Abstract
Sorghum’s ability to exhibit allelopathy is linked to the secretion of lipophilic exudates from its roots. Sorgoleone, a member of the quinone class, constitutes a substantial part of these exudates. While studies typically focus on testing the exudate or crude extract, other compounds are also present, although in lesser quantities. Initially suspected molecular target sites affected by sorgoleone include photosynthetic and mitochondrial electron transport processes, along with p-hydroxyphenylpyruvate dioxygenase. Despite acting as a Photosystem II inhibitor in isolated chloroplasts, its impact on overall photosynthesis remains uncertain. Proposed mechanisms suggest inhibition of root H+-ATPase activity and water uptake, but questions persist regarding sorgoleone’s absorption, transportation to the shoot, and entry into chloroplasts. The spatial separation between sorgoleone exudation and its presumed site of action presents a notable challenge. This review delves into the characteristics and effects of sorgoleone, critically assessing its role in allelopathy. Furthermore, it explores the role of sorgoleone in signaling to facilitate the establishment of a symbiotic relationship between plants and arbuscular mycorrhizal fungi, as well as its impact on the microbiota. However, key questions regarding its mode of action, specific activity and selectivity, bioactive concentration, persistence and release in the rhizosphere, as well as its absorption and translocation, remain to be fully elucidated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The allelopathic potential of sorghum is attributed to the release of phytotoxic lipophilic exudates from its roots. Sorgoleone, a compound found in the exudate produced by sorghum root hairs, is believed to play a role in the observed allelopathic effects. However, the majority of studies assessing the allelopathic potential of sorghum species typically test the exudate or crude extract. While sorgoleone makes up a significant portion of this extract, constituting up to 90%, the exudate also contains lipid benzoquinone, along with a resorcinol analogue, and several other compounds, albeit in much lower quantities (Netzly et al. 1988; Erickson et al. 2001; Czarnota et al. 2003; Dayan et al. 2003; Kagan et al. 2003).
The initial suspected molecular target sites affected by sorgoleone were the photosynthetic and mitochondrial electron transport processes (Rasmussen et al. 1992; Einhellig and Souza 1992; Nimbal et al. 1997; Rimando et al. 1998), as well as the enzyme p-hydroxyphenylpyruvate dioxygenase (Meazza et al.2002). Although sorgoleone acted as an inhibitor of PSII in isolated chloroplasts, the photosynthesis of 7 to 10-day-old plants did not seem to be affected (Hejl and Koster 2004). Subsequently, it was proposed that sorgoleone’s mode of action involved inhibiting root H+-ATPase activity and water uptake. However, a crucial aspect that remains to be clarified is the mechanism by which sorgoleone is absorbed by roots, transported to the shoot, and enters the chloroplast to inhibit PSII in the thylakoid membrane (Hejl and Koster 2004).
Thus, while sorgoleone disrupts various physiological and biochemical processes in vitro, its primary mechanism of action in plants remains unclear. One significant challenge is the spatial separation between where sorgoleone is exuded (soil) and its presumed site of action (foliage) as a PSII inhibitor, a question that has not been adequately addressed so far. In this review, we will present the characteristics and impacts of sorgoleone and critically examine its role in allelopathy. However, there are essential questions that still need further clarification.
2 Chemical structure and discovery of sorgoleone
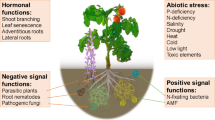
Sorgoleone, a constituent of sorghum exudate, falls within the quinone class and is known for its biological activity (Chang et al. 1986; Netzly et al. 1988; Weston and Czarnota 2001; Weston et al. 2013; Pan et al. 2018). This secondary metabolite is exuded through the root hairs of sorghum roots (Weston and Czarnota 2001; Weston et al. 2013; Jesudas et al. 2014; Wang et al. 2021) (Fig. 1).
Chemically, sorgoleone is a polyunsaturated fatty acid with a benzoquinone ring, specifically 2-hydroxy-5-methoxy-3-[(8-Z,11-Z)-8,11,14-pentadecatriene]-p-benzoquinone [CAS 105018-76-6] (Głąb et al. 2017; Fig. 2). Alongside sorgoleone, sorghum root exudate contains various structurally related lipophilic p-benzoquinones pre-sent in small quantities, as well as a comparable amount of non-quinoid lipid resorcinols. These compounds vary in the length or degree of saturation in the aliphatic side chain and in the substitution pattern of the quinone ring (Erickson et al. 2001; Kagan et al. 2003; Rimando et al. 2003; Dayan et al. 2009, 2010; Pan et al. 2018).
Chemical structures of a sorgoleone and b dihydroquinone of sorgoleone (Głąb et al. 2017)
3 Sorgoleone biosynthesis
The exclusive site for the biosynthesis of the biologically active benzoquinone sorgoleone is the root hairs of sorghum (Weston and Czarnota 2001; Yang et al. 2004; Dayan et al. 2007; Pan et al. 2007; Baerson et al. 2008). Within mature sorghum root hairs lies the complete genetic material and biochemical machinery necessary for the production of this bioactive benzoquinone (Czarnota et al. 2003; Dayan et al. 2007; Pan et al. 2007; Baerson et al. 2008). Root hairs, specialized cells abundant in mitochondria, rough endoplasmic reticulum, and vesicles, play a crucial role in sorgoleone synthesis (Weston and Czarnota 2001). Sorgoleone can be visualized through transmission electron microscopy as densely osmiophilic globules within the cytoplasm of root hair cells, deposited between the plasmalemma and the cell wall (Weston and Czarnota 2001).
The process of sorgoleone biosynthesis has been clarified using retrobiosynthetic nuclear magnetic resonance (NMR) analysis (Fate and Lynn 1996; Dayan et al. 2003). The exudate production from root hairs remains consistent regardless of the root development stage (Dayan 2006), resulting in an accumulation of up to 20 μg of exudate per milligram of root dry weight (Weston and Czarnota 2001; Dayan et al. 2009). The production of sorgoleone can be halted and then restarted after gently rinsing the roots with water. However, although the biosynthesis of lipid benzoquinones and resorcinols is a dynamic process (Dayan et al. 2009), the regulatory mechanism governing root exudate production remains unknown.
The expression of enzymes involved in sorgoleone biosynthesis is induced in a specific root zone, indicating that the secretion is developmentally regulated. Preceding the peak of sorgoleone biosynthesis and secretion, there is an accumulation of internal vesicles, suggesting that these vesicles are involved in precursor storage rather than secretion (Maharjan et al. 2023). Plant hormones play a role in regulating sorgoleone production; for instance, abscisic acid (ABA) was found to increase sorgoleone production, while gibberellic acid (GA) decreased it (Bais et al. 2006). This indicates that ABA and GA are part of the signaling pathway that regulates sorgoleone production. Moreover, sorgoleone production was observed to be higher in plants grown under conditions of drought stress or nutrient deficiency (Wen et al. 2019; Abdelhalim et al. 2019; Oliveira et al. 2020; Sarr et al. 2021; Wang et al.2021). This suggests that environmental stresses can trigger sorgoleone production as a defense mechanism.
