Abstract
The study of the biomechanical behavior of a system of dental implant, abutment and surrounding bone is essential for a thorough understanding of the load transmission generated by masticatory forces, to develop and optimize the implant design. This article presents an accurate numerical model of implant-abutment-bone system which is subjected to a masticatory loading simulated by axial and horizontal forces acting on the abutment. It is presented a three-dimensional finite element method calculation of stress, displacement, and safety factor, highlighting the influence of bone quality and critical zones of stress concentration by a numerical model as close as possible to reality. The stress distribution pattern is influenced by the loading type and localization, rather than its intensity. Stress values obtained with oblique loading forces are higher than with vertical ones. The loads acting on the structure may cause damage, micro-cracks, and not immediate failure or rupture. The bone quality has an important influence, obtaining lower stress values when the bone is weaker and less resistant to deformation. The novelty of the study consists in developing exclusively by means of computer programs of a geometric model that respects exactly all dimensions and shapes of an actual implant. Once the geometric model of great accuracy is constructed, simulations of various clinical cases can be performed through various loads, various types of materials, boundary conditions, etc. Our study results are consistent with clinical studies observations and similar results from the literature, highlighting critical areas of high stresses at the implant neck and its surrounding bone, potentially responsible for implant failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the last decades, the finite element method (FEM) has become a usual method to investigate the biomechanical response of the bone-implant-abutment system [1, 2]. A considerable number of papers are devoted to the analysis of this response, as a major instrument to evaluate the stress distribution [3, 4], osseointegration progress [5], the most appropriate implant design [6], implant stability [7, 8], oral rehabilitation [9], etc.
The implant prosthodontics studies based on FEM are numerous, and they are dedicated to a complex correlation with laboratory and clinical data with a view to considering optimal therapeutic decisions [10].
On the other hand, some other important parameters in evaluating the implant stability are studied by FEM in different papers, such as: bone quality on stress distribution at the bone–implant interface during occlusal loading [11], bone quantity to observe the variation in all mechanical parameters of the bone for accurate design of patient-specific dental implants [12] and bone loss which may affect the bone remodeling process, together with excessive loading [13].
The biomechanics response of the whole structure (bone, with two different layers, the implant system and the abutment) is relevant in determining the location of critical zones of high stress concentrations and displacement. Thus, an optimal design of the implant could be achieved for better stability and implant success.
This paper is devoted to the study of dental implant insertion in a mandible portion using FEM. The overall objective is to determine and analyze on one hand, the distribution of stress and displacement in the implant and surrounding bone, and on the other hand, the safety factor (FOS) and influence of bone quality as well.
In recent years, this article authors have conducted several studies on the biomechanical response of the bone-implant-abutment system, prosthesis presence as well, in various situations to simulate actual clinical cases. Also, they approached in their study the period during the osseointegration process [14], defined as a direct and functional connection between bone and an artificial implant [15]. In this article, the study addresses the post-osseointegration period, highlighting possible factors that may affect the (secondary) stability of the implant, for example the quality of the bone in which the insertion is made, but also high stresses that can affect various areas of the system under consideration.
Regarding the implants, the most critical zone is that one in which the maximum stress concentration occurs. That critical area is the implant neck and the surrounding area, i.e., the marginal bone (neck edge). Therefore, this zone must be clinically preserved to keep a structural and functional bone–implant interface.
2 Materials and methods
In this article, the dental implants and surrounding bone behavior are modeled using exclusively computer programs. This study examines the stress and displacement state due to the insertion of a dental implant into a part of the mandible. Special attention is given to highlight and assess the zones of stress concentration both in the cancellous (trabecular) and the cortical bone, and the implant as well, within various clinical situations of loading due to the process of mastication. These zones represent the most endangered zones in which possible damage, failure, or fracture can take place [7, 9].
2.1 The numerical model of dental implant
The first stage of FEM modeling is the developing of the geometric model of bone—implant—abutment system. Then, the next step is the materials set for these system components by their material constants (Table 1). In this study, the model is static and the materials are considered elastic.
