Abstract
In the present study, we investigate the composition, morphological characteristics and ecological aspects of Euastrum taxa associated with three species of aquatic macrophytes (Utricularia foliosa L., Cabomba caroliniana A. Gray, and Eichhornia azurea Kunth) of the Marimbus do Baiano (Chapada Diamantina), “Caatinga” domain, Bahia State, Brazil. 180 sampling units of periphytic material were analyzed, collected between May/2017–March/2018, revealing a total of 32 taxa. The highest richness was observed in the periphyton of U. foliosa (30), followed by C. caroliniana (24) and E. azurea (23). Nineteen Euastrum taxa were considered rare, whereas five taxa (Euastrum abruptum Nordst., E. abruptum var. chapadae F.M. Costa, G.J.P. Ramos and C.W.N. Moura, E. elegans var. prescottii (Förster) F.M. Costa and C.W.N. Moura, E. evolutum var. integrius West and G.S. West, and E. ornatiscrobiculatum F.M. Costa, I.B. Oliveira and C.W.N. Moura) have stood out for having the highest frequencies of occurrence, being common to the three macrophytes. Most taxa were recorded in an environment with slightly alkaline pH, low electrical conductivity and high dissolved oxygen level. This study highlights the importance of aquatic macrophytes for periphytic communities and expands knowledge about Euastrum in the “Caatinga” domain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Euastrum Ehrenberg ex Ralfs is considered one of the most representative genera of Desmidiaceae, comprising about 649 taxonomically accepted species (Guiry and Guiry 2021), and so like other groups of the family, has a wide geographic distribution, mainly in tropical and subtropical regions (Coesel 1996; Moresco et al. 2009), occurring in oligo-mesotrophic aquatic environments, with low conductivity and slightly acidic (Coesel and Meesters 2007; Stastny 2010). The genus is polyphyletic (Gontcharov and Melkonian 2005; Hall et al. 2008) and polymorphic, characterized by having apical lobes with a U- or V-shaped medial incision (Brook 1981; Anissimova 2016; Bicudo and Menezes 2017).
In Brazil, there are reported 64 species and 53 non-typical Euastrum varieties (Araújo et al. 2015; Menezes et al. 2015; Flora do Brasil 2020); of these, 37 species and 41 non-typical varieties were listed for the state of Bahia. Part of these taxa appears in the study performed by Förster (1964), who analyzed material associated with Utricularia L. (Lentibulariaceae) from the Chapada Diamantina region. Oliveira et al. (2011, 2017) also studied periphyton from several macrophytes in lakes and rivers on the north coast of Bahia, and Costa et al. (2018, 2020) carried out a study on material associated with macrophytes from the Pantanal dos Marimbus, Chapada Diamantina.
In lotic and lentic environments, aquatic macrophytes function as natural substrates for colonization of the periphytic community (Ferreiro et al. 2014). These plants have great ecological importance, influencing nutrient availability for the periphyton (Felisberto and Murakami 2013), allelopathic substances production, and shading promoted light reduction (Erhard and Gross 2006; Meerhoff et al. 2007). The macrophytes with certain characteristics tend to favor the occurrence of desmids, for example, macrophyte that have cut leaves usually support a more diverse desmids community (Laugaste and Reunanen 2005; Mutinová et al. 2016). Among the periphytic algae community, desmids emerge as one of the predominant groups (Díaz-Olarte et al. 2007; Díaz-Olarte and Duque 2009; Menezes et al. 2013), having genera with high specific diversity such as Cosmarium Corda ex Ralfs and Euastrum.
The characteristics physical (architecture, composition, orientation, roughness) and chemical (allelopathy and excretion of nutrients) of macrophytes affect the periphyton structure (Gross 2003; Zhang et al. 2013), being as interactions between these plants and the desmids community potentially higher in floodplains (Domozych and Domozych 2007; Rosen et al. 2019). Therefore, given the necessity of taxonomic studies on desmids from the Caatinga, we investigated the composition and morphological characteristics of Euastrum taxa associated with three species of aquatic macrophytes (Utricularia foliosa Linnaeus, Cabomba caroliniana A. Gray, and Eichhornia azurea Kunth) in a floodplain area (Pantanal dos Marimbus) in northeastern Brazil. Our main question was whether the: (a) does taxa composition of Euastrum change between macrophytes studied? (b) What are the main morphological characteristics of the taxa studied in the Pantanal dos Marimbus? (c) Do environmental variables influence of Euastrum variability?
2 Material and methods
Study area
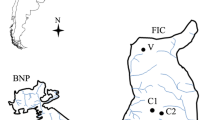
– The study was carried out in Marimbus do Baiano, southern region of the Pantanal dos Marimbus, located in the municipality of Andaraí (Fig. 1a), in Chapada Diamantina, Bahia, Brazil. The Pantanal dos Marimbus is formed by the confluence of the Santo Antônio, Utinga, and São José rivers, characterized by flooded areas where several species of aquatic macrophytes occur (Utricularia foliosa, Cabomba caroliniana, Eichhornia azurea, Nymphaea amazonum Mart & Zucc and Salvinia auriculata Aublet) (França et al. 2010; Ramos et al. 2014; Lima et al. 2018).