Understanding the regulatory mechanism of sorgoleone production holds significant value for various reasons. Firstly, it could facilitate the development of novel approaches to manipulate sorgoleone production in sorghum plants, enabling enhancement or reduction of sorgoleone levels. This modulation could be instrumental in bolstering sorghum’s resistance against pests and diseases or amplifying its allelopathic effects. Secondly, it may shed light on the regulation of other root exudates, offering opportunities to optimize plant nutrition and enhance overall crop productivity.
Sorgoleone exudation is subject to variation based on sorghum genotype and specific cultivation conditions. The optimal temperature range for sorgoleone production is typically between 25 and 35 °C, with significantly reduced production observed at lower temperatures, potentially dropping by as much as 95% (Dayan 2006). Additionally, the presence of other plants appears to stimulate sorgoleone production (Dayan 2006).
The biosynthetic pathway leading to the formation of sorgoleone involves several key enzymatic steps. It begins with the production of an alkylresorcinole intermediate, SbARS2, which utilizes a fatty acyl-CoA starter unit generated from consecutively catalyzed palmitoleoyl-CoA by Sorghum bicolor fatty acid denaturases, SbDES2 and SbDES3. This intermediate undergoes methylation by O-methyltransferase SbOMT3. In the final step of the sorgoleone biosynthesis pathway, a cytochrome P450 enzyme (CYP71AM1) catalyzes the formation of dihydrosorgoleone, using 5-pentadecatrienyl resorcinol-3-methyl ether as a substrate. Once released into the soil from the rhizosphere as a chemically unstable hydroquinone, dihydrosorgoleone undergoes auto-oxidation in vivo to produce sorgoleone, a more stable benzoquinone (Pan et al. 2018, 2021). All identified and functionally characterized genes (SbDES2, SbDES3, SbARS2, SbOMT3, CYP71AM1) in this process encode enzymes that facilitate every biosynthetic step leading to the production of dihydrosorgoleone, the precursor of sorgoleone (Pan et al. 2018; 2021) (Fig. 3). However, the regulation of genes in this pathway remains incompletely understood. A recent question has emerged regarding whether the simultaneous expression of all genes associated with the sorgoleone biosynthesis pathway could produce dihydrosorgoleone, the precursor of sorgoleone. Additionally, there is interest in understanding how heterologous host cells would respond physiologically to the synthesized compound and how the expression of the host gene would be affected (Pan et al. 2021).
Sorgoleone is synthesized through a series of enzymatic reactions, commencing with palmitoleoyl-CoA. The process leads to the formation of dihydrosorgoleone, which, when released into the soil, undergoes auto-oxidation to yield sorgo-leone, a more stable benzoquinone. The key enzymes involved are cytochrome P450 (CYP71AM1), alkylresorcinol synthase (SbARS), fatty acid desaturase (SbDES), and O-methyltransferase (SbOMT) (Pan et al. 2021)
4 Stimulatory effect of water extracts of sorghum (Sorgaab)
Sorgoleone, categorized as a phytotoxin, represents a class of biodegradable and environmentally safe molecules with potential for enhancing crops in both controlled and field conditions. Research examining weed control using water extracts of sorghum (also know as Sorgaab) has shown a significant increase in crop yield post-treatment with plant-released phytotoxins (Cheema et al. 2001, 2003; Ashraf and Akhlaq 2007; Jamil et al. 2009; Khan et al.2015). This increase may stem from effective weed control, reducing competition between weeds and crops, or from hormetic growth stimulation in crop plants. Consequently, applying phytotoxins during vulnerable weed stages, aligning with optimal crop growth periods, could offer both crop protection and enhancement. Abbas et al. (2017) suggested that the optimal timing for weed control coincides with peak of crop growth stimulation.
While few studies have explored the stimulatory responses of plant-released phytotoxins, research on the inhibitory effects of various plant phytotoxins indicates that the response of tested plants depends on the type of phytotoxins involved (Manandhar et al. 2010; Farooq et al. 2011a,b). The extent of growth inhibition varied among toxic compounds, with some showing no significant impact on growth (Abbas et al. 2017). In a study by Kamran et al. (2016), a 3% water extract of sorghum, maize, rice, and moringa, applied alone or in combination, was used to enhance maize growth. Results revealed that the phytotoxins released by these crops differed in their ability to initiate stimulatory responses in morphological and yield traits. The variance in the potential of plant phytotoxins to induce crop stimulation (hormesis) likely relates to the mechanisms underlying the production of growth stimulants.
Timing of plant phytotoxin application is crucial for achieving increased harvest yields. Early application may not sustain stimulatory responses over an extended period. Utilizing plant-released phytotoxins as growth promoters is preferable to herbicide hormesis due to their environmental safety, biodegradability, and absence of residual effects on food quality (Petroski and Stanley 2009). Exogenous application of plant-released phytotoxins via foliar spray can effectively enhance crop growth directly or indirectly. For instance, Jahangeer (2011) demonstrated that a foliar spray containing 3% aqueous extracts of moringa, sorghum, and brassica increased maize grain yield by 52%, 42%, and 42%, respectively.
5 Allelopathy effect of sorghum root extract
In recent years, extensive research has been dedicated to exploring the allelopathic effects of sorghum root extract, with a particular emphasis on sorgoleone (Einhellig and Souza 1992; Nimbal et al. 1997; Weston and Czarnota 2001; Erickson et al. 2001; Kagan et al. 2003; Rimando et al. 2003; Dayan et al. 2009; Dayan et al. 2010; Uddin et al. 2010; Uddin et al. 2014; Sabahie et al. 2014; Pan et al. 2018; Campos et al. 2020; Besançon et al. 2020; Naby and Ali 2021; Tibugari et al. 2021; Gomes et al. 2023).
Allelopathy refers to the production and release of one or more biologically active compounds, termed allelochemicals, which affect the growth, survival, and reproduction of other organisms (Pan et al. 2018). Evidence suggests that allelopathic activity results from the collective action of mixtures of allelochemicals rather than a single allelochemical. For example, pure artemisinin demonstrates less inhibition of redroot pigweed growth compared to an annual wormwood leaf extract (Lydon et al. 1997).
Allelochemicals have been identified in economically significant cereals like wheat, rice, and sorghum (Bertin et al. 2003; Duke 2003; Inderjit and Duke 2003). In sorghum leaves, phenolic compounds serve as the primary allelochemicals. Although Vaughan and Ord (1991) initially expressed skepticism regarding the allelopathic activity of phenolic acids in nature, subsequent studies have demonstrated that, under appropriate concentrations and conditions, certain phenolic acids can indeed be considered allelochemicals (Blum 1995, 1996; Inderjit 1996; Blum et al. 1999; Dalton 1999).