For every material involved in the studied structure, it is indicated the values of the following material constants: Young's modulus, Poisson's ratio, density, tensile strength, and yield strength (Table 2 for the implant and its components, and Table 3 for the two types of bone). Usually, the implant is made of titanium alloy and the abutment of magnesium alloy.
The third step of numerical modeling is to set the boundary conditions and next apply the loading to simulate, in our case, the mastication forces.
Then the numerical model is used to calculate by Cosmos program the distribution of displacement and stress in the whole system made up of bone tissue, implant, and abutment.
2.1.1 Geometrical model
The geometric model consists of two parts: the biological one, the bone, and the one regarding the implant and the abutment. The three-dimensional (3D) geometrical model developed in this study is created entirely using SolidWorks software and uses tetrahedral elements both in the implant and bone.
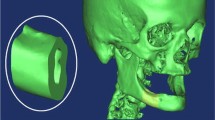
Consider the case of a structure composed of an implant [17] of 11 mm length, a maximum thickness of 2.8 mm inserted in a part of the mandible, extending about 10 mm from the center of the implant and comprising a layer of cancellous bone bordering both the upper and lower sides of a thinner cortical bone layer, as in Fig. 1a.
Figure 1 represents the geometric model of the whole system, bone, implant, and abutment, while Fig. 2a and b presents only the implant geometric model.
The implant is conical shaped with a threaded area in the lower part representing an insertion zone of 11 mm, and in the upper area, the implant contains an abutment of 7 mm with a maximum diameter of 2.3 mm (Fig. 2a and b).
Implantation medium represents a part of the mandible around the implant at a distance from it, which cannot affect the stress and displacement state of the whole system.
The model of the mandible part considers two distinct types of the bone (cortical and cancellous) by assigning those areas to corresponding material properties. All components are modeled taking into account in detail all real technical elements (threads, undercuts, contacts, connection radii, release cutting, etc.).
The geometric model is meshed and used for various finite element (FE) calculations performed by setting appropriate boundary conditions and applying different axial and lateral loads simulating the masticatory forces.
2.1.2 Numerical model characteristics
The geometrical model presented in the previous paragraph is meshed using tetrahedral elements both in the implant and in the two types of bone tissue. Our study used different mesh options, and for instance one of it is as follows: number of elements 184,562, number of nodes 268,244, element size 0.4 mm, tolerance 0.02 mm, time to complete the mesh (hh;mm;ss): 00:02:03.
Then, the type of materials and material constants are set for each component, such as the bone (cancellous and cortical bone) and implant components. In this analysis we used as input data, the values available in [10, 16] and those provided by the technical presentation of the implants.
The mesh used in this model has a big number of finite elements owing to the high fineness of the structural elements of the implant such threads, recesses, etc.
2.2 Contact modeling
The contact modeling between all the parts of the model is very important in the numerical calculations as it influences notably the results. It differs a lot if the study concerns an analysis during the osseointegration period or a post-osseointegration period.
The contact areas are the threaded parts of the implant, mandible, and screw. The phenomenon of contact in these areas is modeled by special finite elements—contact elements, modeling the actual behavior of the system.
In our study, as we refer to a post-osseointegration period, the contact between the implant threads and bone is considered a bonded contact, so without slipping, without friction. The numerical model was built in order to capture the interaction between all the components of the implant and the bone, and to study the whole system.
2.2.1 Boundary conditions
Boundary conditions are usually set in terms of displacements and/or forces in those zones where these quantities are considered known and they must be restricted to remain fixed during the simulation.
Null displacement conditions must be placed on a certain boundaries to provide the solution balance, for instance in Fig. 3a, in which also the boundary conditions are represented by green arrows.
In addition, the restrictions must be placed in those nodes that are far enough from the region of interest, which is in our analysis, the vicinity of the implant. It proceeds in this manner on account of avoiding the overlap of the stress fields corresponding to the reaction forces of the bone–implant interface.