Sampling
– Periphytic samples were collected bimonthly (May, July, September and November/2017; January and March/2018), in 10 sampling stations along the floodplain lake (Fig. 1b). Based on life forms, three species of macrophytes were selected: Cabomba caroliniana, macrophyte rooted submerged, characterized by light branching and highly cut leaves; Eichhornia azurea, macrophyte emerged floater characterized by branched roots and entire leaves; and Utricularia foliosa, macrophyte free submerged, characterized by dense branching and numerous smaller leaves.
We obtained a total of 180 sampling units of periphytic material from the squeeze of macrophyte species whole for qualitative analysis. All samples were preserved in the Transeau solution (Bicudo and Menezes 2017).
Abiotic water and meteorological variables
– In each sampling station, we also measured the abiotic variables of the water such as temperature (°C), pH, conductivity (µS cm−1), and total dissolved solids using the portable Hanna HI98130 probe, dissolved oxygen (mg L−1) using the portable Instrutherm probe (MO-910), and water transparency by secchi disk.
Historical precipitation data (30 years) for the collection period in the municipality of Andaraí were obtained from the National Institute of Meteorology—INMET (2018).
Microscopic analysis
– We analyzed the Euastrum specimens in an Olympus BX45 microscope and photographed using a MicroPublisher-QImaging digital camera and Image-Pro Premier 9.1.4 software. The methodology proposed by Mann et al. (2007) and Pickett-Heaps (1974) was adapted, separating aliquots of the material, which were transferred to Eppendorf and added sodium hypochlorite (NaClO) at 2% or sodium hydroxide (NaOH) at 20% (1:1, v/v) for 24 h to show the ornamentation of the cell wall of taxa under light microscopy. We incorporated all sampling units into the liquid collection in the herbarium of the Universidade Estadual de Feira de Santana (HUEFS).
Taxonomic identification and analyzed attributes
– The specimens were identified based on morphological characteristics, such as cell and semi-cell shape; cell size identified by its maximum length, maximum width, maximum polar lobe width, and isthmus width; number and shape of apical and basal lobes; the shape of the lateral margins of the semi-cell; type of midline apical incisions; type of cell wall ornamentation. All identifications were performed based on specialized literature (Krieger 1937; Förster 1964, 1969; Prescott et al. 1977; Růžička 1977, 1981; Croasdale and Flint 1986; Coesel and Meesters 2007). Species richness was determined by the number of taxa in each sample.
The taxa occurrence frequency was calculated according to the formula: F = n·100/N, where: n = number of samples in which a species was recorded, and N = total samples analyzed. The frequency categories were determined according to Matteucci and Colma (1982): > 70%—very frequent (MF); ≤ 70% to > 40%—frequent (F); ≤ 40% to > 10%—uncommon (I); ≤ 10%—rare (R).
Data analysis
– We applied the Kruskal–Wallis test to detect significant differences (α = 0.05) in the abiotic variables between the collection months, after checking normality (Shapiro–Wilk test) and homoscedasticity (Levene test) (Hammer et al. 2001).
Non-metric multidimensional scaling (NMDS) was applied to the data matrix of Euastrum composed of five categories (incision deep, wall with granules and spines, wall only with scrobicules, size < 59 µm, size > 60 µm), filled with 1 (presence of category) and 0 (absence of category). This analysis aimed to detect differences between the morphological characteristics and the taxa size, using the Jaccard similarity index (Legendre and Legendre 1998), performed in e R software vegan package (R Development Core Team 2009).
We constructed the Venn diagram to demonstrate Euastrum taxa distribution richness among the three macrophyte species studied, using software available on The Bioinformatics and Evolutionary Genomics group website at the University of Ghent, Belgium (http://bioinformatics.psb.ugent.be/webtools/Venn/).
3 Results
Meteorological conditions and abiotic water variables
– The average precipitation of the sampled period presented values below the historical average of the last 30 years (Fig. 2). The months of July and September/17 had a low volume of precipitation (around 45 mm), characteristic of the dry season, while November/17 and March/18 were typical of the rainy season, with 161 and 230 mm, respectively.
All abiotic water variables showed significant differences between the sampled months (Fig. 3). Water temperature recorded the lowest mean value in July/17 (26.6 °C) and highest in January and March/18 (30.3 and 31.0 °C, respectively). We verified the highest dissolved oxygen concentration in March/18 (14.8 mg L−1), followed by July and September/17 (6.2 and 8.7 mg L−1, respectively). During the collection period, water conditions were slightly alkaline, except for May/17 and March/18, where they were slightly acidic (mean 6.7). The conductivity values, as well as the total dissolved solids, were considerably different throughout the study period and with a wide range of variation, especially in July and September/17 and January/18; in both variables, the lowest means (48 µS cm−1, 0.02 ppt) were recorded in the month with the highest rainfall (March/18). Regarding water transparency, we registered the highest average in January/18 (1.34 m) and the lowest in July (0.47 m) and September/17 (0.50 m).