The allelopathic properties of sorghum have been a subject of study for many years. Among the initial investigations into the biologically active elements of sorghum root exudates, it was revealed that these exudates hindered the growth of lettuce (Lactuca sativa) seedlings as well as invasive weed species (Netzly and Butler 1986). The primary component of these exudates, sorgoleone, has been pinpointed, constituting approximately 40% to 90% (w/w) in various sorghum accessions (Nimbal et al. 1997; Weston and Czarnota 2001; Dayan et al. 2009).
Three sorghum varieties, namely Dhlakama, Shirikure, and Macia, exhibit autotoxicity in sorghum. The extent of toxicity varies based on the genotype (Tibugari et al. 2021). However, in Tibugari et al. (2021) study, the researchers utilized the total sorghum exudate instead of purified sorgoleone. Another research using a formulation containing 4.6% of sorgoleone demonstrated its effectiveness in inhibiting weed growth (Uddin et al. 2014). Sorghum root extract displays significant inhibitory effects on various plant species, including Abutilon theophrasti, Datura stramonium, Amaranthus retroflexus, Setaria viridis, Digitaria sanguinalis, and Echinochloa crusgalli (Einhellig and Souza 1992), as well as on caruru germination rate and percentage (Sabahie et al. 2014), early development of canola plants (Campos et al. 2020) and weed growth in wheat fields (Naby and Ali 2021). These findings highlight that sorghum exudate exhibits biological activity even at very low concentrations, suggesting a significant contribution to sorghum allelopathy.
The impact of allelopathic effects on crops succeeding sorghum cultivation or under sorghum straw, and the optimal duration of fallow straw before introducing a new crop, has been a subject of prolonged study. In a greenhouse study, the allelopathic effects on soybean establishment and biomass production were evaluated at various time intervals between sweet sorghum harvest and soybean sowing (Silva et al. 2016). Differences were observed in both culture establishment and biomass; however, while the authors attributed these effects to sorgoleone, no subsequent research was conducted to conclusively establish sorgoleone as the causative agent.
In another greenhouse experiment, two sweet sorghum cultivars (BRS 506 and BRS 511) were utilized to investigate the optimal timing for sowing soybeans after sorghum (Garcia and Sutier 2016). The analyses were conducted at 65 days after emergence and 15 days after plant cutting, assessing sorgoleone levels. The findings indicated that a nine-day interval between sorghum management and soybean sowing was sufficient to mitigate the negative effects. Soybeans sown on the day of sorghum management exhibited symptoms of leaf bleaching at stage V2, which were absent in soybeans sown 15 days after management.
In a separate greenhouse study, Olibone et al (2006) investigated the impact on early soybean growth and root system development in the presence of guinea sorghum and forage residues. The presence of guinea sorghum straw led to a reduction in soil base saturation, and both types of straw hindered soybean growth.
In field conditions, Biesdorf (2017) explored the allelopathic impact of sorghum on soybeans planted at various intervals after sorghum harvesting, and its influence on the infesting plant community. The study revealed that sorghum cultivation influenced the phytosociology and reduced weed incidence. Regarding soybeans, planting within 40 days after sorghum harvest had a negative impact on initial development, although it did not affect overall productivity. In a related study, Paixão (2019) investigated the allelopathic effect of sorghum regrowth on succeeding soybean crops at different intervals after sorghum desiccation in the field. The analysis did not observe significant alterations in the evaluated parameters. The research concluded that soybean development was enhanced in the presence of regrowth straw, possibly due to in-creased nutrient availability, indicating the absence of a toxic allelopathic effect under the experimental conditions.
In sorghum-cultivated areas, a substantial amount of plant material remains after harvest, especially with no-tillage and minimum tillage practices. This straw can serve as an effective tool for weed control. However, it may also have a detrimental impact on the establishment and growth of succeeding crops. Allelopathic chemical substances identified in sorghum include chlorogenic acid, m-coumaric acid, and caffeic acid (Cheema et al. 2009); p-hydroxybenzoic acid, p-coumaric acid, ferulic acid, vanillic acid, serpidric acid, and p-hydroxybenzaldehyde (Sene et al. 2020); and gallic acid, p-coumaric acid, and syringic acid (Alsaadawi and Dayan 2009), as well as p-hydroxybenzoic acid (Alsaadawi et al. 2007).
Notably, the allelopathic effect may not solely be attributed to sorgoleone. Sorgoleone is a phenolic compound exhibiting allelochemical properties, but sorghum also synthesizes other chemicals that inhibit the growth of neighboring plants (Shehzad and Okuno 2020). Further studies focusing on the absorption of substances released by sorghum straw and their mechanisms of action are crucial to comprehend their behavior. Understanding how these substances are diluted, incorporated into the soil volume, and their concentration is vital, as the intensity of allelopathic effects is contingent on the concentration of allelochemicals.
6 Differences in exudation and composition of sorghum extracts among genotypes
In recent years, research has been focused on understanding the variability in extract production among sorghum genotypes, exploring the composition of these extracts, and delving into the biochemical interactions between the compounds. The hydrophobic crude root extracts from 41 sorghum genotypes were quantified, revealing a variability ranging from 0.35 to 2.98 mg per 100 rootlets (Trezzi et al. 2005). Within this range, researchers selected five genotypes, purified sorgoleone using column chromatography, and confirmed its presence through H-NMR analysis. Chromatograms showed five distinct peaks with varying areas, with sorgoleone being the most predominant compound, constituting 73 to 82% of the total extract.
Quantification of crude extract production from 50 sorghum accessions revealed a variation between 1.88 and 8.55 mg per gram of fresh root mass (Franco et al. 2011). The analysis exhibited three to ten peaks with retention times falling within 1.3 to 17.5 min. Sorgoleone stood out with the highest absorption peak and a retention time between 8.4 and 8.7 min, constituting 60.3 to 89.9% of the crude extracts. Notably, 68% of the evaluated genotypes showed production ranging from 1.0 to 2.0 mg per gram of fresh root mass (Franco et al. 2011). However, the production of sorgoleone exhibits variance based on the cultivar studied. May et al (2016) reported this variance, where crude extract production ranged from 5.03 mg g−1 to 10.60 mg g−1 and sorgoleone production ranged from 32.64 mg g−1 to 615.31 mg g −1, depending on the cultivar. Uddin et al (2009) also observed a range of sorgoleone production between 0.41 µg −1 and 6.98 µg −1 of fresh root mass.
Several other works observed variations in the production of sorgoleone (Hess et al. 1992; Ferreira et al. 1999) with values ranging from 11 to 32 mg 100 rootlets−1. Nimbal et al (1997) found differences of 96% between the highest and lowest sorgoleone content per root dry mass, among the 25 sorghum genotypes evaluated. An important piece of information observed in all these works is that the variation in the amount of extract and sorgoleone produced did not have a direct relationship with the sorghum quality (biomass, saccharine, grain), indicating that this trait is dependent both by genetic and environment interactions.