2.2.2 Loading application
Setting the loads in a FE model represents a significant stage of the study as it must simulate the mastication loading. The type of masticatory forces could be of compression, tensile, bending, and shear. The most dangerous forces which may increase the stress around the interface bone-implant are tensile and shear forces, which can damage the integrity of the material and cause stress concentration that could mean implant failure. Generally, the whole system implant—bone adapts to compressive forces [18].
The relation between excessive occlusal forces and the marginal bone loss in the peri-implant region is a complex phenomenon implying investigations and comprehensive research, which include the biomechanical relationships of living type material and mechanical properties of bone around the implant, engineering principles, etc.
Under effective mastication, the repetitive pattern of cyclical forces distributes the loading over the peri-implant bone through the implant. That develops a stress around the ridge. Still, the cyclical random forces of mastication are not easy to simulate. Accordingly, the majority of FE studies use axial and/or non-axial forces—horizontal or oblique, or a combination of them.
The masticatory force magnitude may be fluctuating depending on sex, age, edentation, parafunctional habits and can differ from anterior to posterior even for the same patient.
The loads simulating the masticatory forces develop stress concentration which must be evaluated and thus an appropriate risk must be taken into account [18].
In our FE study, static loads are applied to the abutment by an axial and horizontal forces, acting as in Fig. 3b, or axial forces only. In our study, calculations using different values for loading forces, between 50 and 400 N have been performed. For instance, the numerical results presented in the next section are obtained for an axial force of 100 N and a horizontal force of 20 N and 40 N, respectively.
The Cosmos program determines the structure distribution of stress, strain, displacement. We use the elastic constitutive law that links the stress tensor σ to the strain tensor \({{\varvec{\upvarepsilon}}}\) through the elastic matrix E by Hooke law:
The strain tensor \({{\varvec{\upvarepsilon}}}\) is derived from the displacement vector u through the kinematic relation:
Concerning the convergence tests, let mention that few mesh versions were achieved with a fineness that does not present errors higher than a 3.5% solution [19].
3 Results
3.1 Results concerning the state of stress and displacement
Stress concentration in the implant and surrounding bone structure may occur under the effect of masticatory forces. As the stress is directly proportional to the force and inversely proportional to the surface area to which the force is applied, it is relevant to evaluate the surface on which the force is acting. For instance, the area of the occlusal surface on which rehabilitation is performed is less than 4 mm, such that the stress magnitude in many cases rests in the order of Mpa [20].
The FEM calculation refers to von Mises stress (equivalent stress) values, a scalar quantity which designates the stress magnitude which is useful in damage criteria formulation, plasticity, failure, etc. In our analysis, it is employed to assess the result of loading forces on the region near the dental implant.
It is used as the convention according to which positive stress values signify traction, whereas negative values mean compressive stress.
The results of numerical calculation by FEM are presented in the figures below by diagrams of the isovalues of the physical quantities under consideration, from the areas of minimum value to the areas of maximum value. As much, the most advantageous zones from a biomechanical point of view are those with minimum values of stress and displacement, whereas zones with the most significant damage and high risk are signalized by higher values.
The main quantities of the analysis are: displacement and von Mises stress distribution and they are shown in the next figures for the case of combined axial and horizontal loads, for the whole bone—implant system and for the components as well.
For instance, Fig. 4 shows the displacements distribution in the whole structure: bone, implant, abutment, in the transversal section, for axial and horizontal forces acting simultaneously.
We observe the asymmetry of the stress distribution due to the loading type and bigger values of the displacement in the opposite side of the load application.
If a detailed analysis of the system components is required, we present Figs. 5 and 6 which represent the displacement field for bone only and for implant only, respectively.
Figure 7 presents the von Mises stress in the whole system in the transversal section under combined axial and horizontal loadings. In this case, bigger values are observed in the opposite zone of load action as well as in the case of displacement.
The asymmetry of these figures may be observed.
Calculations for different values of elasticity modulus E for the cortical bone reveal some information on the behavior of bone-implant-abutment system according to the bone quality. We consider, as frequently that the more bone quality is better, the elasticity modulus is higher. Figures 8 and 9 present the maximum displacement and maximum stress evolution vs bone quality, respectively.
