Abstract
Purpose of Review
This systematic review summarizes evidence of single nucleotide polymorphism (SNPs) associations with smoking cessation focusing on dopamine receptor or dopamine metabolism genes. Summary odds ratios (ORs) of SNP associations were calculated and stratified by ancestry, pharmacotherapy, and sex where feasible.
Recent Findings
The 30 included articles reported the results of 32 studies. The minor allele of DRD2/ANKK1 SNP rs1800497 was associated with lower odds of cessation among people of European ancestry [OR = 0.88, 95% CI 0.82 – 0.95, n= 16 studies]; this association was not observed among people of non-European ancestry. Heterogeneity by sex in rs1800497 associations was present [female: OR = 0.91, 95% CI 0.85 – 0.97, n = 9 studies; male: OR = 1.16, 95% CI 0.85 – 1.55, n = 7 studies]. Recipients of nicotine replacement therapy (NRT) with the minor allele of DRD2 SNP rs6277 [OR = 1.43, 95% CI 1.06 – 1.92, n = 2 studies] or COMT SNP rs4680 [OR = 1.61, 95% CI 1.11 – 2.35, n = 3 studies] had increased odds of cessation. Heterogeneity by sex in rs4680 associations was observed [combined sex: OR = 0.73, 95% CI 0.57 – 0.93, n = 3 studies; male: OR = 0.74, 95% CI 0.53 – 1.02, n = 1 study; female: OR = 1.01, 95% CI 0.77 – 1.32, n = 5 studies].
Summary
Associations between rs1800497 and rs4680 and cessation may differ by biological sex. Limited evidence suggests some genetic associations may differ by ancestry and that rs4680 or rs6277 genotype may influence NRT efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cigarette smoking prevalence among U.S. adults declined from 20.9% to 11.5% between 2005 and 2021, however, smoking remains a leading cause of premature death and preventable illness [1, 2•]. Furthermore, cigarette smoking disproportionally affects specific population groups of people, including residents of rural communities, military veterans, sexual minorities, people with lower socioeconomic status, and those with disabilities [2•, 3]. Though quitting smoking provides short- and long-term health benefits [4], the highly addictive property of nicotine creates a significant barrier to smoking cessation. More than half of U.S. adult smokers try to quit smoking each year; more than 90% of these quit attempts result in relapse within 6-months [5, 6]. Additionally, more than one-third of those reaching one full year of smoking abstinence eventually relapse [7].
Precision medicine strategies for smoking cessation and relapse prevention could increase the rates of successful quit attempts. To appropriately inform the development and implementation of precision medicine interventions, the risk factors associated with cessation failure or relapse must be identified. Several smoking phenotypes (e.g. nicotine dependence, persistent smoking, and severity of withdrawal symptoms) were shown to have substantial heritability (23 to 70%) in twin and family studies [8]. Due to the significant role that genetic susceptibility plays in smoking behavior and cessation, studies to identify which specific genetic variants are associated with the likelihood of quitting smoking hold promise for advancing precision medicine approaches.
Nicotine, the main psychoactive substance in tobacco [9], binds to nicotinic acetylcholine receptors which activates the receptors stimulating dopamine release [10]. Dopamine actuates the mesolimbic dopamine pathway contributing to neurological reward [11] and conditioning drug-seeking behavior [12]. Dopaminergic signals are transmitted through dopamine receptor binding. Thus, the five dopamine receptor genes: DRD1, DRD2, DRD3, DRD4, and DRD5, which encode the D1, D2, D3, D4, and D5 receptors, respectively [12], are biologically plausible candidate genes for studies related to smoking or nicotine addiction. In addition, dopamine is metabolized into inactive and active metabolites, a process that is partially responsible for regulating dopamine levels. Thus, the three genes that encode enzymes responsible for dopamine metabolism, COMT, DBH, and MAOA [13], are additional candidate genes for smoking addiction or cessation studies.
Within the existing body of evidence relating to genetic studies of smoking cessation, DRD2 and DRD4 have been the predominant genes studied among the five dopamine receptor genes while studies including dopamine metabolism genetic variants have focused on COMT and DBH. Of these four genes, the most researched is DRD2 and for the most part studies have focused on DRD2/ANKK1 single nucleotide polymorphism (SNP) rs1800497, which is frequently referred to as the Taq1A variant. In relation to DRD4, studies of smoking behavior have mainly examined a 48 base pair variable number tandem repeat (VNTR) within this gene [14, 15]. High throughput SNP genotyping arrays provide SNP genotype data for thousands of single base pair polymorphisms [16], whereas genotyping VTNR polymorphisms is a more complex and computationally intensive process [17]. Thus, genetic research, such as genome wide association studies, have focused on utilizing SNP genotyping. For this reason, the aim of the present study was to synthesize the existing evidence related to associations of the Taq1A SNP and other less studied SNPs within DRD2, COMT, and DBH with smoking cessation outcomes. In doing so, we aimed to consolidate the evidence on the associations of SNPs within these genes and identify areas or gaps for future investigation. Additionally, in synthesizing the evidence, we have also given special consideration to differences in associations by ancestry and biological sex where feasible.
Methods
The independent variable of this systematic review was defined as SNPs within dopamine receptor and metabolism genes. The specific genes of focus were DRD2, COMT, and DBH due to a dearth of studies examining SNPs within other dopamine receptor or metabolism genes. The dependent variable was smoking cessation measured as abstinence from smoking at a study endpoint or observational timepoint for prospective studies, former versus current smoking status for cross-sectional studies, and time to smoking cessation for survival analyses.
Inclusion Criteria
To be eligible for inclusion, a manuscript had to report on a human study, be published in English, and report on the association between one or more SNPs within DRD2, COMT, or DBH and a smoking cessation outcome. Randomized-controlled trials, non-randomized trials, open label trials, cohort studies, case-control studies, and cross-sectional studies were all eligible for inclusion.
Exclusion Criteria
Studies were excluded if they only used a genetic risk score (i.e., no associations reported for individual SNPs) as the independent variable or did not have a smoking cessation outcome. Also excluded were news stories, commentaries, letters to the editor, review papers, heritability or linkage studies (e.g. twin and family studies), studies with < 1-month follow-up, and studies reporting results for novel SNPs or haplotypes. Studies reporting novel SNPs, defined as SNPs reported in only one citation, were excluded because conducting a meta-analysis across studies was not feasible [18,19,20,21, 22•, 23•, 24]. However, we report these novel SNPs in Supplementary table 1. Published reports that used the same data from the same study population and tested the same SNPs as another published report were also excluded. One of these excluded studies utilized data from a placebo-controlled bupropion trial [25]; however, this trial data was pooled with a second bupropion trial for use in another study [26] and the latter study was included in this review. Two other studies utilized the same study population to test associations between Taq1A and cessation [27]; the study with a primary aim of testing for the Taq1A interaction with a serotonin genetic variant was excluded [28]. Another three studies were excluded for using the same study population as another study. Two studies that used Patch II trial data to test associations between COMT SNP rs4680 [29] and ANKK1/DRD2 SNP rs1800497 [30] and cessation, along with a third study using Patch-in-Practice trial data to test associations between rs4680 and time to relapse [31] were excluded because a fourth citation included the associations between these SNPs and abstinence from smoking in both the Patch II and Patch-in-Practice trials [32]. Because this latter study did not present data stratified by biological sex, results reported in two additional manuscripts from the Patch-in-Practice trial [31] and the Patch II trial [33] were used for the results stratified by biological sex. The combined results from these trials were used in the primary systematic review synthesis and the studies stratifying results by sex were used for synthesis of results by biological sex.