In recent research, the quantification of three sorghum root extracts using high-performance liquid chromatography (HPLC) revealed a production range of 43.36 to 67.8 mg per gram of root dry mass, with sorgoleone production ranging from 20.56 to 28.67 mg per gram of fresh root mass (Gomes et al. 2023). Interestingly, genotypes with longer and denser root hairs were found to exude more and produce higher amounts of sorgoleone. The chromatographic profiles displayed five to six distinct substances with varying peaks and areas. Moreover, when allelopathy evaluations were conducted using extracts with standardized sorgoleone amounts; different results were observed, suggesting that other compounds within the extract play a significant role in influencing the outcomes (Gomes et al. 2023).
The majority of studies assessing the allelopathic potential of sorghum species utilize sorghum extract, often attributing its effects to sorgoleone, which can constitute up to approximately 90% of these extracts. However, gaining a deeper understanding of the specific effects of sorgoleone compared to the whole extract is essential for assessing allelopathic effects accurately. Another crucial aspect is identifying the compounds present in sorghum root extracts. Weston et al (2013) conducted a chemical analysis using HPLC on extracts from two sorghum cultivars and identified several phenolic compounds, including protocatecheic, hydroxybenzoic, vanillic, syringic, p-coumaric, ferulic, and sinapic acids, along with four unidentified compounds. However, further research is needed to comprehend the relationship between extract production, composition, and their allelopathic effects.
7 Potential allelopathic effect of sorgoleone
The allelopathic activity of substances can be elucidated through various modes of action and by targeting multiple molecular components affected by these allelochemicals. An example is observed in a freshwater microphyte called Myriophyllum spicatum (Haloragaceae), which releases thelymagrandin II, a compound with algaecidal and cyanobactericidal properties (Gross 1999). Leu et al (2002) further elucidated that thelymagrandin II operates through two distinct modes of action: inhibition of Photosystem II and inhibition of microalgal exoenzyme formation, demonstrating a combined action involving these pathways. Understanding such multifaceted modes of action is critical in comprehending the mechanisms underlying allelopathic effects (Leu et al. 2002).
The suggested allelopathic activity of sorgoleone is primarily linked to its ability to inhibit photosynthesis in higher plant systems. It achieves this by competing for the plastoquinone binding site in Photosystem II, a key component of the photosynthetic machinery (Dayan et al. 2003). Moreover, sorgoleone has been shown to hinder electron trans-fer reactions integral to mitochondrial respiration and to inhibit the enzyme p-hydroxyphenylpyruvate dioxygenase, a crucial enzyme for plastoquinone synthesis (Rasmussen et al. 1992).
The activity of sorgoleone can be attributed to both direct and indirect effects, given its multiple modes of action (Netzly and Butler 1986; Einhellig and Souza 1992; Nimbal et al. 1997; Rimando et al. 1998; Czarnota et al. 2001; Bertin et al. 2003; Duke 2003). Studies on the translocation of carbon-14-labeled sorgoleone in velvet plants (Abutilon theophrasti) suggested uncertainty regarding whether sorgoleone exuded from sorghum roots is absorbed and translocated to its foliage, where it should enter the chloroplast and inhibit Photosystem II (Dayan et al. 2009). Experiments in this domain revealed that the inhibitory activity of sorgoleone on photosynthesis is strongly contingent on the age of the leaf tissue, inhibiting photosynthesis in germinating seedlings but not in older plants. Sorgoleone’s mode of action may involve inhibiting photosynthesis in young seedlings while also affecting other molecular targets in older plants (Dayan et al. 2009).
Apart from sorgoleone, sorghum root extract contains smaller analogues with various substitutions in the quinone moiety and different configurations of carbon atoms and double bonds in the aliphatic side chain. Through high-performance chromatography coupled with mass spectrometry and 1H nuclear magnetic resonance, researchers have isolated sorgoleone and three other quinone compounds. These compounds share the p-quinone fraction with sorgoleone but exhibit distinctions in the number of double bonds or carbon atoms in the aliphatic side chain. These compounds are collectively termed sorgoleones and include sorgoleone-358, sorgoleone-360 (with two double bonds in the C15 side chain), sorgoleone-362 (with a double bond in the C15 side chain), and sorgoleone-386 (with three double bonds in a C17 side chain) (Netzly and Butler 1986).
Examination of the sorghum root extract using gas chromatography coupled with mass spectrometry reveals peaks in the chromatogram with molecular ions not only at 358, as expected for sorgoleone, but also at 359, 360, 362, 363, 364, 365, and 366. This indicates the presence of sorgoleone-like compounds with varying degrees of unsaturation in the side chain (Erickson et al. 2001). These findings support the notion that these analogues of sorgolene may collectively contribute to the overall allelopathic effects observed in sorghum.
It was hypothesized that the biosynthesis of sorgoleone and related compounds is the outcome of the convergence of two pathways: the fatty acid biosynthetic pathway for creating the aliphatic tail and the activity of polyketide synthase-type enzymes for forming the quinone fraction of the molecule (Dayan et al. 2003). Although this convergence of pathways has been confirmed for aflatoxin biosynthesis in fungi, similar examples in plants have not been fully clarified, relying on assumptions that have not been conclusively demonstrated for these compounds (Dayan et al. 2003). For sorgoleone, exogenous acetate labeled with isotopes is integrated into the quinone head instead of the tail (Fate and Lynn 1996). This finding implies that the allelopathic effect attributed to sorgoleone might be specifically linked to the quinone fraction of sorgoleone. In an investigation involving the synthesis and assessment of eight resorcinol lipid derivatives and ten quinones with varying side chain sizes identified from sorghum root extracts, the results revealed that quinones possess phytotoxicity, while resorcinolic lipids do not. This suggests that different fractions constituting the chemical structure of the sorgoleone molecule can exhibit distinct effects (Mizuno et al. 2010).
8 Influence of sorgoleone on the soil microbial community
Plants employ a diverse array of mechanisms to absorb and transport bioactive compounds to the rhizosphere (Wen et al. 2019). The rhizosphere is a critical zone where extensive microorganism-plant-soil interactions take place, encompassing both agricultural and natural systems (Wang et al. 2021). The unique physical, chemical, and biological properties of molecules exuded into the rhizosphere can influence the growth and proliferation of specific groups of microorganisms while inhibiting others. Microbial populations, given their versatile metabolic capabilities, can exhibit variability from one cultivar to another due to differences in root exudation and significant metabolic modifications, influencing the interactions between plants and microorganisms. Even with the same isolate and plant genotype, alterations in the plant’s exudation pattern can lead to different interactions. Root exudation not only determines the microbial community residing in the rhizosphere but also confers physical and chemical advantages to plants, such as those observed in sorghum (Vejan et al. 2016; Wen et al. 2019; Liu et al. 2017).