3.2 Results concerning the factor of safety
Material damage in zones where stresses are greater than a certain level. These areas are signaled based on the application of a failure criterion by calculating the factor of safety (FOS). There is a section in Cosmos program for the FOS determination as a ratio of the admissible limit values and calculated values by FEM model. Limit admissible stress values are distinctive for every material and have been set in specific conditions. If FOS < 1, the calculated stress values exceed the material limit and the structure may fail [21].
Figures representing FOS distribution are useful and they signify the necessity for appropriate constructive solutions to remove risk zones with a low factor of safety.
Figure 10a and b shows the FOS distribution in the implant only in the case of only axial loads and axial and horizontal loads simultaneously
Low values of FOS are obtained in the implant neck, which is well known and found in clinical practice as a critical zone.
4 Discussion
A 3D FEM study is highly adequate for the biomechanical behavior assessment of a system made up of bone, implant and abutment subjected to different loading conditions. The novelty of this study consists in developing entirely by the computer programs of a geometric model which has exactly all dimensions and shapes of an actual implant. As soon as the geometric model of great accuracy is built, simulations of different clinical situations may be developed, through various loads, various types of materials, boundary conditions, etc.
The stress pattern is not changed by an increase in load, but generally it just increases its value [9]. The loading the system is subjected to may provoke damage, microcracks in certain parts of the system, but not an immediate failure or rupture [18].
Concerning the bone quality influence on the results of the study, we obtained higher stress values in the cortical bone around the implant [22], being maximum on the opposite side to the application of force. Consequently, the implant placement must be considered carefully in the cortical bone with greater thickness since the stress in the cancellous bone is low, which may lead to atrophy in that zone [19].
Furthermore, the stress values obtained in the case of oblique loading force are higher than in the case of vertical loading force only [4]. In terms of FOS, this is lower when oblique forces are applied, compared with vertical forces only.
The concept that the interface of materials with different elasticity modulus is a vulnerability of rehabilitation systems is confirmed by FEM analysis results [23]. For example, rehabilitations involving materials that have an elasticity modulus similar to the tooth are able to preserve and consolidate the remaining tooth structure [18]. Putting together a fatigue laboratory tests with FEM studies, it may eliminate or at least diminish the experimental limitations by relating fatigue failure to stress, rather than to an appropriate test configuration [24].
There are some limitations concerning on one hand the FEM application generally, and on the other hand some limitations concerning this study.
General limitations may refer to the fact that the simulation of a real clinical case may not be applied straightforward in a FE program [23], but we can develop a model to simulate an actual situation as accurate as possible, and some hypotheses and simplifications have to be done. For instance, more convenient for the numerical calculation is to use elastic, isotropic, and homogenous materials, even it is not the case for real materials and processes [23]. At the same time, the FEM model is a static situation at a certain moment of load application and not an actual clinical situation. Consequently, the FEM results must be comprehended and interpreted carefully.
Clinicians should, however, be noted that these FEM applications are generated by computer calculations; thus they have hypotheses and important restraints that clearly will influence the application of these results to an actual case [25]. One more aspect of the FEM model is that the results are overestimated, due to the applied simplifications [25].
There are some findings that can be withdrawn from this study regarding the sensitive area of the implant neck. It should have enough distance from the soft tissue and possible implant adherence would not influence the mucous membrane, since marginal bone resorption and soft tissue inflammation may lead to implant failure [22]. Additionally, the neck of implants should have enough sturdiness to take over the maximum stress concentrations taking place in the neck of the implant. The implant stability can be affected if the implant is not robust enough in this zone [10]. For patients presenting a narrow alveolar crest bone, it is an overload risk in the mesial and distal zone [18].
Consequently, the stress distribution in the whole system of implant, bone, and abutment depends on numerous factors such as loading type, biomechanical characteristics of bone, cortical bone thickness, density of trabecular bone and some other factors [23].