Literature Search
We searched the PubMed and Scopus databases for evidence of associations between SNPs within dopaminergic genes and smoking cessation (Fig. 1). The original search was conducted on February 7, 2020 for journal articles published from January 1, 1990 through February 7, 2020, updated searches were conducted periodically to identify articles meeting search criteria published from February 7, 2020 through May 22, 2023. The PubMed and Scopus searches retrieved a total of 917 unique articles (removing duplicates) for evaluation of exclusion/inclusion criteria. (Search terms are detailed in Appendix 1).
Article Screening and Data Extraction
Of the 917 articles, 861 were excluded after abstract screening (Fig. 1). After full text review of the remaining 56 articles, 32 citations were excluded. By checking all the references cited in the originally identified records, we identified an additional 6 articles that met inclusion criteria for a total of 30 included articles. For each included article, data were extracted for study design, sample size, participant ancestry, intervention protocols, SNP associations with smoking cessation, and results of any pharmacogenomic analysis.
Statistical Analysis
Associations of SNPs with smoking cessation were organized by gene, study design, ancestry, biological sex, and pharmacotherapy. Summary measures were obtained using the %METAANAL SAS macro, which produced the Laird and DerSimonian fixed effect estimators and tested for between-study heterogeneity [34, 35]. Random effects pooling was assumed weighting the studies proportionate to the inverse of the study variance plus the between-study variances.
Results
There were 30 articles included in the final meta-analysis for the systematic review. These articles reported results from 32 unique studies: 12 randomized trials, 5 open label trials, 7 cohort studies, 4 case-control studies, and 4 cross-sectional studies (Table 1). The sample sizes of the 12 randomized trials ranged from 76 to 2,374. Eleven of these trials comprised populations that were entirely or predominantly (85 - 98.9%) of European ancestry [26, 32, 33, 36,37,38,39,40,41,42,43,44,45,46] and one was of people of Chinese ancestry that was also 93.6% male [47]. Of the five open label trials three were of European populations comprised of 371 to 804 people [27, 32, 43], one comprised of 225 Korean men [42], and the fifth stratified results by ancestry for the 178 women of European ancestry and 112 women of African ancestry [40] included in the trial. All seven cohort studies were of people of European ancestry with sample sizes ranging from 419 to 10,017 [48,49,50,51,52, 53••]. Two of the four case-control studies stratified results by ancestry: one stratified by women of European ancestry (n = 541) and women of African ancestry (n = 241) [40], whereas the other stratified by Mexican ancestry (n = 59) and African ancestry (n = 72) [54]. Two other case-control studies studied 881 people [55] and 283 people [56] of European ancestry. The four cross-sectional studies were all conducted in people of Asian ancestry, three were studies of Japanese people [57,58,59] and one was a study of Chinese men [60]; these ranged in size from 96 to 359 people.
There was sufficient evidence to perform meta-analyses of the associations between four SNPs and smoking cessation. Two SNPs, DRD2/ANKK1 SNP rs1800497 G>A and DRD2 SNP rs6277 G>A, are associated with D2 dopamine receptor density [61, 62], and two SNPs, COMT SNP rs4680 and DBH SNP rs77905, are within dopamine metabolism genes. We reported the results of the meta-analyses by SNP and ancestry, and by sex and pharmacotherapy where feasible.
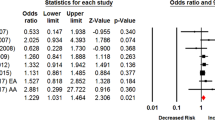
DRD2/ANKK1 SNP rs1800497 (Fig. 2)
Associations With Smoking Cessation Among European Ancestral Populations
The meta-analysis of 16 studies of people of European ancestry) [26, 27, 32, 33, 37,38,39,40,41,42,43,44,45,46, 52, 53••, 55, 56] showed those with at least one copy of the minor allele of SNP rs1800497 (commonly annotated as the A1 allele) had lower odds of smoking cessation [Summary OR = 0.88, 95% CI 0.82 - 0.95] compared to those with homozygous common allele genotypes (commonly annotated as A2/A2 genotypes) (Fig. 2, part a). In seven of the 16 studies, at least some of the participants received a pharmaceutical intervention; however, in these studies the smoking cessation outcome was assessed at least 3 months and up to 3 years after the intervention ended.
For four of the trials, it was feasible to stratify the associations with abstinence at end of treatment by intervention arm (Fig. 2, part b). In the two studies with a placebo arm [26, 32], those with at least one copy of the minor allele had higher odds of smoking cessation at end-of-treatment (10-12 weeks post-cessation) [Summary OR = 1.37, 95% CI 0.70 – 2.70], but the association was not statistically significant. Although the test for heterogeneity between these two studies was not statistically significant (p = 0.09), the findings between the two studies were inconsistent [David et al. (2011) OR = 0.96, 95% CI 0.54 – 1.72 [32], whereas David et al. (2007) OR = 1.92, 95% CI 1.13 – 3.28 [26]]. There was less evidence of an association between SNP rs1800497 genotype and end-of-treatment abstinence among recipients of nicotine replacement therapy (NRT) [Summary OR = 1.11, 95% CI 0.79 – 1.54 (n = 2 studies)] [32] or bupropion [OR = 0.91, 95% CI 0.59 – 1.40] (n = 1 study)] [26].
Associations With Smoking Cessation Among Non-European Ancestral Populations (Fig. 2, Part C)
Korean men with the A1 allele who received bupropion in an open label trial had significantly lower odds of smoking cessation than men without the minor allele [OR = 0.56, 95% CI 0.32 - 0.99 (n = 1 study)] [42]. In studies with no pharmacotherapy intervention, Japanese study participants with the A1 allele had increased odds of smoking cessation but the association was not statistically significant [Summary OR = 1.17, 95% CI 0.61 – 2.24 (n = 3 studies)] [57,58,59]. Some heterogeneity between these three cross-sectional studies of Japanese people was indicated (test for heterogeneity p = 0.07) because one study [58] observed an association between the A1 allele and the likelihood of former versus current smoker status that was in the opposite direction of the other two studies. Synthesis of the two studies with concordant results showed a strong association between the A1 allele and increased likelihood of being a former smoker [Summary OR = 1.64, 95% CI 1.09 – 2.49] [57, 59]. In contrast, the third study found those with the A1 allele had lower, though not statistically significant, odds of being a former smoker [OR = 0.65, 95% CI 0.32 – 1.29] [58]. One study evaluated SNP rs1800497 associations with smoking cessation among people of African or Mexican ancestry finding no genetic association among either population [African ancestry OR = 1.03, 95% CI 0.40 – 2.66, Mexican ancestry OR = 1.03, 95% CI 0.31 – 3.43] [54].