Taxonomic composition and morphology
– We identified a total of 32 Euastrum taxa from the periphyton of the macrophytes studied (Table 1). In all months sampled, Utricularia foliosa was the macrophyte with the highest taxonomic richness (30 taxa), followed by C. caroliniana (24 taxa) and E. azurea (23 taxa).
Comparing the occurrence of the taxa over the months, it was evident that U. foliosa presented the highest richness values, with emphasis on May/17 (26 taxa) and November/17 (22 taxa). On the other hand, we registered the lower values in March/18 for all studied macrophytes: U. folisosa (14 taxa), C. caroliniana (11 taxa), and E. azurea (9 taxa) (Fig. 4). Considering the months of lowest and highest rainfall, we noticed that ten taxa were exclusive to the driest months: Euastrum abruptum var. lagoense (Nordstedt) Willi Krieger, E. angolense var. brasiliense Willi Krieger, E. attenuatum var. brasiliense Grönblad, E. brasiliense Borge, E. ciastonii Raciborski, E. denticulatum var. rectangulare West and G.S. West, E. evolutum var. monticulosum (W.R. Taylor) Willi Krieger, E. groenbladii A.M. Scott and H. Croasdale, E. obesum Joshua, and E. westenii F.M. Costa, I.B. Oliveira and C.W.N. Moura. No taxon was registered exclusively in the rainy months (Table 2).
Five taxa (Euastrum abruptum Nordstedt, E. abruptum var. chapadae F.M. Costa, G.J.P. Ramos and C.W.N. Moura, E. elegans var. prescottii (Förster) F.M. Costa and C.W.N. Moura, E. evolutum var. integrius West and G.S. West, and E. ornatiscrobiculatum F.M. Costa, I.B. Oliveira and C.W.N. Moura) stood out for having the highest frequency of occurrence and for being present in the three studied macrophytes (Table 2). Euastrum ansatum Ehrenberg ex Ralfs and E. praemorsum var. foersteri F.M. Costa, C.E.M. Bicudo and C.W.N. Moura were frequent in U. foliosa and E. azurea. However, we observed that most taxa studied were classified in the rare category (18), followed by the uncommon (6). Most taxa occurred in well-oxygenated waters, slightly alkaline and with an average conductivity of 80 or 90 µS cm−1. However, some were recorded in slightly acidic waters (E. abruptum var. lagoense, E. obesum, and E. westenii).
Considering all registered taxa, 19 of them occurred in the three species of macrophytes (Fig. 5, Table 2). Regarding the macrophyte-exclusive taxa, we detected only five in Utricularia foliosa (E. abruptum var. lagoense, E. angolense var. brasiliense, E. brasiliense, E. obesum, and E. westenii). On the other hand, E. ciastonii and E. attenuatum var. brasiliense Grönblad were exclusive to Cabomba caroliniana and Eichhornia azurea, respectively.
As for morphology, the NMDS revealed the formation of two clusters that brought together most of the taxa (Fig. 6). Group I, with 19 taxa, included those smaller than 59 μm in length and with a cell wall ornamented mainly by granules and spines, and a deep apical incision; Euastrum abruptum (Fig. 7a), E. abruptum var. chapadae (Fig. 7b), E. abruptum var. lagoense (Fig. 7c), E. bidentatum var. scottii (Fig. 7h), E. ciastonii (Fig. 7j), E. denticulatum var. rectangulare (Fig. 7k), E. elegans var. prescottii (Fig. 7m), E. evolutum (Fig. 7n), E. evolutum var. integrius (Fig. 7o), E. fissum var. nordestinum (Fig. 8b), E. gemmatum (Fig. 8d), E. informe var. oculatum (Fig. 8f), E. marimbusense (Fig. 8h), E. ornatiscrobiculatum (Fig. 8j), E. praemorsum var. forsteri (Fig. 8m), E. sibiricum (Fig. 9a), E. subornatum var. brasiliense (Fig. 9c), E. westenii (Fig. 9d). Group II gathered five taxa lengths greater than 60 μm and cell wall ornamented only by scrobicules; E. ansatum (Fig. 7e), E. ansatum var. concavum (Fig. 7f), E. brasiliense (Fig. 7i), E. didelta var. quadriceps (Fig. 7l), E. subintegrum var. brasiliense (Fig. 9b).