To validate the FEM study results, it is necessary to correlate them with preclinical and clinical lengthy trials [26].
5 Conclusions
This paper presents the biomechanical response by a FEM numerical model of a system made up of the implant and surrounding bone. This response is employed to analyze those factors that influence the implant stability. The purpose of this study is to provide a very accurate numerical model, especially regarding to the implant (the geometric model used is one of a real implant) to obtain a response as close to reality as possible.
The geometric model contains the entire system modeling of the problem: the implant, bone support with two types—trabecular and cortical bone, and abutment. The simulation of the masticatory forces is achieved by the application of axial and horizontal forces on a certain surface of the abutment. The results show several important related issues, such as implant insertion, the optimal implant length choice, the sensitive zone of implant neck, but the most significant factor being, in the end, the bone quality.
These results of the study are consistent with clinical observations and the results obtained by other papers. Further studies are already in progress for on one hand, to establish a better connection between the FE analysis and the actual clinical situation and, on the other hand to extend the FE numerical simulations to more complex clinical cases, for instance more elements prostheses.
The clinical significance of this study consists in the fact that without in vivo or laboratory experiments, expensive both in terms of time and money, the clinician can estimate through successive runs of the program, the biomechanics of the complex bone-implant-prosthetics system, to highlight the risk factors involved in the success of the implant dental.
Abbreviations
- 3D:
-
Three dimensional
- FEM:
-
Finite element method
- FE:
-
Finite element
- FOS:
-
Factor of safety
References
Chatterjee S, Sarkar S, Kalidindi SR, Basu B (2019) Periprosthetic biomechanical response towards dental implants, with functional gradation, for single/multiple dental loss. J Mech Behav Biomed Mater 94:249–258. https://doi.org/10.1016/j.jmbbm.2019.03.001
Liang R, Guo W, Qiao X, Wen H, Yu M, Tang W, Liu L, Wei Y, Tian W (2015) Biomechanical analysis and comparison of 12 dental implant systems using 3D finite element study. Comput Meth Biomech Biomed Eng 18:1340–1348
Lee JS, Cho IH, Kim YS et al (2012) Bone-implant interface with simulated insertion stress around an immediately loaded dental implant in the anterior maxilla: a three-dimensional finite element analysis. Int J Oral Maxillofac Implants 27(2):295–302
Bahuguna R, Anand B, Kumar D, Aeran H, Anand V, Gulati M (2013) Evaluation of stress models in bone around dental implant for different blunt angulations under axial and oblique loading: a FE analysis. Natl J Maxillofac Surg 4:46–51
Vanegas-Acosta JC, Landinez PN, Garzon-Alvarado DA, Casale RM (2011) A finite element method approach for the mechanobiological modeling of the osseointegration of a dental implant. Comput Methods Programs Biomed 101:297–314
El-Anwar MI, El-Zawahry MM (2011) A three dimensional finite element study on dental implant design. J Genet Eng Biotechnol 9:77–82
Mathieu V, Vayron R, Richard G, Lambert G, Naili S, Meningaud JP, Haiat G (2014) Biomechanical determinants of the stability of dental implants: influence of the bone-implant interface properties. J Biomech 47:3–13
Huang YM, Chou IC, Jiang CP, Wu YS, Lee SY (2014) Finite element analysis of dental implant neck effects on primary stability and osseointegration in a type IV bone mandible. Biomed Mater Eng 24:1407–1415
Santos MBF, Silva Neto JP, Consani RLX, Mesquita MF (2011) Three-dimensional finite element analysis of stress distribution in peri-implant bone with relined dentures and different heights of healing caps. J Oral Rehab 38:691–696
