Abstract
Phytochromes (PHYs) have long been associated with classic photomorphogenic responses and recently implied with the regulation of plant productivity. We aimed to characterize these links in an important agronomic crop such as tomato (cv. Moneymaker) by evaluating biomass partitioning and morphophysiological parameters related to productivity under distinct light conditions in phyA, phyB1 and phyB2 tomato mutants. Under sun, PHY mutants presented lower leaf biomass during the vegetative phase the same way as the wild type (WT) under shading treatment. However, no difference regarding fruit biomass ratio (harvest index) was registered between WT and PHY mutants. phyA was the shortest genotype with lesser lateral branches and smaller xylem vessels and alongside phyB1, presented lesser leaf area. Net photosynthesis rate and photosystem II maximum potential quantum efficiency were not affected by phytochrome loss under sun condition. Nevertheless, PHY mutants showed lesser chlorophyll a content and stomata conductance and transpiration rates. Together, our data reveal that despite some morphophysiological and developmental impairments and the differences in biomass accumulation associated with the distinct PHYs under distinct light conditions, the plant harvest index is not affected by individual PHY losses under sun condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Light is the primary energy resource used by plants for their growth and survival. To withstand a photodynamical environment, plants optimize the light harvesting through a plethora of morphophysiological responses encompassing alterations in plant architecture, biomass reallocation and photosynthesis-related processes. Many core mechanisms related to plant growth and development in response to the environmental light conditions are controlled by photoreceptors such as the phytochromes (Sharrock 2008).
Phytochromes (PHYs) comprise a small family of plant photoreceptors, which are responsive to red and far-red light spectrum. PHYs are synthesized in the cytosol as the inactive red-absorbing Pr form in the dark and converted into the active far-red-absorbing Pfr form after exposure to red light (Han et al. 2007). The active Pfr form is translocated into the nucleus triggering a complex signaling cascade through the interaction with a wide plethora of light signaling partners and transcriptional regulation of photoresponsive genes (Chen and Chory 2011; Wang and Wang 2015).
PHYs are strongly associated with classic photomorphogenic responses such as photocontrol of seed germination, stem elongation, leaf development and transition to flowering (Kami et al. 2010). PHYs are also implied with the regulation of important aspects of plant productivity due to their light-dependent roles (Krahmer et al. 2018). Arabidopsis thaliana (L.) Heynh. PHY mutants present growth deficiencies mainly associated with the negative impacts of PHY deficiency upon chlorophyll levels, carbon assimilation rate and resource allocation, directly affecting plant biomass and carbon partitioning metabolism (Strasser et al. 2010; Yang et al. 2016). Despite the evidences of the association of PHYs with plant productivity, few studies concerning this relationship have been carried out in important agronomic species such as tomato (Solanum lycopersicum L.).
In tomato, PHYs are represented by five members: SlPHYA, SlPHYB1, SlPHYB2, SlPHYE and SlPHYF (Alba et al. 2000). Deficiency of SlPHYA leads to reduced photosynthetic electron transport rates, lower levels of starch in vegetative tissues and impaired shoot biomass accumulation (Kharshiing and Sinha 2016). SlPHYA is also responsible for the control of carbon flux related processes, especially in dark-grown seedlings, optimizing growth rate and biomass partitioning according to the light availability (Carlson et al. 2019). SlPHYB1 is mainly expressed in vegetative tissues and SlPHYB2, in the fruit pericarp (Hauser et al. 1997; Bianchetti et al. 2017), and therefore, their impacts on the productivity must be analyzed throughout the tomato life cycle. SlPHYE functions are associated with shade avoidance responses (Schrager-Lavelle et al. 2016) and specific roles for SlPHYF remains elusive. Mutants for both SlPHYE and SlPHYF are not yet available in tomato backgrounds.