Nonmetric multidimensional scaling (NMDS) ordination of Euastrum taxa based on their morphological characteristics. Abbreviations: S size, ID incision deep, WGS wall with granules and spines, WOS wall only with scrobicles. Numbers represent the taxa: (1) Euastrum abruptum, (2) E. abruptum var. chapadae, (3) E. abruptum var. lagoense, (4) E. angolense var. brasiliense, (5) E. ansatum, (6) E. ansatum var. concavum, (7) E. attenuatum var. brasiliense, (8) E. bidentatum var. scottii, (9) E. brasiliense, (10) E. ciastonii, (11) E. denticulatum var. rectangulare, (12) E. didelta var. quadriceps, (13) E. elegans var. prescottii, (14) E. evolutum, (15) E. evolutum var. integrius, (16) E. evolutum var. monticulosum, (17) E. fissum var. nordestinum, (18) E. gayanum var. angulatum, (19) E. gemmatum, (20) E. groenbladii, (21) E. informe var. oculatum, (22) E. luetkemuellerii var. carniolicum, (23) E. marimbusense, (24) E. obesum, (25) E. ornatiscrobiculatum, (26) E. pectinatum var. pinheirense, (27) E. platycerum var. groenbladii, (28) E. praemorsum var. forsteri, (29) E. sibiricum, (30) E. subintegrum var. brasiliense, (31) E. subornatum var. brasiliense, (32) E. westenii
Euastrum taxa. a E. abruptum; b E. abruptum var. chapadae; c E. abruptum var. lagoense; d E. angolense var. brasiliense; e E. ansatum; f E. ansatum var. concavum; g E. attenuatum var. brasiliense; h E. bidentatum var. scottii; i E. brasiliense; j E. ciastonii; k E. denticulatum var. rectangulare; l E. didelta var. quadriceps; m E. elegans var. prescottii; n E. evolutum; o E. evolutum var. integrius. Scale Bars:10 μm
Euastrum taxa. a E. evolutum var. monticulosum; b E. fissum var. nordestinum; c E. gayanum var. angulatum; d E. gemmatum; e E. groenbladii; f E. informe var. oculatum; g E. luetkemuellerii var. carniolicum; h E. marimbusense; i E. obesum; j E. ornatiscrobiculatum; k E. pectinatum var. pinheirense; l E. platycerum var. groenbladii; m E. praemorsum var. forsteri. Scale Bars: 10 μm
Seven taxa were not included in the groups above, possibly because they have distinct morphological combinations, such as length greater than 60 μm and wall ornamented by granules and spines; E. evolutum var. monticulosum (Fig. 8a) and E. platycerum var. groenbladii (Fig. 8l). And shorter than 59 μm in length with a smooth cell wall; E. angolense var. brasiliense (Fig. 7d) and E. attenuatum var. brasiliense (Fig. 7g), or ornamented by scrobicules; E. groenbladii (Fig. 8e), E. luetkemuellerii var. carniolicum (Fig. 8g) and E. pectinatum var. pinheirense (Fig. 8k).
4 Discussion
Periphyton analysis of macrophytes with different morphological and ecological characteristics revealed an interesting diversity of Euastrum taxa associated with natural substrates in the Marimbus do Baiano. Our data show that the taxonomic composition was more outstanding in the periphyton of Utiricularia foliosa when compared to that of Cabomba caroliniana and Eicchornia azurea. Recently, Santos et al. (2022) evaluated the changes in the desmid community on three macrophytes in the Marimbus do Baiano. The authors measured fractal dimension (df) of Nymphaea amazonum Mart & Zucc (df = 1.59), C. caroliniana (df = 1.65) and U. foliosa (df = 1.73). The macrophyte with high structural complexity, U. foliosa, presented the highest richness, density and diversity of desmids. In contrast, rooted macrophyte with low structural complexity, N. amazonum, registered the lowest values for these attributes. Other studies also indicate the greater richness of desmids on U. foliosa than in other macrophytes (Pellegrini and Ferragut 2012; Santos et al. 2013; Souza et al. 2015; Ramos et al. 2021). Our results can probably be attributed to the structural complexity of the plants, especially the branching of leaves, as the bigger the branch, the greater the periphytic material retaining capacity (Rovira et al. 2016; Fernandes et al. 2016; Casartelli and Ferragut 2017).
The macrophyte architecture can be a strong driver of periphytic algal structure. But our results showed that the seasonality too could have influenced the variability of Euastrum. Several registered taxa occurred only in the months with less rainfall, while the lowest richness was verified in the wettest month (March). Studies suggest that the periphytic biomass accumulation can be strongly influenced by seasonality, with the highest rate during the dry season since the water volume increase can cause the periphyton detachment from the substrates (Felisberto and Rodrigues 2005, 2010; Casartelli et al. 2016).