Stratification of SNP rs1800497 Associations by Biological Sex (Fig. 3)
The association between SNP rs1800497 and smoking cessation may differ by biological sex. Women with the A1 allele had lower odds of smoking cessation [Summary OR = 0.91, 95% CI 0.85 - 0.97, n = 9 studies] [27, 31, 33, 45, 46, 57, 58]. The association was similar when the results were limited to populations of women with European ancestry [Summary OR = 0.88, 95% CI 0.80 – 0.98, n = 7 studies] [27, 31, 33, 45, 46]. In contrast, men with the A1 allele had increased odds of smoking cessation [Summary OR = 1.16, 95% CI 0.87 - 1.55, n = 7 studies], [27, 31, 33, 45, 57,58,59] but the association was not statistically significant. No association was observed when considering only studies of men of European ancestry [Summary OR = 1.06, 95% CI 0.85 – 1.33] [27, 31, 33, 45].
DRD2 SNP rs6277 (Fig. 4)
Five studies, all of people of European ancestry, reported associations between DRD2 SNP rs6277 and smoking cessation. Among people who did not receive a pharmaceutical intervention for cessation no difference in odds of cessation between rs6277 genotypes was found [Summary OR = 0.99, 95% CI 0.91 - 1.08, n = 5 studies] [32, 44, 53, 55]. Among those who received NRT, the minor allele was associated with increased odds of cessation [Summary OR = 1.43, 95% CI 1.06 – 1.92, n = 3 studies] [32, 43]. In a study that analyzed the combined bupropion and placebo arms, no association was observed between rs6277 genotype and smoking cessation [OR = 1.05, 95% CI 0.64 - 1.72] [43].
COMT SNP rs4680
Associations Among People of European Ancestry (Fig. 5, Part A)
Among people of European ancestry, no statistically significant association between the minor allele of COMT SNP rs4680 and the age of smoking cessation [HR = 0.97, 95% CI 0.83 – 1.12, n = 1 study] [48] or the likelihood of smoking cessation [Summary OR = 0.95, 95% CI 0.83 - 1.09, n = 8 studies] [32, 40, 46, 49,50,51] was observed. Heterogeneity across the eight studies of smoking cessation was statistically significant (p = 0.004). After removing the five studies with all-female study populations from the meta-analysis [32, 49, 51, 53], we no longer observed significant heterogeneity across the remaining studies (p = 0.31) (the all-female studies were analyzed separately as described below). Among the studies of both men and women that remained, those with the minor allele of SNP rs4680 had significantly lower odds of smoking cessation compared to those with homozygous common allele genotypes [Summary OR = 0.73 95% CI 0.57 - 0.93, n = 3 studies].
The association between the minor allele of the SNP rs4680 and smoking cessation among recipients of NRT was in the opposite, more favorable, direction [Summary OR = 1.61, 95% CI 1.11 - 2.35, n = 3 studies] [32, 40] to that noted above. In a single study among people of European ancestry receiving bupropion, no statistically significant association between SNP rs4680 genotype and end-of-treatment abstinence was observed [OR = 1.09, 95% CI 0.86 - 1.38, n = 1 study] [41] (Fig. 5, part b).
Associations Among People of Non-European Ancestry (Fig. 5, Part C)
Five studies of non-European ancestral populations reported associations between SNP rs4680 and smoking cessation: two studies of women of African ancestry [40], one study of Korean men [42], and two studies of Chinese people who were predominantly male [47, 60]. A non-statistically significant association that was close to the null value of 1.0 was observed among women of African ancestry who did not receive a pharmaceutical intervention [OR = 1.08, 95% CI 0.51 – 2.29, n = 1 study], whereas among women receiving NRT, those with the minor allele were nearly two times more likely to quit smoking at end of treatment [OR = 2.02, 95% CI 0.84 – 4.85, n = 1 study] but the association was not statistically significant [40]. Among people of Chinese ancestry, those with the minor allele of SNP rs4680 had higher, but not statistically significant, odds of smoking cessation than those with homozygous common allele genotypes [Summary OR = 1.48, 95% CI 0.94 - 2.32, n = 2 studies]. The combined study population of these two studies was 96.9% male. In contrast, Korean men with the minor allele of SNP rs4680 receiving bupropion in an open label trial had significantly lower odds of smoking cessation [OR = 0.49, 95% CI 0.28 - 0.84, n = 1 study].
Stratification of SNP rs4680 Associations by Biological Sex (Fig. 6)
To assess the association among women, we evaluated the association of SNP rs4680 with smoking cessation using results from the six all-female studies and from one study with results available stratified by biological sex. Among the seven studies without a pharmaceutical intervention, no association between SNP rs4680 and cessation was observed [Summary OR = 0.98, 95% CI 0.86 - 1.12, n = 7 studies] [40, 50, 51], though there was significant heterogeneity between these studies (p = 0.02). After removing the study by Omidvar et al [51], a study of older people aged ≥55 years at recruitment and followed for 12 years, the heterogeneity between studies was no longer statistically significant (p = 0.09) but still the association between rs4680 genotype and smoking cessation was null [Summary OR = 1.02, 95% CI 0.91 - 1.14, n = 6 studies]. Among women receiving an NRT intervention, those with the minor allele were significantly more likely to quit smoking than those without the minor allele [Summary OR = 1.95, 95% CI 1.07 – 3.57, n = 2 studies] [40].
In addition to the results previously presented from studies of male or predominantly (97%) male populations of Asian ancestry, it was feasible to obtain SNP rs4680 genotype associations among men from two studies of European ancestry populations. Among untreated, older men (55+ years of age at recruitment) of European ancestry, those with the minor allele were less likely to quit smoking during the 12-years of follow-up [Summary OR = 0.74, 95% CI 0.53 – 1.02, n = 1 study] [51]. No association between SNP rs4680 genotype and abstinence was observed among men of European ancestry receiving NRT in an open label trial [Summary OR = 1.08, 95% CI 0.53 – 2.18, n = 1 study] [40].
DBH SNP rs77905 (Fig. 7)
Three studies, all of people of European ancestry, reporting associations between SNP rs77905 and smoking cessation were included in the review. Among those who did not receive a pharmaceutical intervention, the minor allele was associated with increased odds of smoking cessation [Summary OR = 1.79, 95% CI 1.17 - 2.73, n = 2 studies] [32, 37]; however, no association between SNP rs77905 and smoking cessation was observed for those receiving NRT [Summary OR = 0.95, 95% CI 0.66 - 1.36, n = 2 studies] [32].
Additional GWAS Findings for DRD2 and DBH
Three large GWAS (N = 547,219 to 3,383,199) were identified in the literature but were not included in the meta-analysis because they report novel variants (variants not reported in another citation) within DRD2 or DBH. For genome-wide significant associations with smoking cessation, Xu et al. [22•] identified DRD2 SNP rs113344912 C<T (p-value = 2.9 x 10-11); Liu et al. [24] identified DBH SNP rs1611124 T<C (p-value = 5.26 x 10-9); and Saunders et al. [23•] identified DRD2 SNPs rs2734839 (p-value = 6.66 x 10-16), rs2734837 (p-value = 5.55 x 10 -16), rs2734338 (p-value = 8.87 x 10-15), and DBH SNP rs1108581 (p-value = 6.67 x 10-25).