Chen L (2012) Application of finite element analysis in implant dentistry. Finite element analysis of the stress on the implant-bone interface of dental implants with different structures. Finite element analysis—New trends and developments. Intech
Taharou B, Merdji A, Hillstrom R, Benaissa A, Roy S, Della N, Aid A, Mukdadi O (2021) Biomechanical evaluation of bone quality effect on stresses at bone-implant interface: a finite element study. J Appl Comput Mech 7(3):1266–1275. https://doi.org/10.22055/JACM.2020.32323.2005
Bhattacharyya S, Choudhury S, Datta P, Pal AK, Roy S, Chatterjee R, De R, Chakraborty A, Saha S, Chowdhury AR (2021) Assessment of jaw bone quality for designing patient-specific dental implant using computed tomography data. J Long Term Eff Med Implants 31(1):4958. https://doi.org/10.1615/JLongTermEffMedImplants.2021036995
Ouldyerou A, Merdji A, Aminallah L, Msomi V, Chong PL, Roy S (2022) biomechanical evaluation of marginal bone loss in the surrounding bone under different loading: 3d finite element analysis study. Int J Multiscale Comput Eng 20(4):43–56. https://doi.org/10.1615/IntJMultCompEng.2022043707
Roateşi I, Roatesi S (2020) Modeling of dental implant osseointegration progress by three-dimensional finite element method. Appl Sci 10:5561. https://doi.org/10.3390/app10165561
Giudice A, Bennardo F, Antonelli A, Barone S, Wagner F, Fortunato L, Traxler H (2020) Influence of clinician’s skill on primary implant stability with conventional and piezoelectric preparation techniques: an ex-vivo study. J Biol Regul Homeost Agents Mar-Apr 34(2):739–745. https://doi.org/10.23812/20-96-L-53
Gislason MK, Nash D (2012) Finite element modelling of a multi-bone joint: the human wrist. Finite element analysis—New trends and developments. Intech
Roateşi I (2016) Finite elements method in implant prosthetics, Chapter 10, Perusal of the Finite Element Method. Intech. https://doi.org/10.5772/64969
Misch CE (2008) Comtemporary implant dentistry. Clinical biomechanics in implant dentistry. Mosby. Elsevier
Marcian P, Borak L, Valasek J, Kaiser J, Florian Z, Wolf J (2014) Finite element analysis of dental implant loading on atrophic and non-atrophic cancellous and cortical mandibular bone—a feasibility study. J Biomech 47:3830–3836
Winter W, Klein D, Karl M (2013) Effect of model parameters on finite element analysis of micromotions in implant dentistry. J Oral Implantol 39:23–29
Moller N, Hansson SO, Holmberg JE, Rollenhagen C (2017) Handbook of safety principles. Ch. 6. Wiley
Chrcanovic BR, Albrektsson T, Wennerberg A (2017) Bone quality and quantity and dental implant failure: a systematic review and meta-analysis. Int J Prosthodont 30(3):219–237
Ormianer Z, Palti A, Demiral B, Heller G, Lewinstein I, Khayat PG (2012) Implant-supported first molar restorations: correlation of finite element analysis with clinical outcomes. Int J Oral Maxillofac Implants 27:1–12
Shemtov-Yona K, Rittel D (2016) Fatigue of dental implants: facts and fallacies. Dent J 4(2):16
Verri FR, Cruz RS, de Souza Batista VE, Almeida DA, Verri AC, Lemos CA, Santiago Júnior JF, Pellizzer EP (2016) Can the modeling for simplification of a dental implant surface affect the accuracy of 3D finite element analysis? Comput Methods Biomech Biomed Engin 15:1–8
Lisiak-Myszke M, Marciniak D, Bieliński M, Sobczak H, Garbacewicz L, Drogoszewska B (2020) Application of finite element analysis in oral and maxillofacial surgery—a literature review. Materials (Basel) 13(14):3063
Acknowledgements
We are thankful to Prof. E. Avram for his important support in achieving the numerical model and FEM calculations and equally warm thanks to Prof. V. Năstăsescu for his expertise and high professionalism on FEM use.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Technical Editor: João Marciano Laredo dos Reis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roatesi, I., Roatesi, S. Biomechanics study of dental implant-bone system by finite element method. J Braz. Soc. Mech. Sci. Eng. 45, 345 (2023). https://doi.org/10.1007/s40430-023-04170-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40430-023-04170-5