The analysis of abiotic factors showed that most taxa occurred in well-oxygenated, slightly alkaline, and moderately conductive waters. However, the highest richness of Euastrum was reported in May, when water was with slightly low pH. As desmids are widely reported in environments under acidic conditions (Brook 1981; Coesel 2000; Coesel and Meesters 2007), possibly such factor could be contributed to high richness in that month. Nevertheless, various studies of tropical environments have reported desmids in alkaline waters (Araújo and Bicudo 2006; Souza et al. 2015; Casartelli et al. 2016; Bicudo and Menezes 2017; Santos et al. 2022). The water transparency was somewhat low due to the high concentration of humic substances, especially in the driest months giving the brown aspect to the water. This color is typical of the most aquatic ecosystems from the Chapada Diamantina region, including the Santo Antônio River, which is considered the main river that supplies the Pantanal dos Marimbus (Ramos et al. 2021).
The studied area presents a rich diversity, with endemism of E. abruptum var. chapadae, E. fissum var. nordestinum, E. marimbusense, E. ornatiscrobiculatum, E. praemorsum var. foersteri, and E. westenii, the former variety being classified as very frequent in Marimbus do Baiano (Costa et al. 2018, 2020). Among the taxa considered uncommon or rare, E. ansatum var. concavum, E. groenbladii, and E. sibiricum had their geographic distribution expanded to the northeast region of Brazil (Costa et al. 2020). The other identified taxa, except for E. angolense var. brasiliense, had already been referred to other areas of Bahia (Bicudo and Martins 1989; Oliveira et al. 2011; Ramos et al. 2011; Oliveira et al. 2017; Ramos et al. 2018).
The traditional taxonomy, based entirely on morphological characteristics, has been deconstructed by molecular studies revealing the non-monophyly of many desmid genera (Kouwets 2008; Skaloud et al. 2012). According to Gontcharov and Melkonian (2008, 2011), Desmidiaceae consists of at least 22 independent phylogenetic lineages. However, molecular data are still insufficient for several traditional genera with distinct morphology (Hall et al. 2008).
For the Euastrum genus, molecular studies conducted by Gontcharov and Melkonian (2008, 2011) revealed two distinct strains reflecting the morphology of the taxa: strain 1, comprising large taxa (> 50–60 μm long) with porous cell surface, large bulges facials, and scrobicules; and lineage 2 (smaller than < 50 µm in length), with a varied ornate wall, and the apical lobe with a less pronounced incision, often V-shaped.
Assessing the morphology of the taxa identified in Marimbus do Baiano, we observed that most are shorter than 59 μm and have a cell wall with more than one type of ornamentation. Those larger than 60 µm are ornamented by scrobicules. Cell size is probably related to competition for space and ornamentation to the adhesion process in the periphyton, as an adaptive strategy, thus making them less susceptible to predation (Coesel 2003; Černá and Neustupa 2009; Bestová et al. 2018).
Thus, we expanded knowledge about Euastrum taxa associated with macrophytes in shallow tropical lake of Bahia State, especially in floodplains, where studies are scarce and emphasized the need to promote conservation actions in the Pantanal dos Marimbus. The results presented highlight the importance of aquatic macrophytes for periphytic communities and as repositories of new species. We agree with Ramos et al. (2021) as to expanding the limits of the APA Marimbus-Iraquara to encompass the Marimbus do Baiano, aiming to protect the local biodiversity regarding the pressures caused by touristic activities exploitation.
References
Anissimova OV (2016) Architecture of cell wall of Euastrum Ralfs: new genus critheria. Mosc Univ Biol Sci Bull 71:155–159. https://doi.org/10.3103/S0096392516030019
Araújo A, Bicudo CEM (2006) Criptógamos do Parque Estadual das Fontes do Ipiranga, São Paulo, SP. Algas, 22: Zygnemaphyceae (gêneros Actinotaenium, Cosmarium e Heimansia). Hoehnea 33(2):219–237
Araújo A, Oliveira IB, Peres CK, Fajar A, Moura CWN (2015) Conjugatophyceae in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. http://floradobrasil2015.jbrj.gov.br/FB119508