Discussion
This systematic review synthesized the available evidence for associations between smoking cessation and DRD2/ANKK1 SNP rs1800497 (Taq1A), DRD2 SNP rs6277, COMT SNP rs4680, and DBH SNP rs77905. The majority of the evidence was from studies of people with European ancestry and showed that the minor alleles of Taq1A and rs4680 were associated with lower odds of smoking cessation, whereas the evidence indicated that the minor allele of rs77905 was associated with increased odds of smoking cessation and that there was no evidence of an association between rs6277 and smoking cessation. The existing evidence suggests that the direction of the associations for Taq1A and rs4680 may differ by ancestry and biological sex. No studies examined associations between rs6277 or rs77905 among non-European ancestral populations or by biological sex, thus, it was not feasible to assess whether associations for these SNPs followed a similar pattern in relation to ancestry and sex. Lastly, some of the genetic associations differed between those who received no pharmacotherapy, bupropion, and NRT.
In addition to the meta-analysis, we cite three large GWAS [22•, 23•, 24] for which it was not feasible to include the findings in the meta-analysis because the identified SNPs were not found in another citation. These GWAS replicated findings in terms of genes (DRD2 and DBH) identified but did not replicate specific genetic variants within these genes. This demonstrates that GWAS have a primary and crucial role for identifying genes and gene regions associated with phenotypes, but that the whole body of evidence, including candidate gene and single SNP studies, should be considered to fully understand the genetic influences for complex disease. While the candidate gene approach has come under scrutiny for potentially spurious associations that do not replicate, replication can be a concern between GWAS studies, and some genetic associations with complex diseases may not be detected through GWAS, despite very large sample sizes, potentially due to complex environmental interactions. Despite the criticism, candidate gene studies can play an important role in uncovering the functional causal pathways and interactions through which genes influence more complex phenotypes [63]. Thus, although most of the studies cited in this review utilized a candidate gene or single SNP approach to examine genetic associations, synthesizing the findings from these studies is important for understanding the current knowledge and identifying scientific gaps related to dopaminergic genes and smoking cessation. However, as the evidence synthesized across four specific SNPs within genes influencing the dopaminergic pathway is discussed below, the convergence of candidate gene and GWAS should be noted and considered [64].
Genetic Associations by Ancestry and Sex
DRD2/ANKK1 SNP rs1800497 (Taq1A)
The Taq1A (A1) allele has been associated with reduced D2 dopamine receptor density in postmortem human brain studies, and researchers hypothesize that the A1 allele’s association with substance use may result from compensatory behavior due to deficient dopaminergic responses [65]. The present review showed that the A1 allele was associated with lower odds of cessation in people of European ancestry, but that this association may not be consistent by biological sex and ancestry. When considering study results among females only, we found the A1 allele was associated with lower likelihood of having quit smoking. In contrast, among male populations, the association was in the opposite direction but was not statistically significant. Examination of studies of Japanese males suggested that Japanese men with the A1 allele may be more likely to have quit smoking compared to those without the A1 allele; however, significant heterogeneity across studies makes it difficult to make strong inferences.
Two of the studies of Japanese males found that the A1 allele was associated with increased odds of being a former smoker whereas a third study reported the inverse association. Heterogeneity was detected only in the results for male participants. Two of the discordant studies both recruited the study participants from first-visit patients to the Aichi Cancer Center and did so within temporal proximity. Despite this similarity in source population, the prevalence of smoking status among the males participating in these two studies differed in terms of current, former, and never smokers. Among the male participants in Hamajima et al [57], 48%, 31%, and 21% were current, former, or never smokers, respectively. In contrast, in Yoshida et al [58], the proportion of male participants reporting current, former and never smoker status was evenly distributed at 34%, 33%, and 33%, respectively. The disparity in prevalence, despite the same source population, suggests potential systematic differences, potentially due to different inclusion criteria. For example, Hamajima et al. recruited first-visit patients to the outpatient hospital inclusive of cancer patients, whereas Yoshida et al. recruited patients visiting the outpatient clinic for either cancer screening or treatment of Helicobacter pylori infection. The prevalence of smoking can affect the relative genetic heritability of smoking within a specific population [8], which could be a factor that partially explains the disparate study results.
Two previous meta-analyses reported associations between Taq1A and smoking cessation. These studies observed a significant association among people of European ancestry but found no association between Taq1A and cessation among people of Asian ancestry [66, 67]. Both prior studies also reported significant heterogeneity in studies reporting results for people with Asian ancestry. Neither of these prior studies considered pharmacotherapy cessation interventions or biological sex, and other potential sources of bias were present. For example, the results of one prior review included the results of a study of Korean schizophrenia patients with the results of two studies of Japanese people; the inclusion of a population with comorbid mental illness may have biased the smoking outcome [67]. Additional bias from inclusion of the aforementioned study of schizophrenia patients could also have been introduced because this study excluded former smokers, thus, the study compared current smokers to never smokers which does not effectively measure smoking cessation [68]. The second previous review synthesized the results of three cross-sectional studies of Japanese people with a study of short-term smoking cessation (4 – 10 weeks post quit) among Korean male smokers who all received bupropion. While our present review included these four studies, we considered the study of Korean men receiving bupropion therapy separately due to potential differences in genetic associations by ancestry and pharmacotherapy [69].
It is difficult to validate the findings in Japanese people due to heterogeneity across the few existing studies. However, genetic associations can differ by ancestry and the discordance we observed between the associations for Japanese and European populations in the present review could be partially explained by different minor allele frequencies (MAF). Based on data from the 1000 Genomes Project, the A1 allele has a MAF of 0.2020 among Americans of European decent (CEU) whereas the MAF is 0.3894 among people with Japanese ancestry (JPT) [70]. Additionally, among the JPT population, the Taq1A SNP is located within a large haplotype block [70] that is not observed within the CEU population, thus, Taq1A may have high linkage (correlation) with other functional SNPs among people with Japanese ancestry.
Though genetic differences by ancestry and sex are biologically plausible, other factors complicate associations across ancestry and sex. Sex differences in smoking prevalence are greater in Asian countries than in the United States. In 2019, the age standardized smoking prevalence among Japanese women and men, respectively, was 10.2%, and 33.4%, whereas the prevalence was 15.3% among U.S. women and 19.9% among U.S men [71•]. This may be explained by sex differences in societal attitudes toward smoking in Japan where smoking is more likely to be considered an acceptable part of social etiquette for men than women [72]. Studies have also shown that Japanese male smokers inhale cigarette smoke moderately in comparison to American men with European ancestry, but that the strength of inhalation does not differ between female smokers of Japanese or European ancestry [73]. Adding further complexity, interest in quitting smoking may be lower among people who smoke in East Asia than in the United States or Europe [5, 74,75,76]. These phenomenon indicate that environmental and cultural factors may moderate genetic risk and partially explain differences by ancestry and sex.