As an avenue to understand how sorghum and its root microbiome may be connected through root exudates. Oda et al (2023) identified the molecular determinants of microbial sorgoleone degradation and the distribution of this trait among microbes. They isolated and studied from sorghum-associated soils, three bacterial strains classified as Acinetobacter, Burkholderia, and Pseudomonas species that grow with sorgoleone as a sole carbon and energy source.
In a recent study involving five Bacillus isolates, it was observed that sorgoleone strongly inhibited the growth of three isolates, while stimulating the growth of two. Among the inhibited isolates, two were identified as B. safenensis and one as B. cereus. On the other hand, the two isolates that showed growth stimulation were classified as B. flexus (Wang et al. 2021).
Additionally, the role of root exudation as a signaling mechanism for establishing effective symbiosis between plants and arbuscular mycorrhizal fungi (AMF) has been established in various plant species, including maize, soybean, and sorghum (Yoneyama et al. 2015; Kobae et al. 2018; Abdelhalim et al. 2019). Recent research has demonstrated sorgoleone’s ability to influence mycorrhizal colonization in sorghum plants (Oliveira et al. 2020; Sarr et al. 2021). Furthermore, sorgoleone has shown potential to significantly enhance plant biomass and phosphorus (P) content in mycorrhizal plants compared to non-mycorrhizal ones, especially when grown under low P (Oliveira et al. 2020; Sarr et al. 2021). Importantly, sorgoleone has been found to affect the microbial community structure in the sorghum rhizosphere soil (Wang et al. 2021; Ortas and Bilgili 2022).
The concentration of P in the soil significantly influences AMF colonization (Kobae et al. 2018). This is due to the impact on root exudation of fungal signaling compounds, a key aspect of the symbiotic response. Low-P concentrations in the soil promote increased root exudation and subsequent mycorrhizal colonization. Conversely, high-P concentrations suppress exudation, reducing the level of plant-fungus signaling during symbiosis and hindering colonization (Chiu and Paszkowski 2019). In cases of P deficiency, plants deficient in root exudate biosynthesis exhibit lower levels of colonization (MacLean et al. 2017; Lanfranco et al. 2018). When soil P availability is abundant and comparable to what a non-mycorrhizal plant can absorb, rendering the fungus an energy burden for the plant with no additional nutritional benefit in terms of P absorption, it can lead to a depressive effect on plant development in the presence of AMF (Pedersen and Sylvia 1996; Siqueira et al. 1998; De Novais et al. 2014).
9 Final considerations and perspectives
Since its discovery, sorgoleone has emerged as a pivotal secondary metabolite with extensive applications. Understanding the hormesis of this plant-released phytotoxin is crucial for comprehending its role in microorganism-plant-soil interactions. It is crucial to focus efforts on identifying compounds within sorghum root extract and understanding the chemical characteristics of sorgoleone-related analogs found in the extract. The practical application of post-emergence sorgoleone through leaf spraying, a common field practice, presents challenges. There is a lack of evidence demonstrating the translocation of sorgoleone from leaves to roots, where it is subsequently exuded into the soil. Exploring genes and functional genomics becomes imperative to unravel the role of transcriptional modulation of genes and regulatory factors involved in sorgoleone biosynthesis. This foundational work is essential before advancing technologies aimed at reducing dependence on synthetic herbicides. Ongoing research in this field holds promise in unveiling the intricacies of sorgoleone’s biosynthesis and function, with potential applications in agriculture and ecology. This continual exploration contributes to a deeper understanding of plant biology, enabling us to leverage it to enhance agricultural productivity.
References
Abbas T, Nadeem MA, Tanveer A, Chauhan S (2016) Can hormesis of plant-released phytotoxins be used to boost and sustain crop production? Crop Prot 93:69–76. https://doi.org/10.1016/j.cropro.2016.11.020
Abdelhalim T, Jannoura R, Joergensen RG (2019) Mycorrhiza response and phosphorus acquisition efficiency of sorghum cultivars differing in strigolactone composition. Plant Soil 437:55–63. https://doi.org/10.1007/s11104-019-03960-y
Alsaadawi IS, Dayan FE (2009) Potentials and prospects of sorghum allelopathy in agroecosystems. Allelopath J 24:255–270
Alsaadawi IS, Al-Ekelle MHS, Al-Hamzawi MK (2007) Differential allelopathic potential of grain sorghum genotypes to weeds. Allelopath J 19:153–159
Ashraf M, Akhlaq M (2007) Effects of sorghum leaves, roots and stems water extract, hand weeding and herbicide on weeds suppression and yield of wheat. Sarhad J Agric 2:321e327
Baerson SR, Dayan FE, Rimando AM, Nanayakkara ND, Liu CJ, Schroder J, Duke SO (2008) A functional genomics investigation of allelochemical biosynthesis in Sorghum bicolor root hairs. J Biol Chem 283:3231–3247. https://doi.org/10.1074/jbc.M706587200
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266. https://doi.org/10.1146/annurev.arplant.57.032905.105159
Bertin C, Yang X, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil. https://doi.org/10.1146/annurev.arplant.57.032905.105159
Besançon TE, Dayan FE, Gannon TW, Everman WJ (2020) Conservation and divergence in sorgoleone production of sorghum species. J Environ Qual 49:368–377. https://doi.org/10.1002/jeq2.20038
Biesdorf EM (2017) Alelopatia do sorgo granífero sobre a soja e as plantas daninhas. Universidade Federal de Viçosa, Dissertação
Blum U (1995) The value of model plant-microbe-soil systems for understanding processes associated with allelopathic interaction: One example. ACS Symposium Series 582. https://doi.org/10.1021/bk-1995-0582.ch009.
Blum U (1996) Allelopathic interactions involving phenolic acids. J Nematol 28:259–267
Blum U, Shafer SR, Lehman ME (1999) Evidence for inhibitory allelopathic interactions involving phenolic acids in field soils: concepts vs. an experimental model. Crit Rev Plant Sci. https://doi.org/10.1080/07352689991309441
Campos TS, Sousa AGV, Rego JS, Santos W, Sousa, Benett CGS, Arruda N (2020) Allelopathic effect of Sorghum bicolor and Digitaria insularis on germination and initial development of Canola. Rev Agric Neotrop 7:65–72. https://doi.org/10.32404/rean.v7i4.4230
Chang M, Netzly DH, Butler LG, Lynn DG (1986) Chemical regulation of distance. Characterization of the first natural host germination stimulant for Striga asiatica. J Am Chem Soc 108:7858–7860. https://doi.org/10.1021/ja00284a074
Cheema ZA, Khaliq A, Akhtar S (2001) Use of sorgaab (sorghum water extract) as a natural weed inhibitor in spring mungbean. Int J Agric Biol 3:515–518
Cheema ZA, Khaliq A, Mubeen M (2003) Response of wheat and winter weeds to foliar application of different plant water extracts of sorghum (Sorghum bicolor). Pak J Weed Sci Res 9:89–97
Cheema ZA, Mushtaq MN, Farooq M, Hussain A (2009) Purple nutsedge management with allelopathic sorghum. Allelopath J 23:305–312
Chiu CH, Paszkowski U (2019) Mechanisms and impact of symbiotic phosphate acquisition. Cold Spring Harb Perspect Biol 11:a034603. https://doi.org/10.1101/cshperspect.a034603
Czarnota MA, Rimando AM, Weston LA (2003) Evaluation of seven sorghum (Sorghum sp) accessions. J Chem Ecol 29:2073–83. https://doi.org/10.1023/a:1025634402071
Dalton BR (1999) The occurrence and behavior of plant phenolic acids in soil environments and their potential involvement in allelochemical interference interactions: Methodological limitations in establishing conclusive proof of allelopathy. In Principles and practices in plant ecology pp 57–74. CRC Press.