Despite the impacts of the different PHYs on plant development, the light intensity and the spectra quality are also important factors that interfere in the effective action of PHYs upon photomorphogenic responses (Shinomura et al. 2000; Chen et al. 2004), leading to the investigation of the effects of these photoreceptors over the morphophysiology of plants grown under sun and shading conditions. Light limitation or lower-red–far-red wavelength ratio (R-to-FR ratio) are environmental promoters of the stem growth, leading to the allocation of relatively more biomass into these organs (Poorter et al. 2012; Cagnola et al. 2012), presumably driving the resources away from agriculturally important organs such as leaves and fruits. In tomato, negative impacts of shading upon fruit yield depend upon the levels of light limitation (Abdel-Mawgoud et al. 1996; Sandri et al. 2003). As for the light quality, low R-to-FR ratios increased the accumulation of biomass into the stems at the expense of leaves. However, fruit production was improved under these light conditions possibly due to a positive impact on flowering acceleration (Kalaitzoglou et al. 2019).
Being a fruit-bearing crop, unraveling the light and PHY influence over the plant morphophysiology and dynamics of biomass allocation into the harvestable organs is of fundamental importance for the tomato yield improvement. In this study, we evaluated biomass partitioning and morphophysiological parameters related to productivity of phyA, phyB1 and phyB2 tomato mutant plants under sun and partial shading light conditions. Our data reveal that, despite the differences in biomass accumulation and partitioning between the organs during the vegetative phase in the different light conditions, the ratio between fruit biomass and total plant biomass (harvest index) is not affected in the tomato PHY mutants.
2 Materials and methods
Plant material and treatments
– The experiment was performed in the greenhouse of the Universidade Federal de Goiás’ Botany Department (Goiânia, Brazil, 716 m altitude, 16° 35′ 39″ S, 49° 17′ 16″ W). Seeds of wild-type (WT), phyA, phyB1 and phyB2 tomato phytochrome mutants (Solanum lycopersicum cv. Moneymaker) were sown in germination trays containing Bioplant® substrate under the sun (conditions described below). After emergency, 1-week-old seedlings similar in size and vigor were transplanted to 10-L pots containing dark red latosol with the following features: pH 5.2, 2.3 mg kg−1 disponible P, 50 mg kg−1 K, 5.7 cmolc kg−1 Ca, 0.5 cmolc kg−1 Mg, 9.4 cmolc kg−1 cation exchange capacity and 30 g kg−1 organic matter. Pots were daily watered, and the substrate was supplemented monthly with 5 g NPK 10:10:10 and bimonthly with Dimy® foliar fertilizer 1:10.
The plants were grown in greenhouse with transparent cover, considered the sun treatment (1050 μmol m−2 s−1 average photon flux density, 29–44 °C day, 22–26 °C night, 35–60% relative air humidity), and in shaded environment with black polyethylene screens, considered the shading treatment (660 μmol m−2 s−1 average photon flux density, 29–33 °C day, 22–26 °C night, 53–70% relative air humidity). The effective PAR radiation was measured by a line quantum sensor (LI-191, LI-COR Biosciences) at noon at the average plant height.
Morphometric and plant biomass analyses
– Height, lateral branch number and leaf area were measured in five plants of each genotype and light treatment 45 days after treatment (DAT). For leaf area analysis, all leaves of the plant were measured by LI-3100 leaf area meter (LI-COR Biosciences).
At the end of the vegetative (45 DAT) and reproductive (130 DAT) stages, five plants of each genotype and treatment were harvested for plant biomass measurements. The plants were divided into roots, stems, leaves and fruits and dried under 65 °C until constant biomass weight.
Anatomical analyses
– At 37 DAT, leaflets of the 6th or 7th node and stem portions between the 5th and 6th node from base to apex from five plants of each genotype and light treatment were collected and fixed in 50% FAA solution (formaldehyde, acetic acid and ethanol) for 48 h and later transferred to 50% ethanol.
Paradermal sections of the middle third leaf blades were performed to measure stomatal density and index in both abaxial and adaxial surfaces using Image-Pro Plus software®. All stomata present in five random fields of approximately 0.077 mm2 were counted for each biological replicate. The stomatal index was performed following the equation: stomatal index (SI) = [NE/(CE + NE)] × 100, in which NE is the stomatal number and CE is the number of epidermal cells.