COMT SNP rs4680
The minor allele of COMT SNP rs4680 is functionally associated with lower catechol-o-methyltransferase (COMT) enzyme activity. Lower COMT slows dopamine metabolism resulting in increased dopamine levels in the prefrontal cortex [77]. COMT enzyme activity differs by sex, potentially because estrogen downregulates activity [77]. Our findings suggest that SNP rs4680 associations with smoking cessation may also differ by sex. Among females only, the minor allele of SNP rs4680 was not associated with likelihood of smoking cessation, whereas, within studies of both males and females, people with the allele had lower odds of quitting smoking. Evaluation of associations specifically among males was feasible from two studies of people of European ancestry, one study of older males 55 years of age or older at recruitment and one study in which all received NRT. Though limited, these studies showed the minor allele of rs4680 may be associated with reduced odds of cessation in males, but that NRT may attenuate the association. Two studies of Chinese men showed an inverse association with the minor allele associated with smoking abstinence. Though these results indicate potential genetic differences by ancestry and sex, more research is necessary.
Though the findings showed that the minor allele of rs4680 was not associated with odds of cessation among females only, we observed significant heterogeneity across studies. One of the possible sources of heterogeneity stemmed from a study of older women (55+ years of age at recruitment). In this study, women had significantly lower odds of having quit smoking over the 12-years follow-up. We no longer observed significant heterogeneity across the studies of all-female populations after removing this study from the meta-analysis. Because estrogen downregulates COMT activity [77], the genetic associations for rs4680 may be different between older and younger women as hormone levels change between pre-, peri-, and post-menopause. This hypothesis is supported by findings showing the association between rs4680 and the degree of smoking relapse was stronger among postmenopausal women [78•].
Genetic Associations by Pharmacotherapy
Though based on a limited number of randomized controlled or open label trials, the findings of the present review indicate that some genetic associations may differ at end of treatment between recipients of pharmacotherapy compared with non-recipients. While the minor allele of COMT SNP rs4680 was generally associated with lower odds of abstinence, the inverse association was observed among recipients of NRT suggesting NRT may be a more effective smoking cessation aid for people with this minor allele. In limited evidence from only two studies of women and one of men, this association with increased abstinence among recipients of NRT appeared to be concentrated among women, but clearly more evidence is needed to clarify this question.
Differences in genetic associations by pharmacotherapy were also observed for DRD2 SNP rs6277 and DBH SNP rs77905. No association between genotype of rs6277 and likelihood of smoking cessation was observed in untreated smokers; however, among recipients of NRT, the minor allele was associated with significantly increased odds of smoking cessation at end of treatment. Conversely, the results showed that the minor allele of rs77905 was associated with increased odds of abstinence in untreated smokers, but the association was not observed at end of treatment among recipients of NRT. The evidence-base is clearly not sufficient to draw firm conclusions, but these findings suggest that NRT may be more effective for those with the minor allele of rs6277 or for those with the common allele genotype of rs77905. This question, with its clinical implications for precision approaches, clearly merits further research.
The results concerning DRD2 SNP Taq1A genotype associations stratified by NRT, bupropion, and placebo arms are difficult to interpret due to the limited number of studies and the somewhat disparate results in the placebo arms of the studies. However, both Taq1A and rs6277 have been associated with the density of D2 dopamine receptors [61, 62, 79] indicating both SNPs may influence the same biological mechanism. Additional research could uncover the potential for genetically informed precision medicine smoking cessation interventions based on dopamine receptor and other dopamine-related genes.
Furthermore, SNPs within non-dopaminergic genes are also important to consider. For example, both the nicotinic acetylcholine receptor (nAChR) and nicotine metabolism pathways play important roles in smoking behavior and nicotine dependence; the genetic evidence in relation to smoking cessation within these pathways has been previously reviewed and documented [80•, 81•]. Precision interventions informed by single SNPs have been somewhat successful, such as a trial showing that nAChR variant CHRNA5 SNP rs16969968 predicts response to the smoking cessation pharmacotherapy varenicline [82•]. Nonetheless, the complexity of the results from the four SNPs presented in the present review, combined with the strong associations observed for SNPs within genes influencing other important pathways, suggests composite genetic risk models, such as those using polygenic risk scores, could more effectively combine the risk associated with multiple genetic variants. Precision medicine approaches informed by polygenic risk scores hold promise but research to further this line of inquiry is still in its beginning stages [83•].
Conclusion
Most studies of associations between SNPs within dopaminergic genes and smoking cessation have reported results for DRD2/ANKK1 SNP rs1800497 (Taq1A) and/or COMT SNP rs4680; synthesis of these studies found the minor alleles of these SNPs are associated with lower odds of abstinence in people of European ancestry. Evidence suggests these associations may differ by biological sex and among people of non-European ancestry. In relation to DRD2 SNP rs6277 and DBH SNP rs77905, studies were sparse but suggested that these SNPs could be indicators of pharmacotherapy cessation intervention efficacy. Additional studies are needed to enable stronger inferences to be made. If these findings were found to be genuine, due to the complexity in the direction of the genetic associations by pharmacotherapy findings across individual SNPs, polygenic risk models that combine the risk from multiple variants may be better suited to inform precision medicine approaches. Future research should aim to further the understanding of genetic risk within genes influencing dopaminergic function well beyond the four SNPs for which evidence has been synthesized. This line of inquiry is important because of the key role the dopamine pathway plays in addiction. When planning and conducting future studies, the findings of this review highlight the importance of considering differences by ancestry and sex.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
National Center for Chronic Disease Prevention and Health Promotion. The health consequences of smoking: 50 years of progress: a report of the surgeon general. Atlanta, GA: Centers for Disease Control and Prevention; 2014.
. Cornelius ME, Loretan CG, Jamal A, Davis Lynn BC, Mayer M, Alcantara IC, et al. Tobacco product use among adults - United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(18):475–83. https://doi.org/10.15585/mmwr.mm7218a1. Recent CDC statistics on tobacco use in U.S.
Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco product use among military veterans - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7–12. https://doi.org/10.15585/mmwr.mm6701a2.
United States Public Health Service Office of the Surgeon General, National Center for Chronic Disease Prevention and Health Promotion; Office on Smoking and Health. Smoking cessation: a report of the surgeon general. Washington (DC): US Department of Health and Human Services; 2020.
Creamer MR, Wang TW, Babb S, Cullen KA, Day H, Willis G, et al. Tobacco product use and cessation indicators among adults - United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(45):1013–9. https://doi.org/10.15585/mmwr.mm6845a2.
Babb SMA, Schauer G, Asman K, Jamal A. Quitting smoking among adults — United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2017;65:1457–64. https://doi.org/10.15585/mmwr.mm6552a1.
García-Rodríguez O, Secades-Villa R, Flórez-Salamanca L, Okuda M, Liu S-M, Blanco C. Probability and predictors of relapse to smoking: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2013;132(3):479–85. https://doi.org/10.1016/j.drugalcdep.2013.03.008.
Munafo MR, Johnstone EC. Genes and cigarette smoking. Addiction. 2008;103(6):893–904. https://doi.org/10.1111/j.1360-0443.2007.02071.x.
National Center for Chronic Disease Prevention and Health Promotion; Office on Smoking and Health. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the surgeon general. Atlanta, GA: Centers for Disease Control and Prevention; 2010.