Dayan FE (2006) Factors modulating the levels of the allelochemical sorgoleone in Sorghum bicolor. Planta 224:339–346. https://doi.org/10.1007/s00425-005-0217-5
Dayan FE, Kagan IA, Rimando AM (2003) Elucidation of the biosynthetic pathway of the allelochemical sorgoleone using retrobiosynthetic NMR analysis. J Biol Chem 278:28607–28611. https://doi.org/10.1074/jbc.M304185200
Dayan FE, Watson SB, Nanayakkara ND (2007) Biosynthesis of lipid resorcinols and benzoquinones in isolated secretory plant root hairs. J Exp Bot 58:3263–3272. https://doi.org/10.1093/jxb/erm173
Dayan FE, Howell JL, Weidenhamer JD (2009) Dynamic root exudation of sorgoleone and its in planta mechanism of action. J Exp Bot 60:2107–2117. https://doi.org/10.1093/jxb/erp082
Dayan FE, Rimando AM, Pan Z, Baerson SR, Gimsing AL, Duke SO (2010) Sorgoleone. Phytochemistry 71:1032–1039. https://doi.org/10.1016/j.phytochem.2010.03.011
De Novais CB, Borges WL, da Conceicão JE, Júnior OJS, Siqueira JO (2014) Inter-and intraspecific functional variability of tropical arbuscular mycorrhizal fungi isolates colonizing corn plants. Appl Soil Ecol 76:78–86. https://doi.org/10.1016/j.apsoil.2013.12.010
De Oliveira IF, Simeone MLF, De Guimarães CC, Garcia NS, Schaffert RE, De Sousa SM (2021) Sorgoleone concentration influences mycorrhizal colonization in sorghum. Mycorrhiza 31:259–264. https://doi.org/10.1007/s00572-020-01006-1
Duke SO (2003) Weeding with transgenes. Trends Biotechnol 21:192–195. https://doi.org/10.1016/S0167-7799(03)00056-8
Einhellig FA, Souza IF (1992) Phytotoxicity of sorgoleone found in grain sorghum root exudates. J Chem Ecol 18:1–11. https://doi.org/10.1007/BF00997160
Erickson J, Schott D, Reverri T, Muhsin W, Ruttledge T (2001) GC-MS analysis of hydrophobic root exudates of sorghum and implications on the parasitic plant Striga asiatica. J Agric Food Chem 49:5537–5542. https://doi.org/10.1021/jf0111099
Farooq M, Jabran K, Cheema ZA, Wahid A, Siddique KHM (2011a) The role of allelopathy in agricultural pest management. Pest Manag Sci 67:493–506. https://doi.org/10.1002/ps.2091
Farooq M, Habib M, Rehman A, Wahid A, Munir R (2011b) Employing aqueous allelopathic extracts of sunflower in improving salinity tolerance of rice. J Agric Soc Stud 7:75–80
Fate GD, Lynn DG (1996) Xenognosin methylation is critical in defining the chemical potential gradient that regulates the spatial distribution in Striga pathogenesis. J Am Chem Soc 118:11369–11376. https://doi.org/10.1021/ja961395i
Ferreira ML, de Almeida Barbosa LC, Demuner AJ, da Silva AA, Wakil J (1999) Análise e quantificação da sorgoleona em diferentes cultivares de sorgo (Sorghum bicolor L.). Acta Sci Agron 21:565–570. https://doi.org/10.4025/actasciagron.v21i0.4288
Franco FHS, Machado Y, Takahashi JA, Karam D, Garcia QS (2011) Quantification of sorgoleone in sorghum extracts and roots under different storage periods. Planta Daninha 29:953–962. https://doi.org/10.1590/S0100-83582011000500001
Garcia RA, Sutier GAS (2016) Alelopatia de sorgo-sacarino na soja cultivada em sucessão. Dourados: Embrapa Agropecuária Oeste, 28 p. (Boletim de Pesquisa e Desenvolvimento, 74).
Głąb L, Sowiński J, Bough R, Dayan FE (2017) Allelopathic potential of sorghum (Sorghum bicolor (L.) Moench) in weed control: a comprehensive review. Adv Agron 145:43–95. https://doi.org/10.1016/bs.agron.2017.05.001
Gomes TC, Simeone MLF, Karam D, Dias LC (2023) The allelopathic effect of sorghum and its links to the extract composition and the sorgoleone. Rev Agri-Environ Sci 9:e023009. https://doi.org/10.36725/agries.v9i1.8453
Gross EM (1999) Allelopathy in benthic and littoral areas: case studies on allelochemicals from benthic cyanobacteria and submersed macrophytes. In: Principles and practices in plant ecology. CRC Press. pp 179–199.
Hejl AM, Koster KL (2004) The allelochemical sorgoleone inhibits root H+-ATPase and water uptake. J Chem Ecol 30:2181–2191. https://doi.org/10.1023/b:joec.0000048782.87862.7f
Hess DE, Ejeta G, Butler LG (1992) Selecting sorghum genotypes expressing a quantitative biosynthetic trait that confers resistance to Striga. Phytochemistry 31:493–497. https://doi.org/10.1016/0031-9422(92)90023-J
Inderjit (1996) Plant phenolics in allelopathy. Bot Rev 62:86–202
Inderjit, Duke SO (2003) Ecophysiological aspects of allelopathy. Planta 217:529–539. https://doi.org/10.1007/s00425-003-1054-z
Jahangeer A (2011) Response of Maize (Zea mays L.) to Foliar Application of Three Plant Water Extracts. MSc Thesis. Department of Agronomy, University of Agriculture, Faisalabad, Pakistan.
