(10):1784–93. https://doi.org/10.1093/aje/kwz156.
•• Llop S, Tran V, Ballester F, et al. CYP3A genes and the association between prenatal methylmercury exposure and neurodevelopment. Environ Int. 2017;105:34–42. https://doi.org/10.1016/j.envint.2017.04.013. Data from three large cohorts were combined using meta analysis to investigate whether cyp3A genetic polymorphisms modified the association between prenatal Hg and Bayley Scales of Infant Development scores.
Wang B, Liu J, Liu B, Liu X, Yu X. Prenatal exposure to arsenic and neurobehavioral development of newborns in China. Environ Int. 2018;121:421–7. https://doi.org/10.1016/j.envint.2018.09.031.
•• Signes-Pastor AJ, Vioque J, Navarrete-Muñoz EM, et al. Inorganic arsenic exposure and neuropsychological development of children of 4–5 years of age living in Spain. Environ Res. 2019;174:135–42. https://doi.org/10.1016/j.envres.2019.04.028. Authors measured speciated As levels and were therefore able to exclude arsenobetaine, a non-toxic arsenical derived from fish and seafood, from the As measure. Possible differences by child sex were also evaluated.
• Desai G, Barg G, Queirolo EI, et al. A cross-sectional study of general cognitive abilities among Uruguayan school children with low-level arsenic exposure, potential effect modification by methylation capacity and dietary folate. Environ Res. 2018;164:124–31. https://doi.org/10.1016/j.envres.2018.02.021. This paper measured speciated As levels and were therefore able to exclude arsenobetaine, a non-toxic arsenical derived from fish and seafood, from their As measure. They also investigated several modifying factors, including child sex, dietary folate, and %MMA, a possible indicator of inefficient As metabolism.
Zhou T, Guo J, Zhang J, et al. Sex-specific differences in cognitive abilities associated with childhood cadmium and manganese exposures in school-age children: a prospective cohort study. Biol Trace Elem Res. 2020;193(1):89–99. https://doi.org/10.1007/s12011-019-01703-9.
Gunier RB, Arora M, Jerrett M, et al. Manganese in teeth and neurodevelopment in young Mexican-American children. Environ Res. 2015;142:688–95. https://doi.org/10.1016/j.envres.2015.09.003.
García-Chimalpopoca Z, Hernández-Bonilla D, Cortez-Lugo M, et al. Verbal memory and learning in schoolchildren exposed to manganese in Mexico. Neurotox Res. 2019;36(4):827–35. https://doi.org/10.1007/s12640-019-00037-7.
Mora AM, Arora M, Harley KG, et al. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5years in the CHAMACOS cohort. Environ Int. 2015;84:39–54. https://doi.org/10.1016/j.envint.2015.07.009.
de Carvalho CF, Oulhote Y, Martorelli M, et al. Environmental manganese exposure and associations with memory, executive functions, and hyperactivity in Brazilian children. Neurotoxicology. 2018;69:253–9. https://doi.org/10.1016/j.neuro.2018.02.002.
Claus Henn B, Bellinger DC, Hopkins MR, et al. Maternal and cord blood manganese concentrations and early childhood neurodevelopment among residents near a mining-impacted superfund site. Environ Health Perspect. 2017;125(6):1–9. https://doi.org/10.1289/EHP925.
Haynes EN, Sucharew H, Hilbert TJ, et al. Impact of air manganese on child neurodevelopment in East Liverpool, Ohio. Neurotoxicology. 2018;64:94–102. https://doi.org/10.1016/j.neuro.2017.09.001.
Oulhote Y, Mergler D, Barbeau B, et al. Neurobehavioral function in school-age children exposed to manganese in drinking water. Environ Health Perspect. 2014;122(12):1343–50. https://doi.org/10.1289/ehp.1307918.
Bouchard MF, Surette C, Cormier P, Foucher D. Low level exposure to manganese from drinking water and cognition in school-age children. Neurotoxicology. 2018;64:110–7. https://doi.org/10.1016/j.neuro.2017.07.024.
• Mora AM, Córdoba L, Cano JC, et al. Prenatal mancozeb exposure, excess manganese, and neurodevelopment at 1 year of age in the infants’ environmental health (ISA) study. Environ Health Perspect. 2018;126(5):1–9. https://doi.org/10.1289/EHP1955. Multiple Mn biomarkers in early and late parts of pregnancy were used to estimate associations between prenatal exposure and Bayley Scales of Infant Development scores.
•• Joo H, Choi JH, Burm E, et al. Gender difference in the effects of lead exposure at different time windows on neurobehavioral development in 5-year-old children. Sci Total Environ. 2018;615:1086–92. https://doi.org/10.1016/j.scitotenv.2017.10.007. Multiple time windows of prenatal Pb exposure and childhood Pb exposure were measured, and the authors assessed sex differences in the association between Pb and child neurobehavior.
Rodrigues JLG, Araújo CFS, dos Santos NR, et al. Airborne manganese exposure and neurobehavior in school-aged children living near a ferro-manganese alloy plant. Environ Res. 2018;167(May):66–77. https://doi.org/10.1016/j.envres.2018.07.007.
Arbuckle TE, Davis K, Boylan K, Fisher M, Fu J. Bisphenol a, phthalates and lead and learning and behavioral problems in Canadian children 6-11 years of age: CHMS 2007-2009. Neurotoxicology. 2016;54:89–98. https://doi.org/10.1016/j.neuro.2016.03.014.