A transversal section of the stem portions was performed in microtome (Leica RM2245), and the five biggest xylem vessel tubes were measured (Zhang et al. 2016) using Image-Pro Plus software.
Gas exchange, fluorescence measurements and photosynthetic pigments quantification
– Photosynthetic rate (A, µmol CO2 m−2 s−1), stomatal conductance (gs, mol H2O m−2 s−1) and transpiration rate (E, mmol H2O m−2 s−1) were measured with a portable LI-6400XTR infrared gas analyzer (LI-COR Biosciences, Lincoln, NE, USA) between 8:00 a.m. and 10:30 a.m. on the 6th or 7th fully expanded leaves from base to apex of five plants of each genotype and light treatments at 37 DAT. Measurements were taken 1 min after stabilization of gas exchange parameters. Equipment was configured as described by Alves et al. (2016). Photosystem II potential photochemical efficiency (Fv/Fm) was measured with a portable fluorometer (Hansatech PEA MK2 model, Kings Lynn, England) on the same leaves chosen for the gas exchange analysis. Measurement protocol and Fv/Fm derived calculations were performed as described by Alves et al. (2016). Following gas exchange and fluorescence measurements, the same leaves were harvested for chlorophyll a and b quantification through pure acetone extraction according to Lichtenthaler (1987).
Statistical analyses
– The design of the experiment was completely randomized. Data were analyzed using two-way ANOVAs followed by Tukey’s HSD post hoc test (α = 0.05).
3 Results
Light conditions perceived via phytochromes alter photosynthate partitioning patterns in tomato
– Phytochromes regulate biomass accumulation, and the photosynthate partitioning control is also dependent upon the environmental radiation conditions. Loss of phytochromes resulted in contrastingly lower biomass content during the vegetative phase (Fig. 1a, Online Resource 1). Despite being lighter, at 45 DAT, the phytochrome mutants cultivated under sun displayed an increased accumulation of biomass in the roots and stems and decreased accumulation in the leaves (related to total dry mass) compared to WT. Nevertheless, under shading conditions, all tomato genotypes presented the same partitioning pattern between the organs despite a slight increase in root biomass for the phytochrome mutants (Fig. 1b–d). Therefore, the shading treatment influenced the biomass partitioning patterns as much as the loss of phytochromes did.
Biomass accumulation and photosynthate partitioning analyses of wild-type (WT) tomato plants and phytochrome mutants (phyA, phyB1 and phyB2) under sun and shade conditions after 45 days (A–D) and 130 days of treatment (E–I). Bars indicate standard deviation (n = 5). Groups not connected by same letters are significantly different (ANOVA/Tukey’s HSD post hoc test, α = 0.05)
Differences in the total biomass content were still remarkable in the reproductive stage for the phyA and phyB1 mutant (Fig. 1e). Once there was no fruit set in tomato plants cultivated under shading, at the 130 DAT, end of reproductive stage, we could assess biomass partitioning only in plants cultivated under sun aiming to check a possible allocation into the fruits. All phytochrome mutants presented higher allocation of biomass only in the leaves (Fig. 1h) and no differences regarding fruit biomass ratio (harvest index) were registered between the WT and the mutants (Fig. 1i). We conclude that, despite the distinct biomass partitioning pattern between the organs during the vegetative phase, the plant harvest index was not affected in the studied phytochrome mutants.
Light conditions and distinct phytochromes differentially affect tomato plant architecture and anatomical traits
– A crop yield is directly dependent upon the plant architecture and anatomical traits, such as stomata density and xylem vessels area, once they could limit light absorption, carbon gain through assimilation and the distribution of resources through the plant. We investigated the impact of light conditions and phytochrome loss upon these traits in tomato.
Under shading, all tomato genotypes were taller than under sun (Fig. 2a). phyA was the shortest regardless of the light conditions, also presenting lesser lateral branches (Fig. 2b). All phytochrome mutants presented lesser leaf area than the WT under shading. However, only phyA and phyB1 mutants presented lesser leaf area when cultivated under sun (Fig. 2c).