Bestová H, Munoz F, Svoboda P, Škaloud P, Violle C (2018) Ecological and biogeographical drivers of freshwater green algae biodiversity: from local communities to large-scale species pools of desmids. Oecologia 186:1017–1030. https://doi.org/10.1007/s00442-018-4074-x
Bicudo CEM, Martins DV (1989) Desmídias (Zygnemaphyceae) de Itanagra, estado da Bahia, Brasil. Rev Bras Biol 49:309–324
Bicudo CEM, Menezes M (2017) Gêneros de algas de águas continentais do Brasil: chave para identificação e descrições, 3rd edn. RiMa, São Carlos, p 552
Bioinformatics & Evolutionary Genomics (2017) Ghent, Ghent University. Publishing: http://bioinformatics.psb.ugent.be/webtools/Venn/. Accessed 06 Au 2018
Brook AJ (1981) Biology of desmids. Blackwell Scientific Publications, London, p 246
Casartelli MR, Ferragut C (2017) The effects of habitat complexity on periphyton biomass accumulation and taxonomic structure during colonization. Hydrobiologia 807:233–246. https://doi.org/10.1007/s10750-017-3396-8
Casartelli MR, Lavagnolli GJ, Ferragut C (2016) Periphyton biomass accrual rate changes over the colonization process in a shallow mesotrophic reservoir. Acta Limnol Bras 28:e9. https://doi.org/10.1590/S2179-975X0116
Černá K, Neustupa J (2009) The PH-related morphological variations of two acidophilic species of Desmidiales (Viridiplantae) isolated from a lowland peat bog, Czech Republic. Aquat Ecol 44:409–419. https://doi.org/10.1007/s10452-009-9296-x
Coesel PFM (1996) 5. Biogeography of desmids. Hydrobiologia 336:41–53
Coesel PFM (2000) Desmids (Chlorophyta, Desmidiaceae) from Thale Noi (Thailand). Nord J Bot 20:369–382. https://doi.org/10.1111/j.1756-1051.2000.tb00751.x
Coesel PFM (2003) Desmid flora data as a tool in conservation management of Dutch freshwater wetlands. Biol Bratisl 58:717–722
Coesel PFM, Meesters K (2007) Desmids of the lowlands: Mesotaeniaceae and Desmidiaceae of the European lowlands. KNNV Publishing, Zeist, p 351
Costa FM, Ramos GJP, Oliveira BO, Bicudo CEM, Moura CWN (2018) Five new taxa and a new record of Euastrum (Desmidiaceae) from the Chapada Diamantina region, Bahia State, Brazil. Phytotaxa 372:193–202. https://doi.org/10.11646/phytotaxa.372.3.2
Costa FM, Ramos GJP, Oliveira BO, Bicudo CEM, Moura CWN (2020) Notes on the genus Euastrum (Desmidiaceae) in Brazil, with description of a new species. Phytotaxa 451:034–044. https://doi.org/10.11646/phytotaxa.451.1.3
Croasdale H, Flint EA (1986) Flora of the New Zealand: freshwater algae, Chlorophyta, Desmids with comments on their habitats. DSIR, Botany Division, Chistchurch
Díaz-Olarte J, Duque SR (2009) Ensambles algales en un microecosistema natural de la planta carnívora tropical Utricularia foliosa L. Caldasia 31:319–337
Díaz-Olarte J, Valoyes-Valois V, Guisande C, Torres NN, González-Bermúdez A, Sanabria Aranda L, Hernández AMM, Duque SR, Marciales LJ, Nunez-Avellaneda M (2007) Periphyton and phytoplankton associated with the tropical carnivorous plant Utricularia foliosa. Aquat Bot 87:285–291. https://doi.org/10.1016/j.aquabot.2007.06.010
Domozych DS, Domozych CR (2007) Desmids and biofilms of freshwater wetlands: development and microarchitecture. Microb Ecol 55:81–93. https://doi.org/10.1007/s00248-007-9253-y
Erhard D, Gross EM (2006) Allelopathic activity of Elodea canadensis and Elodea nuttallii against epiphytes and phytoplankton. Aquat Bot 85:203–211. https://doi.org/10.1016/j.aquabot.2006.04.002
Felisberto SA, Murakami EA (2013) Papel do perifíton na ciclagem de nutrientes e na teia trófica. RiMa Editora, p 44
Felisberto SA, Rodrigues L (2005) Influência do gradiente longitudinal (rio-barragem) na similaridade das comunidades de desmídias perifíticas. Braz J Bot 28:241–254. https://doi.org/10.1590/S0100-84042005000200005
Felisberto SA, Rodrigues L (2010) Periphytic algal community in artificial and natural substratum in a tributary of the Rosana reservoir (Corvo Stream, Paraná State, Brazil). Acta Sci 32:373–385. https://doi.org/10.4025/actascibiolsci.v32i4.4627
Fernandes UL, Oliveira ECC, Lacerda SR (2016) Role of macrophyte life forms in driving periphytic microalgal assemblages in a Brazilian reservoir. J Limnol 75:44–51. https://doi.org/10.4081/jlimnol.2015.1071
Ferreiro N, Giorgi A, Feijoo C (2014) Effects of macrophyte architecture and leaf shape complexity on structural parameters of the epiphytic algal community in a Pampean stream. Aquat Ecol 47:389–401. https://doi.org/10.1007/s10452-013-9452-1
Förster K (1964) Desmidiaceae naus Brasilien, 2: Bahia, Goyaz, Piauhy und Nord-Brasilien. Hydrobiologia 23:321–505