Littleton J. Receptor regulation as a unitary mechanism for drug tolerance and physical dependence - not quite as simple as it seemed. Addiction. 2001;96(1):87–101. https://doi.org/10.1046/j.1360-0443.2001.961877.x.
Trifilieff P, Martinez D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacol. 2014;76(Pt B):498–509. https://doi.org/10.1016/j.neuropharm.2013.06.031.
Foll BL, Gallo A, Strat YL, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol. 2009;20(1):1–17. https://doi.org/10.1097/fbp.0b013e3283242f05.
Opmeer EM, Kortekaas R, Aleman A. Depression and the role of genes involved in dopamine metabolism and signalling. Prog Neurobiol. 2010;92(2):112–33. https://doi.org/10.1016/j.pneurobio.2010.06.003.
David SP, Munafò MR, Murphy MFG, Proctor M, Walton RT, Johnstone EC. Genetic variation in the dopamine D4 receptor (DRD4) gene and smoking cessation: Follow-up of a randomised clinical trial of transdermal nicotine patch. Pharmacogenomics J. 2008;8(2):122–8. https://doi.org/10.1038/sj.tpj.6500447.
Lerman C, Caporaso N, Main D, Audrain J, Boyd NR, Bowman ED, et al. Depression and self-medication with nicotine: the modifying influence of the dopamine D4 receptor gene. Health Psychol. 1998;17(1):56–62. https://doi.org/10.1037/0278-6133.17.1.56.
Brookes KJ. The VNTR in complex disorders: The forgotten polymorphisms? A functional way forward? Genomics. 2013;101(5):273–81. https://doi.org/10.1016/j.ygeno.2013.03.003.
Bakhtiari M, Park J, Ding Y-C, Shleizer-Burko S, Neuhausen SL, Halldórsson BV, et al. Variable number tandem repeats mediate the expression of proximal genes. Nat Commun. 2021;12(1):2075. https://doi.org/10.1038/s41467-021-22206-z.
Breitling LP, Müller H, Illig T, Rujescu D, Winterer G, Dahmen N, et al. Dopamine-related genes and spontaneous smoking cessation in ever-heavy smokers. Pharmacogenomics. 2011;12(8):1099–106. https://doi.org/10.2217/pgs.11.74.
Hirvonen K, Korhonen T, Salomaa V, Männistö S, Kaprio J. Association of the DBH Polymorphism rs3025343 With Smoking Cessation in a Large Population-Based Sample. Nicotine Tob Res. 2017;19(9):1112–5. https://doi.org/10.1093/ntr/ntx066.
Robinson JD, Lam CY, Minnix JA, Wetter DW, Tomlinson GE, Minna JD, et al. The DRD2 TaqI-B polymorphism and its relationship to smoking abstinence and withdrawal symptoms. Pharmacogenomics J. 2007;7(4):266–74. https://doi.org/10.1038/sj.tpj.6500427.
Leventhal AM, Lee W, Bergen AW, Swan GE, Tyndale RF, Lerman C, et al. Nicotine dependence as a moderator of genetic influences on smoking cessation treatment outcome. Drug Alcohol Depend. 2014;138:109–17. https://doi.org/10.1016/j.drugalcdep.2014.02.016.
. Xu K, Li B, McGinnis KA, Vickers-Smith R, Dao C, Sun N, et al. Genome-wide association study of smoking trajectory and meta-analysis of smoking status in 842,000 individuals. Nat Commun. 2020;11(1):5302. https://doi.org/10.1038/s41467-020-18489-3. Recent GWAS on smoking phentoypes.
. Saunders GRB, Wang X, Chen F, Jang S-K, Liu M, Wang C, et al. Genetic diversity fuels gene discovery for tobacco and alcohol use. Nature. 2022;612(7941):720–4. https://doi.org/10.1038/s41586-022-05477-4. Most recent GWAS on smoking phenotypes.
Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51(2):237–44. https://doi.org/10.1038/s41588-018-0307-5.
Lerman C, Wileyto EP, Audrain J, Pinto A, Kucharski S, Niaura R, et al. Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychol. 2003;22(5):541–8. https://doi.org/10.1037/0278-6133.22.5.541.
David SP, Strong DR, Munafò MR, Brown RA, Lloyd-Richardson EE, Wileyto PE, et al. Bupropion efficacy for smoking cessation is influenced by the DRD2 Taq1A polymorphism: Analysis of pooled data from two clinical trials. Nicotine Tob Res. 2007;9(12):1251–7. https://doi.org/10.1080/14622200701705027.
Swan GE, Valdes AM, Ring HZ, Khroyan TV, Jack LM, Ton CC, et al. Dopamine receptor DRD2 genotype and smoking cessation outcome following treatment with bupropion SR. Pharmacogenomics J. 2005;5(1):21–9. https://doi.org/10.1038/sj.tpj.6500281.
Swan GE, Jack LM, Valdes AM, Ring HZ, Ton CC, Curry SJ, et al. Joint effect of dopaminergic genes on likelihood of smoking following treatment with bupropion SR. Health Psychol. 2007;26(3):361–8. https://doi.org/10.1037/0278-6133.26.3.361.
Johnstone EC, Elliot KM, David SP, Murphy MF, Walton RT, Munafò MR. Association of COMT Val108/158Met genotype with smoking cessation in a nicotine replacement therapy randomized trial. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1065–9. https://doi.org/10.1158/1055-9965.epi-06-0936.
Johnstone EC, Yudkin PL, Hey K, Roberts SJ, Welch SJ, Murphy MF, et al. Genetic variation in dopaminergic pathways and short-term effectiveness of the nicotine patch. Pharmacogenetics. 2004;14(2):83–90. https://doi.org/10.1097/00008571-200402000-00002.
Munafò MR, Johnstone EC, Murphy MFG, Aveyard P. Lack of association of DRD2 rs1800497 (Taq1A) polymorphism with smoking cessation in a nicotine replacement therapy randomized trial. Nicotine Tob Res. 2009;11(4):404–7. https://doi.org/10.1093/ntr/ntp007.
David SP, Johnstone EC, Churchman M, Aveyard P, Murphy MFG, Munafò MR. Pharmacogenetics of smoking cessation in general practice: Results from the Patch II and Patch in Practice trials. Nicotine Tob Res. 2011;13(3):157–67. https://doi.org/10.1093/ntr/ntq246.
Yudkin P, Munafo M, Hey K, Roberts S, Welch S, Johnstone E, et al. Effectiveness of nicotine patches in relation to genotype in women versus men: randomised controlled trial. BMJ. 2004;328(7446):989–90. https://doi.org/10.1136/bmj.38050.674826.ae.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. https://doi.org/10.1016/0197-2456(86)90046-2.
DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–45. https://doi.org/10.1016/j.cct.2015.09.002.
Wilcox CS, Noble EP, Oskooilar N. ANKK1/DRD2 locus variants are associated with rimonabant efficacy in aiding smoking cessation: pilot data. J Investig Med. 2011;59(8):1280–3. https://doi.org/10.2130/jim.0b013e31823581fa.