Ji Y, Hong X, Wang G, et al. A prospective birth cohort study on early childhood lead levels and attention deficit hyperactivity disorder: new insight on sex differences. J Pediatr. 2018;199:124–131.e8. https://doi.org/10.1016/j.jpeds.2018.03.076.
Ng S, Lin CC, Jeng SF, Hwang YH, Hsieh WS, Chen PC. Mercury, APOE, and child behavior. Chemosphere. 2015;120:123–30. https://doi.org/10.1016/j.chemosphere.2014.06.003.
•• Patel NB, Xu Y, McCandless LC, et al. Very low-level prenatal mercury exposure and behaviors in children: the HOME Study. Environ Health. 2019;18(1). https://doi.org/10.1186/s12940-018-0443-5. This study measured multiple time windows of prenatal Hg exposure and assessed sex differences in the association of Hg and child neurobehavior.
Rodríguez-Barranco M, Gil F, Hernández AF, et al. Postnatal arsenic exposure and attention impairment in school children. Cortex. 2016;74:370–82. https://doi.org/10.1016/j.cortex.2014.12.018.
Ode A, Rylander L, Gustafsson P, et al. Manganese and selenium concentrations in umbilical cord serum and attention deficit hyperactivity disorder in childhood. Environ Res. 2015;137:373–81. https://doi.org/10.1016/j.envres.2015.01.001.
Menezes-Filho JA, de Carvalho-Vivas CF, Viana GFS, et al. Elevated manganese exposure and school-aged children’s behavior: a gender-stratified analysis. Neurotoxicology. 2014;45:293–300. https://doi.org/10.1016/j.neuro.2013.09.006.
Tinkov AA, Mazaletskaya AL, Ajsuvakova OP, et al. ICP-MS assessment of hair essential trace elements and minerals in Russian preschool and primary school children with attention-deficit/hyperactivity disorder (ADHD). Biol Trace Elem Res. Published online 2019. https://doi.org/10.1007/s12011-019-01947-5
Schwartz J. Low-level Lead exposure and children′s IQ: a meta-analysis and search for a threshold. Environ Res. 1994;65(1):42–55. https://doi.org/10.1006/enrs.1994.1020.
Singh G, Singh V, Sobolewski M, Cory-Slechta DA, Schneider JS. Sex-dependent effects of developmental lead exposure on the brain. Front Genet. 2018;9(MAR):89. https://doi.org/10.3389/fgene.2018.00089
Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25(1):1–24. https://doi.org/10.3109/10408449509089885.
Clarkson TW, Magos L, Myers GJ. The toxicology of mercury-current exposures and clinical manifestations. 2003;18. Accessed September 2, 2020. www.nejm.org
NRC. Toxicological effects of methylmercury. National Academies Press; 2000. https://doi.org/10.17226/9899
Mahaffey KR, Sunderland EM, Chan HM, et al. Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption. Nutr Rev. 2011;69(9):493–508. https://doi.org/10.1111/j.1753-4887.2011.00415.x.
Rothenberg SE, Yu X, Liu J, et al. Maternal methylmercury exposure through rice ingestion and offspring neurodevelopment: a prospective cohort study. Int J Hyg Environ Health. 2016;219(8):832–42. https://doi.org/10.1016/j.ijheh.2016.07.014.
Navas-Acien A, Francesconi KA, Silbergeld EK, Guallar E. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environ Res. 2011;111(1):110–8. https://doi.org/10.1016/j.envres.2010.10.009.
Council NR. Critical aspects of EPA’s IRIS assessment of inorganic arsenic. Published online 2013.
Carignan CC, Karagas MR, Punshon T, Gilbert-Diamond D, Cottingham KL. Contribution of breast milk and formula to arsenic exposure during the first year of life in a US prospective cohort. J Expo Sci Environ Epidemiol. 2016;26(5):452–7. https://doi.org/10.1038/jes.2015.69.
Balachandran RC, Mukhopadhyay S, McBride D, et al. Brain manganese and the balance between essential roles and neurotoxicity. J Biol Chem. 2020;295(19):6312–29. https://doi.org/10.1074/jbc.REV119.009453.
Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Asp Med. 2005;26(4–5 SPEC. ISS):353–62. https://doi.org/10.1016/j.mam.2005.07.003.
National Academy of Science. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Academies Press; 2001. https://doi.org/10.17226/10026
Erikson KM, Thompson K, Aschner J, Aschner M. Manganese neurotoxicity: a focus on the neonate. Pharmacol Ther. 2007;113(2):369–77. https://doi.org/10.1016/j.pharmthera.2006.09.002.
Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA. Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27(3):315–26. https://doi.org/10.1016/j.neuro.2005.10.007.
Guilarte TR. Manganese and Parkinson’s disease: a critical review and new findings. Environ Health Perspect. 2010;118(8):1071–80. https://doi.org/10.1289/ehp.0901748.
Miranda M, Bustamante ML, Mena F, Lees A. Original footage of the Chilean miners with manganism published in neurology in 1967. Neurology. 2015;85(24):2166–9. https://doi.org/10.1212/WNL.0000000000002223.
Funding
The research described in this paper was funded in part by NIEHS grants: F31ES029010, T32ES014562, R00 ES022986, and K99 ES030400.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Environmental Epidemiology
Rights and permissions
About this article
Cite this article
Bauer, J.A., Fruh, V., Howe, C.G. et al. Associations of Metals and Neurodevelopment: a Review of Recent Evidence on Susceptibility Factors. Curr Epidemiol Rep 7, 237–262 (2020). https://doi.org/10.1007/s40471-020-00249-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40471-020-00249-y