Förster K (1969) Amazonische Desmidieen 1. Areal Santarém. Amazoniana 2(1–2):5–116
França F, Melo E, Oliveira IB, Reis ATCC, Alves GL, Costa MF (2010) Plantas vasculares das áreas alagadas dos Marimbus, Chapada Diamantina, Bahia, Brasil. Hoehnea 37(4):719–730. https://doi.org/10.1590/S2236-89062010000400003
Gontcharov AA, Melkonian M (2005) Molecular phylogeny of Staurastrum Meyen ex Ralfs and related genera (Zygnematophyceae, Streptophyta) based on coding and noncoding rDNA sequence comparisons. J Phycol 41(4):887–899. https://doi.org/10.1111/j.0022-3646.2005.04165.x
Gontcharov AA, Melkonian M (2008) In search of monophyletic taxa in the family Desmidiaceae (Zygnematophyceae, Viridiplantae): the genus Cosmarium. Am J Bot 95:1079–1095. https://doi.org/10.3732/ajb.0800046
Gontcharov AA, Melkonian M (2011) A study of conflict between molecular phylogeny and taxonomy in the Desmidiaceae (Streptophyta, Viridiplantae): Analysesof 291 rbcL sequences. Protist 162:253–267. https://doi.org/10.1016/j.protis.2010.08.003
Gross EM (2003) Allelopathy of aquatic autotrophs. Crit Rev Plant Sci 22:313–339. https://doi.org/10.1080/713610859
Guiry MD, Guiry GM (2021) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org
Hall JD, Karol KG, McCourt RM, Delwiche CF (2008) Phylogeny of the conjugating green algae based on chloroplast and mitochondrial nucleotide sequence data. J Phycol 44:467–477. https://doi.org/10.1111/j.1529-8817.2008.00485.x
Hammer O, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Instituto Nacional de Meteorologia-INMET (2018) Publishing: http://www.inmet.gov.br/portal/. Accessed 30 July 2018
Kouwets AC (2008) The species concept in desmids: the problem of variability, infraspecific taxa and the monothetic species definition. Biologia 63:881–887. https://doi.org/10.2478/s11756-008-0135-7
Krieger W (1937) Die Desmidiaceen Europas. In: L. Rabenhorst’s Kryptogamen-Flora von Deutschland, osterreich um der Schweiz, vol 13. Akademische Verlasgsgesellschaft, Leipzig, pp 377–712
Laugaste R, Reunanen M (2005) The composition and density of epiphyton on some macrophyte species in the partly meromictic Lake Verevi. Hydrobiologia 547:137–150. https://doi.org/10.1007/s10750-005-4155-9
Legendre P, Legendre L (1998) Numerical ecology. Elsevier, Amsterdam, p 969
Lima ACP, França F, Jesus TB (2018) Avaliação dos níveis de metais pesados no pantanal dos Marimbus, Bahia, Brasil. Eng Sanit 23:591–598. https://doi.org/10.1590/S1413-41522018164218
Mann DG, Bayer MM, Droop SJM, Higks YA, Marshall AD, Martin RR, Rosin PL (2007) New methods for preparing, imaging and typifying desmids (Chlorophyta, Zygnematophyceae), including extended depth of focus and 3-D reconstruction. Phycologia 46:29–45. https://doi.org/10.2216/04-60.1
Matteucci SD, Colma A (1982) Metodología para el estudio de la vegetación. Série Biologia, Washington, p 167
Meerhoff M, Iglesias C, De Mello FT, Clemente JM, Jensen E, Lauridsen TL, Jeppesen E (2007) Effects of habitat complexity on community structure and predator avoidance behaviour of littoral zooplankton in temperate versus subtropical shallow lakes. Freshw Biol 52:1009–1021. https://doi.org/10.1111/j.1365-2427.2007.01748.x
Menezes CM, Bueno NC, Sobjak TM, Bortolini JC, Temponi LG (2013) Zygnemaphyceae associada à Utricularia foliosa L. no Parque Nacional do Iguaçu, Paraná, Brasil. Iheringia Ser Bot 68:5–26
Moresco C, Biolo S, Bueno NC (2009) O gênero Micrasterias Agardh ex Ralfs (Desmidiaceae, Zygnemaphyceae) em um lago artificial urbano, Paraná, Brasil. Hoehnea 36:349–358. https://doi.org/10.1590/S2236-89062009000200012
Mutinová PT, Neustupa J, Bevilacqua S, Terlizzi A (2016) Host specificity of epiphytic diatom (Bacillariophyceae) and desmid (Desmidiales) communities. Aquat Ecol 50(4):697–709. https://doi.org/10.1007/s10452-016-9587-y
Oliveira IB, Bicudo CEM, Moura CWN (2011) Euastrum (Desmidiaceae, Zygnematophyceae) na planície litorânea do norte da Bahia, Brasil. Sitientibus 11:62–73
Oliveira IB, Bicudo CEM, Moura CWN (2017) Novos registros de táxons dos gêneros Euastrum Ehrenb. ex Ralfs e Micrasterias C. Agardh ex Ralfs (Zygnematophyceae, Desmidiaceae) para a Bahia e o Brasil. Iheringia Ser Bot 72:295–313. https://doi.org/10.21826/2446-8231201772217
Pellegrini BG, Ferragut C (2012) Variação sazonal e successional da comunidade de algas perifíticas em substrato natural em um reservatório mesotrófico tropical. Acta Bot Bras 26:807–818. https://doi.org/10.1590/S0102-33062012000400010