Breitling LP, Twardella D, Hoffmann MM, Witt SH, Treutlein J, Brenner H. Prospective association of dopamine-related polymorphisms with smoking cessation in general care. Pharmacogenomics. 2010;11(4):527–36. https://doi.org/10.2217/pgs.10.1.
Berlin I, Covey LS, Jiang H, Hamer D. Lack of effect of D2 dopamine receptor TaqI A polymorphism on smoking cessation. Nicotine Tob Res. 2005;7(5):725–8. https://doi.org/10.1080/14622200500259176.
Cinciripini PM, Wetter DW, Tomlinson GE, Tsoh JY, De Moor CA, Cinciripini LG, et al. The effects of the DRD2 polymorphism on smoking cessation and negative affect: Evidence for a pharmacogenetic effect on mood. Nicotine Tob Res. 2004;6(2):229–39. https://doi.org/10.1080/14622200410001676396.
Colilla S, Lerman C, Shields PG, Jepson C, Rukstalis M, Berlin J, et al. Association of catechol-O-methyltransferase with smoking cessation in two independent studies of women. Pharmacogenet Genomics. 2005;15(6):393–8. https://doi.org/10.1097/01213011-200506000-00004.
David SP, Strong DR, Leventhal AM, Lancaster MA, McGeary JE, Munafò MR, et al. Influence of a dopamine pathway additive genetic efficacy score on smoking cessation: Results from two randomized clinical trials of bupropion. Addiction. 2013;108(12):2202–11. https://doi.org/10.1111/add.12325.
Han DH, Joe KH, Na C, Lee YS. Effect of genetic polymorphisms on smoking cessation: A trial of bupropion in Korean male smokers. Psychiatr Genet. 2008;18(1):11–6. https://doi.org/10.1097/ypg.0b013e3282df0939.
Lerman C, Jepson C, Wileyto EP, Epstein LH, Rukstalis M, Patterson F, et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology. 2006;31(1):231–42. https://doi.org/10.1038/sj.npp.1300861.
Morton LM, Wang SS, Bergen AW, Chatterjee N, Kvale P, Welch R, et al. DRD2 genetic variation in relation to smoking and obesity in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Pharmacogenet Genomics. 2006;16(12):901–10. https://doi.org/10.1097/01.fpc.0000230417.20468.d0.
Tashkin DP, Rabinoff M, Noble EP, Ritchie TL, Simmons MS, Connett J. Association of Dopamine-related Gene Alleles, Smoking Behavior and Decline in FEV1 in Subjects with COPD: Findings from the Lung Health Study. COPD. 2012;9(6):620–8. https://doi.org/10.3109/15412555.2012.712167.
Ton TGN, Rossing MA, Bowen DJ, Srinouanprachan S, Wicklund K, Farin FM. Genetic polymorphisms in dopamine-related genes and smoking cessation in women: A prospective cohort study. Behav Brain Funct. 2007;3:22. https://doi.org/10.1186/1744-9081-3-22.
Sun H, Guo S, Chen D, Yang F, Zou Y, Di X, et al. Association of functional COMT Val108/Met polymorphism with smoking cessation in a nicotine replacement therapy. J Neural Transm. 2012;119(12):1491–8. https://doi.org/10.1007/s00702-012-0841-8.
Breitling LP, Dahmen N, Illig T, Rujescu D, Nitz B, Raum E, et al. Variants in COMT and spontaneous smoking cessation: retrospective cohort analysis of 925 cessation events. Pharmacogenet Genomics. 2009;19(8):657–9. https://doi.org/10.1097/fpc.0b013e32832fabf3.
David SP, Johnstone E, Griffiths SE, Murphy M, Yudkin P, Mant D, et al. No association between functional catechol O-methyl transferase 1947A>G polymorphism and smoking initiation, persistent smoking or smoking cessation. Pharmacogenetics. 2002;12(3):265–8. https://doi.org/10.1097/00008571-200204000-00011.
Munafò MR, Freathy RM, Ring SM, St Pourcain B, Smith GD. Association of COMT Val(108/158) Met genotype and cigarette smoking in pregnant women. Nicotine Tob Res. 2011;13(2):55–63. https://doi.org/10.1093/ntr/ntq209.
Omidvar M, Stolk L, Uitterlinden AG, Hofman A, Van Duijn CM, Tiemeier H. The effect of catechol-O-methyltransferase Met/Val functional polymorphism on smoking cessation: retrospective and prospective analyses in a cohort study. Pharmacogenet Genomics. 2009;19(1):45–51. https://doi.org/10.1097/fpc.0b013e328317f3f8.
Stapleton JA, Sutherland G, O'Gara C, Spirling LI, Ball D. Association between DRD2/ANKK1 Taq1A genotypes, depression and smoking cessation with nicotine replacement therapy. Pharmacogenet Genomics. 2011;21(8):447–53. https://doi.org/10.1097/fpc.0b013e328347473a.
. Jones SK, Alberg AJ, Wallace K, Froeliger B, Carpenter MJ, Wolf BJ. CHRNA5-A3-B4 and DRD2 genes and smoking cessation throughout adulthood: A longitudinal study of women. Nicotine Tob Res. 2023;25(6):1164–73. https://doi.org/10.1093/ntr/ntad026. Most recent article included in systematic review.
Wu X, Hudmon KS, Detry MA, Chamberlain RM, Spitz MR. D2 Dopamine Receptor Gene Polymorphisms among African-Americans and Mexican-Americans: A Lung Cancer Case-Control Study. Cancer Epidemiol Biomarkers Prev. 2000;9(10):1021–6.
Styn MA, Nukui T, Romkes M, Perkins K, Land SR, Weissfeld JL. The impact of genetic variation in DRD2 and SLC6A3 on smoking cessation in a cohort of participants 1 year after enrollment in a lung cancer screening study. Am J Med Genet B Neuropsychiatr Genet. 2009;150(2):254–61. https://doi.org/10.1002/ajmg.b.30801.
Spitz MR, Shi H, Yang F, Hudmon KS, Jiang H, Chamberlain RM, et al. Case-control study of the D2 dopamine receptor gene and smoking status in lung cancer patients. J Natl Cancer Inst. 1998;90(5):358–63. https://doi.org/10.1093/jnci/90.5.358.
Hamajima N, Ito H, Matsuo K, Saito T, Tajima K, Ando M, et al. Association between smoking habits and dopamine receptor D2 Taql A A2 allele in Japanese males: a confirmatory study. J Epidemiol. 2002;12(4):297–304. https://doi.org/10.2188/jea.12.297.
Yoshida K, Hamajima N, Kozaki K-I, Saito H, Maeno K, Sugiura T, et al. Association between the dopamine D2 receptor A2/A2 genotype and smoking behavior in the Japanese. Cancer Epidemiol Biomarkers Prev. 2001;10(4):403–5.
Ohmoto M, Takahashi T, Kubota Y, Kobayashi S, Mitsumoto Y. Genetic influence of dopamine receptor, dopamine transporter, and nicotine metabolism on smoking cessation and nicotine dependence in a Japanese population. BMC Genet. 2014;15(1) https://doi.org/10.1186/s12863-014-0151-2.