Jamil M, Cheema ZA, Mushtaq MN, Farooq M, Cheema MA (2009) Alternative control of wild oat and canary grass in wheat fields by allelopathic plant water extracts. Agron Sustain Dev 29:475–482. https://doi.org/10.1051/agro/2009007
Jesudas PA, Kingsley SJ, Ignacimuthu S (2014) Sorgoleone from Sorghum bicolor as a potent bioherbicide. Res J Recent Sci 3:32–36
Kagan IA, Rimando AM, Dayan FE (2003) Chromatographic separation and in vitro activity of sorgoleone congeners from the roots of Sorghum bicolor. J Agric Food Chem 51:7589–7595. https://doi.org/10.1021/jf034789j
Kamran M, Cheema ZA, Farooq M, Hassan AU (2016) Influence of foliage applied allelopathic water extracts on the grain yield, quality and economic returns of hybrid maize. Int J Agric Biol 18:577–583
Khan EA, Khakwani AA, Ghazanfarullah A (2015) Effects of allelopathic chemicals extracted from various plant leaves on weed control and wheat crop productivity. Pak J Bot 47:735–740
Kobae Y, Kameoka H, Sugimura Y, Saito K, Ohtomo R, Fujiwara T, Kyozuka J (2018) Strigolactone biosynthesis genes of rice are required for the punctual entry of arbuscular mycorrhizal fungi into the roots. Plant Cell Physiol 59:544–553. https://doi.org/10.1093/pcp/pcy001
Lanfranco L, Fiorilli V, Gutjahr C (2018) Partner communication and role of nutrients in the arbuscular mycorrhizal symbiosis. New Phytol 220:1031–1046. https://doi.org/10.1111/nph.15230
Leu E, Krieger-Liszkay A, Goussias C, Gross EM (2002) Polyphenolic allelochemicals from the aquatic angiosperm Myriophyllum spicatum inhibit photosystem II. Plant Physiol 130:2011–2018. https://doi.org/10.1104/pp.011593
Liu H, Carvalhais LC, Crawford M, Singh E, Dennis PG, Pieterse CM, Schenk PM (2017) Inner plant values: diversity, colonization and benefits from endophytic bacteria. Front Microbiol Front Microbiol 8:2552. https://doi.org/10.3389/fmicb.2017.02552
Lydon J, Teasdale JR, Chen PK (1997) Allelopathic activity of annual wormwood (Artemisia annua) and the role of artemisinin. Weed Sci 45:807–811. https://doi.org/10.1017/S0043174500089001
MacLean AM, Bravo A, Harrison MJ (2017) Plant signaling and metabolic pathways enabling arbuscular mycorrhizal symbiosis. Plant Cell 29:2319–2335. https://doi.org/10.1105/tpc.17.00555
Maharjan B, Vitha S, Okumoto S (2023) Developmental regulation and physical interaction among enzymes involved in sorgoleone biosynthesis. Plant J 115:820–832. https://doi.org/10.1111/tpj.16263
Manandhar S, Shrestha BB, Lekhak HD (2010) Weeds of paddy field at Kirtipur. Kathmandu Sci World 5:100–106. https://doi.org/10.3126/sw.v5i5.2665
May A, Cerdeira A, Queiroz SDN, Santos MDS, Corniani N, Da Silva EHFM (2016) Avaliação do teor de sorgoleone presente em extratos de raízes de cultivares de sorgo por cromatografia líquida de alta eficiência (HPLC). Sete Lagoas: Embrapa Milho e Sorgo, 21 p. (Boletim de Pesquisa e Desenvolvimento, 145).
Meazza G, Scheffler BE, Tellez MR, Rimando AM, Romagni JG, Duke SO, Dayan FE (2002) The inhibitory activity of natural products on plant p-hydroxyphenylpyruvate dioxygenase. Phytochemistry 60:281–288. https://doi.org/10.1016/s0031-9422(02)00121-8
Mizuno CS, Rimando AM, Duke SO (2010) Phytotoxic activity of quinones and resorcinolic lipid derivatives. J Agric Food Chem 58:4353–4355. https://doi.org/10.1021/jf100108c
Naby KY, Ali KA (2021) Allelopathic potential of Sorghum bicolor L root exudates on growth and chlorophyll content of wheat and some grassy weeds. IOP Conf Ser: Earth Environ Sci 761:012085. https://doi.org/10.1088/1755-1315/761/1/012085
Netzly DH, Butler LG (1986) Roots of sorghum exude hydrophobic droplets containing biologically active components 1. Crop Sci 26:775–778. https://doi.org/10.2135/cropsci1986.0011183X002600040031x
Netzly DH, Riopel JL, Ejeta G, Butler LG (1988) Germination stimulants of witchweed (Striga asiatica) from hydrophobic root exudate of sorghum (Sorghum bicolor). Weed Sci 36:441–446. https://doi.org/10.1017/S0043174500075172
Nimbal CI, Pedersen JF, Yerkes CN, Weston LA, Weller SC (1997) Phytotoxicity and distribution of sorgoleone in grain sorghum germplasm. J Agric Food Chem 44:1343–1347. https://doi.org/10.1021/jf950561n
Oda Y, Elmore JR, Nelson WC, Wilson A, Farris Y, Shrestha R, Egbert R (2023) Sorgoleone degradation by sorghum-associated bacteria; an opportunity for enforcing plant growth promotion. bioRxiv. https://doi.org/10.1101/2023.05.26.542311
Olibone D, Calonego JC, Pavinato PS, Rosolem CA (2006) Early growth of soybean as affected by sorghum crop residues. Planta Daninha 24:255–261. https://doi.org/10.1590/S0100-83582006000200007
Ortas I, Bilgili G (2022) Mycorrhizal species selectivity of sweet sorghum genotypes and their effect on nutrients uptake. Acta Agric Scand, Sec B—Soil Plant Sci 72:733–743. https://doi.org/10.1080/09064710.2022.2063167
Paixão MQ (2019). Desempenho da soja cultivada em sucessão a milho e a sorgo. 31 f. Dissertação (Mestrado em Fitotecnia)—Universidade Federal de Viçosa, Viçosa.
Pan Z, Rimando AM, Baerson SR, Fishbein M, Duke SO (2007) Functional characterization of desaturases involved in the formation of the terminal double bond of an unusual 16:3Delta(9,12,150) fatty acid isolated from Sorghum bicolor root hairs. J Biol Chem 282:4326–4335. https://doi.org/10.1074/jbc.M606343200
Pan Z, Baerson SR, Wang M, Bajsa-Hirschel J, Rimando AM, Wang X, Duke SO (2018) A cytochrome P450 CYP 71 enzyme expressed in Sorghum bicolor root hair cells participates in the biosynthesis of the benzoquinone allelochemical sorgoleone. New Phytologis 218:616–629. https://doi.org/10.1111/nph.15037
Pan Z, Bajsa-Hirschel J, Vaughn JN, Rimando AM, Baerson SR, Duke SO (2021) In vivo assembly of the sorgoleone biosynthetic pathway and its impact on agroinfiltrated leaves of Nicotiana benthamiana. New Phytol 230:683–697. https://doi.org/10.1111/nph.17213
Pedersen CT, Sylvia DM (1996) Mycorrhiza: ecological implications of plant interactions. In: Concepts in mycorrhizal research. Dordrecht: Springer Netherlands, pp 195–222. https://doi.org/10.1007/978-94-017-1124-1_8.