Pickett-Heaps JD (1974) Scanning electron microscopy of some Cultured desmids. Transactions of the American Microscopical Society 93(1):1–23. https://doi.org/10.2307/3225214
Prescott GW, Croasdale HT, Vinyard WC (1977) A synopsisof North American desmids: Desmidiaceae, Placodermae. University of Nebraska Press, Lincoln
Ramos GJP, Oliveira IB, Moura CWN (2011) Desmídias de ambiente fitotelmata bromelícola da Serra da Jiboia, Bahia, Brasil. Rev Bra Bioci 9:103–113
Ramos GJP, Bicudo CEM, Góes-Neto A, Moura CWN (2014) New additions of coccoid green algae to the phycoflora of Brazil and the Neotropics. Acta Bot Bras 28:8–16. https://doi.org/10.1590/S0102-33062014000100002
Ramos GJP, Bicudo CEM, Moura CWN (2018) Some new, rare and interesting desmids from bromeliad phytotelmata in Brazil. Phytotaxa 346:59–77. https://doi.org/10.11646/phytotaxa.346.1.3
Ramos GJP, Santos MAD, Moura CWN (2021) How hidden is the diversity of the genus Cosmarium (Desmidiaceae) in the Brazilian Caatinga? Acta Bot Bras 35:188–214. https://doi.org/10.1590/0102-33062020abb0370
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.r-project.org/
Rosen BH, Stahlhut KN, Hall JD (2019) Catalog of microscopic organisms of the Everglades, part 2—The desmids of the Arthur R. Marshall Loxahatchee National Wildlife Refuge. U.S. Geological Survey Scientific Investigations Report, Virginia, pp 277
Rovira A, Alcaraz C, Trobajo R (2016) Effects of plant architecture and water velocity on sediment retention by submerged macrophytes. Freshw Biol 61:758–768. https://doi.org/10.1111/fwb.12746
Růžička J (1977) Die Desmidiaceen Mitteleuropas. E Schweizerbat’sche Verlags Buchhandlung 1:1–291
Růžička J (1981) Die desmidiaceen Mitteleuropas, vol 1. Schweizerbart’sche Verlags buchhandlung, Stuttgart, pp 293–736
Santos TR, Ferragut C, Bicudo CEM (2013) Does macrophyte architecture influence periphyton? Relationships among Utricularia foliosa, periphyton assemblage structure and its nutrient (C, N, P) status. Hydrobiologia 714:71–83. https://doi.org/10.1007/s10750-013-1531-8
Santos MA, Ferragut C, Simões NR, Silva DML, Moura CWN (2022) What are the main environmental predictors of differences in the community structure of periphytic desmids in a semi-arid floodplain lake? Aquat Ecol. https://doi.org/10.1007/s10452-022-09957-7
Škaloud P, Št’astný J, Nemjová K, Mazalová P, Poulíčková A, Neustupa J (2012) Molecular phylogeny of baculiform desmid taxa (Zygnematophyceae). Plant Syst Evol 298:1281–1292. https://doi.org/10.1007/s00606-012-0634-4
Souza ML, Pellegrini BG, Ferragut C (2015) Periphytic algal community structure in relation to seasonal variation and macrophyte richness in a shallow tropical reservoir. Hydrobiologia 755:183–196. https://doi.org/10.1007/s10750-015-2232-2
Šťastný J (2010) Desmids (Conjugatophyceae, Viridiplantae) from the Czech Republic; new and rare taxa, distribution, ecology. Fottea 10:1–74
Zhang N, Li H, Jeppsen E, Li W (2013) Influence of substrate type on periphyton biomass and nutrient state at contrasting high nutrient levels in a subtropical shallow lake. Hydrobiologia 710:129–141. https://doi.org/10.1007/s10750-012-1287-
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES)—Finance Code 001. We are grateful to Projeto Flora da Bahia (483909/2012-2); to Maria A. dos Santos, João T.X. Neto and Adones J.S. Pereira for their help with the field trips; and CAPES for the fellowship granted to FMC.
Author information
Authors and Affiliations
Contributions
FMC, GJPR and CWNM collected the sampling units; FMC analyzed the material and wrote the manuscript with support of GJPR and CWNM; LMS performed the statistical analysis; IBO and CEMB assisted in taxonomic identifications; all authors discussed the results and provided critical feedback to the final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We would like to declare that there is no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Matos Costa, F., Ramos, G.J.P., Santana, L.M. et al. Morphological diversity and ecological aspects of Euastrum taxa (Desmidiaceae) associated with macrophytes from a wetland in the semiarid region of Bahia, Brazil. Braz. J. Bot 45, 1327–1343 (2022). https://doi.org/10.1007/s40415-022-00837-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-022-00837-w