Guo S, Chen DF, Zhou DF, Sun HQ, Wu GY, Haile CN, et al. Association of functional catechol O-methyl transferase (COMT) Val108Met polymorphism with smoking severity and age of smoking initiation in Chinese male smokers. Psychopharmacology (Berl). 2007;190(4):449–56. https://doi.org/10.1007/s00213-006-0628-4.
Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry. 1991;48(7):648–54. https://doi.org/10.1001/archpsyc.1991.01810310066012.
Thompson J, Thomas N, Singleton A, Piggott M, Lloyd S, Perry EK, et al. D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics. 1997;7(6):479–84. https://doi.org/10.1097/00008571-199712000-00006.
Moore SR. Commentary: What is the case for candidate gene approaches in the era of high-throughput genomics? A response to Border and Keller (2017). J Child Psychol Psychiatry. 2017;58(3):331–4. https://doi.org/10.1111/jcpp.12697.
Olfson E, Bierut LJ. Convergence of genome-wide association and candidate gene studies for alcoholism. Alcohol Clin Exp Res. 2012;36(12):2086–94. https://doi.org/10.1111/j.1530-0277.2012.01843.x.
Ritchie T, Noble EP. Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochem Res. 2003;28(1):73–82. https://doi.org/10.1023/a:1021648128758.
Ma Y, Wang M, Yuan W, Su K, Li MD. The significant association of Taq1A genotypes in DRD2/ANKK1 with smoking cessation in a large-scale meta-analysis of Caucasian populations. Transl Psychiatry. 2015;5(12):e686. https://doi.org/10.1038/tp.2015.176.
Ohmoto M, Sakaishi K, Hama A, Morita A, Nomura M, Mitsumoto Y. Association between dopamine receptor 2 TaqIA polymorphisms and smoking behavior with an influence of ethnicity: a systematic review and meta-analysis update. Nicotine Tob Res. 2013;15(3):633–42. https://doi.org/10.1093/ntr/nts196.
Lee H-S, Kim S-H, Lee H-J, Kim L, Lee S-K, Jang D-W, et al. Gender-specific molecular heterosis of dopamine D2 receptor gene (DRD2) for smoking in schizophrenia. Am J Med Genet. 2002;114(6):593–7. https://doi.org/10.1002/ajmg.10641.
Choi HD, Shin WG. Lack of association between DRD2 Taq1A gene polymorphism and smoking cessation therapy: A meta-analysis. Int J Clin Pharmacol Ther. 2015;53(6):415–21. https://doi.org/10.5414/cp202214.
Bress A, Kittles R, Wing C, Hooker SE Jr, King A. Genetic ancestry as an effect modifier of naltrexone in smoking cessation among African Americans: An analysis of a randomized controlled trial. Pharmacogenet Genom. 2015;25(6):305–12. https://doi.org/10.1097/fpc.0000000000000138.
. Kendrick PJ, Abbasi-Kangevari M, Abdoli A, Abhilash ES, Akunna CJ, Allebeck P, et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. 2021;397(10292):2337–60. https://doi.org/10.1016/s0140-6736(21)01169-7. Worldwide comparison of smoking.
Lee C, Gao M, Ryff CD. Conscientiousness and Smoking: Do Cultural Context and Gender Matter? Front Psychol. 2020;11:1593. https://doi.org/10.3389/fpsyg.2020.01593.
Domino EF, Kadoya C, Matsuoka S, Ni L, Fedewa KS. Comparative American and Japanese tobacco smoke uptake parameters after overnight tobacco deprivation. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(6):973–84. https://doi.org/10.1016/s0278-5846(03)00157-x.
Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311(2):183–92. https://doi.org/10.1001/jama.2013.284692.
Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14(9):615–26. https://doi.org/10.1097/00008571-200409000-00006.
Qian J, Cai M, Gao J, Tang S, Xu L, Critchley JA. Trends in smoking and quitting in China from 1993 to 2003: National Health Service Survey data. Bull World Health Organ. 2010;88(10):769–76. https://doi.org/10.2471/blt.09.064709.
Harrison PJ, Tunbridge EM. Catechol-O-methyltransferase (COMT): a gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology. 2008;33(13):3037–45. https://doi.org/10.1038/sj.npp.1301543.
. Jones SK, Alberg AJ, Wallace K, Froeliger B, Carpenter MJ, Wolf B. Genetic associations with smoking relapse and proportion of follow-up in smoking relapse throughout adulthood in pre- and postmenopausal women. Cancer Prev Res. 2023;16(5):269–79. https://doi.org/10.1158/1940-6207.capr-22-0421. Genetic differences in association with smoking by menopausal status.
Markett S, Reuter M, Montag C, Weber B. The dopamine D2 receptor gene DRD2 and the nicotinic acetylcholine receptor gene CHRNA4 interact on striatal gray matter volume: Evidence from a genetic imaging study. Neuroimage. 2013;64:167–72. https://doi.org/10.1016/j.neuroimage.2012.08.059.
. Jones SK, Wolf BJ, Froeliger B, Wallace K, Carpenter MJ, Alberg AJ. Nicotine Metabolism Predicted by CYP2A6 Genotypes in Relation to Smoking Cessation: A Systematic Review. Nicotine Tob Res. 2021;24(5):633–42. https://doi.org/10.1093/ntr/ntab175. Contemporary review of nicotine metabolism gene and smoking cessation.
. Jones SK, Wolf BJ, Froeliger B, Wallace K, Carpenter MJ, Alberg AJ. A systematic review of genetic variation within nicotinic acetylcholine receptor genes and cigarette smoking cessation. Drug Alcohol Depend. 2022;239:109596. https://doi.org/10.1016/j.drugalcdep.2022.109596. Contemporary review of nicotinic acetylcholine receptor genes and smoking cessation.
. Chen LS, Baker TB, Miller JP, Bray M, Smock N, Chen J, et al. Genetic Variant in CHRNA5 and Response to Varenicline and Combination Nicotine Replacement in a Randomized Placebo-Controlled Trial. Clin Pharmacol Ther. 2020;108(6):1315–25. https://doi.org/10.1002/cpt.1971. Study showing that nicotinic receptor SNP is predictive of response to smoking cessation pharmacotherapy.
. Bray M, Chang Y, Baker TB, Jorenby D, Carney RM, Fox L, et al. The Promise of Polygenic Risk Prediction in Smoking Cessation: Evidence From Two Treatment Trials. Nicotine Tob Res. 2022;24(10):1573–80. https://doi.org/10.1093/ntr/ntac043. Study of polygenic risk scores and prediction of abstinence in smoking cessation trials.
Funding
Stephanie Jones received funding from the National Institutes of Health TL1 grant (TL1TR001451) and Medical University of South Carolina’s Hollings Cancer Center Abney Graduate Fellowship to support the completion of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no completing interests to declare that are relevant to the content of this article.
Human and Animal Rights and Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jones, S.K., Wolf, B.J., Wallace, K. et al. Single Nucleotide Polymorphisms Within DRD2, COMT, and DBH and Smoking Cessation: A Systematic Review Considering Genetic Differences by Ancestry and Biological Sex. Curr Addict Rep 11, 736–751 (2024). https://doi.org/10.1007/s40429-024-00580-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40429-024-00580-0