Petroski RJ, Stanley DW (2009) Natural compounds for pest and weed control. J Agric Food Chem 57:8171–8179
Rasmussen JA, Hejl AM, Einhellig FA, Thomas JA (1992) Sorgoleone from root exudate inhibits mitochondrial functions. J Chem Ecol 18:197–207. https://doi.org/10.1007/BF00993753
Rimando AM, Dayan FE, Czarnota MA, Weston LA, Duke SO (1998) A new photosystem II electron transfer inhibitor from Sorghum bicolor. J Nat Prod 61:927–930. https://doi.org/10.1021/np9800708
Rimando AM, Dayan FE, Streibig JC (2003) PSII inhibitory activity of resorcinolic lipids from Sorghum bicolor. J Nat Prod 66:42–45. https://doi.org/10.1021/np0203842
Sabahie M, Vazan S, Oveisi M, Golzardi F (2014) Evaluation of allelopathic effects of aqueous extract of sorghum crops (Sorghum bicolor L.) on germination red root pigweed (Amaranthus retroflexus L.). Bull Environ Pharmacol Life Sci 3:129–132
Sarr PS, Nakamura S, Ando Y, Iwasaki S, Subbarao GV (2021) Sorgoleone production enhances mycorrhizal association and reduces soil nitrification in sorghum. Rhizosphere 17:100283. https://doi.org/10.1016/j.rhisph.2020.100283
Sène M, Doré T, Pellissier F (2000) Effect of phenolic acids in soil under and between rows of a prior sorghum (Sorghum bicolor) crop on germination, emergence, and seedling growth of peanut (Arachis hypogea). J Chem Ecol 26:625–637. https://doi.org/10.1023/A:1005420020135
Shehzad T, Okuno K (2020) Genetic analysis of QTLs controlling allelopathic characteristics in sorghum. PLoS ONE 15:e0235896. https://doi.org/10.1371/journal.pone.0235896
Silva AP, Salton JC, Filho OFL, Ramos FS (2016) Estabelecimento e fitomassa aérea da soja em cultivo subsequente ao sorgo sacarino. In: Jornada de Iniciação à Pesquisa da Embrapa, 2016, Dourados. Resumos... Brasília, DF: Embrapa, 2016. JIPE 2016.
Siqueira JO, Saggin-Júnior OJ, Flores-Aylas WW, Guimarães PT (1998) Arbuscular mycorrhizal inoculation and superphosphate application influence plant development and yield of coffee in Brazil. Mycorrhiza 7:293
Tibugari H, Chiduza C, Mashingaidze AB, Mabasa S (2020) High sorgoleone autotoxicity in sorghum (Sorghum bicolor (L) Moench) varieties that produce high sorgoleone content. South Afr J Plant Soil 37:160–167. https://doi.org/10.1080/02571862.2020.1711539
Trezzi MM, Vidal RA, Dick DP, Peralba MCR, Kruse ND (2006) Sorptive behavior of sorgoleone in ultisol in two solvent systems and determination of its lipophilicity. J Environ Sci Health B 41:345–356. https://doi.org/10.1080/03601230600613780
Uddin MR, Kim YK, Park SU, Pyon JY (2009) Herbicidal activity of sorgoleone from grain sorghum root exudates and its contents among sorghum cultivars. 한국잡초학회지. 29:229–236.
Uddin MR, Park KW, Kim YK, Park SU, Pyon JY (2010) Enhancing sorgoleone levels in grain sorghum root exudates. J Chem Ecol 36:914–922. https://doi.org/10.1007/s10886-010-9829-8
Uddin MR, Park SU, Dayan FE, Pyon JY (2014) Herbicidal activity of formulated sorgoleone, a natural product of sorghum root exudate. Pest Manag Sci 70:252–257. https://doi.org/10.1002/ps.3550
Vaughan D, Ord BG (1991) Extraction of potential allelochemicals and their effects on root morphology and nutrient contents. Plant Root Growth: An Ecol Perspect 399421:399–421
Vejan P, Abdullah R, Khadiran T, Ismail S, Nasrulhaq Boyce A (2016) Role of plant growth promoting rhizobacteria in agricultural sustainability—a review. Molecules 21:573. https://doi.org/10.3390/molecules21050573
Wang P, Chai YN, Roston R, Dayan FE, Schachtman DP (2021) The Sorghum bicolor root exudate sorgoleone shapes bacterial communities and delays network formation. mSystems 6:10–1128. https://doi.org/10.1128/msystems.00749-20
Wen Z, Li H, Shen Q, Tang X, Xiong C, Li H, Shen J (2019) Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytol 223:882–895. https://doi.org/10.1111/nph.15833
Weston LA, Czarnota MA (2001) Activity and persistence of sorgoleone, a long-chain hydroquinone produced by Sorghum bicolor. J Crop Prod 4:363–377. https://doi.org/10.1300/J144v04n02_17
Weston LA, Alsaadawi IS, Baerson SR (2013) Sorghum allelopathy—from ecosystem to molecule. J Chem Ecol. https://doi.org/10.1007/s10886-013-0245-8
Yang X, Owens TG, Scheffler BE, Weston LA (2004) Manipulation of root hair development and sorgoleone production in sorghum seedlings. J Chem Ecol 30:199–213. https://doi.org/10.1023/b:joec.0000013191.35181.03
Yoneyama K, Arakawa R, Ishimoto K, Kim HI, Kisugi T, Xie X, Yoneyama K (2015) Difference in Striga-susceptibility is reflected in strigolactone secretion profile, but not in compatibility and host preference in arbuscular mycorrhizal symbiosis in two maize cultivars. New Phytol 206:983–989. https://doi.org/10.1111/nph.13375
Acknowledgements
This research was funded by Empresa Brasileira de Pesquisa Agropecuária—Embrapa (Grant number 20.20.03.024.00.00), Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq/INCT—‘Plant-Growth Promoting Microorganisms for Agricultural Sustainability and Environmental Responsibility’ (Grant number 465133/2014–2, Fundação Araucaria-STI, Capes). I.F.O. was recipient of a research fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Capes and T.C.G. was recipient of a research fellowship from Universidade Federal de São João del-Rei—UFSJ.
Author information
Authors and Affiliations
Contributions
Conceptualization, supervision, writing and editing, S.M.S.T.; writing—original draft preparation, I.F.O. and T.C.G.; writing—review and editing, M.L.F.S., D.K. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Author SMST is an Associate Editor for Brazilian Journal of Botany and the peer-review process for this article was independently handled by another member of the journal editorial board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira, I.F., Gomes, T.C., Simeone, M.L.F. et al. Sorgoleone unveiled: exploring its biosynthesis, functional perspectives and applications. Braz. J. Bot 47, 723–733 (2024). https://doi.org/10.1007/s40415-024-01026-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-024-01026-7