Morphometric analyses of wild-type (WT) tomato plants and phytochrome mutants (phyA, phyB1 and phyB2) under sun and shade conditions after 45 days of treatment. A Plant height (cm). B Number of lateral branches. C Total leaf area (cm2). Bars indicate standard deviation (n = 5). Groups not connected by same letters are significantly different (ANOVA/Tukey’s HSD post hoc test, α = 0.05)
All genotypes presented higher stomata density (SD) and stomata index (SI) in the abaxial leaf surface under sun conditions, except phyB1, for which no differences were either registered between sun and shading conditions (Table 1). As for the adaxial surface, all phytochrome mutants cultivated under sun presented lower SI than WT. Under shading conditions, tomato plants presented reduced adaxial SD and SI, except for phyA, for which these stomata parameters were the same as when cultivated under sun. These results indicate that distinct phytochromes influence stomata development in the adaxial and abaxial surface of tomato leaves according to the light conditions.
phyA xylem vessels are more irregular when compared to other genotypes (Fig. 3) and they are also smaller, regardless of the light conditions (Fig. 3, Table 1).
Phytochrome loss and shading conditions impact tomato photosynthetic traits
– The remarkably differences registered in the biomass accumulation and growth patterns between the tomato genotypes and light treatments could be associated with changes in certain aspects of photosynthesis-related process such as carbon assimilation, gas exchanges, PSII quantum efficiency and chlorophyll content.
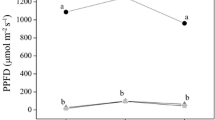
Under sun conditions, there were no registered differences between the genotypes regarding the net photosynthesis rate, but, under shading, phyB1 and phyB2 mutants presented higher rates than WT (Fig. 4a). Phytochrome mutants showed lesser rates of stomatal conductance and transpiration under sun. Under shading, phyB2 presented the highest transpiration rates (Fig. 4b–c).
Gas exchange and fluorescence parameters of wild-type (WT) tomato plants and phytochrome mutants (phyA, phyB1 and phyB2) under sun and shade conditions after 45 days of treatment. A Net photosynthesis rate (A, in µmol CO2 m−2 s−1). B Stomata conductance (gs, in mol H2O m−2 s−1). C Transpiration rate (E, in mmol H2O m−2 s−1). D Photosystem II maximum potential quantum efficiency (Fv/Fm). Bars indicate standard deviation (n = 5). Groups not connected by same letters are significantly different (ANOVA/Tukey’s HSD post hoc test, α = 0.05)
PSII maximum potential quantum efficiency was not affected by the phytochrome loss. However, under shading conditions, phyA and phyB2 PSII maximum potential quantum efficiency was reduced (Fig. 4e).
Phytochrome mutants presented less chlorophyll a content under sun conditions when compared to WT. However, under shading conditions, phyA and phyB1 accumulated the highest levels of chlorophyll a. Although lower, phyB2 did not change its chlorophyll a content due to light conditions, indicating a strong influence of PHYB2 on chlorophyll a accumulation (Fig. 5a). Shading conditions increased chlorophyll b content for all the genotypes, though phyB1 and phyB2 mutants presented the lowest levels regardless of the light conditions (Fig. 5b).
Photosynthetic pigments chlorophyll a (A) and chlorophyll b (B) of wild-type (WT) tomato plants and phytochrome mutants (phyA, phyB1 and phyB2) under sun and shade conditions after 45 days of treatment. Bars indicate standard deviation (n = 5). Groups not connected by same letters are significantly different (ANOVA/Tukey’s HSD post hoc test, α = 0.05)
4 Discussion
Phytochromes are key players integrating the light signaling environmental conditions and plant development, coordinating the influence of the surrounding light signaling and modifying plant architecture and metabolic responses to better achieve the energy resource. As part of a multigenic family and coordinating distinct aspects of plant development influenced by light, such as growth, photosynthesis and resource allocation, we assessed the impacts of distinct light regimes on the phenotypes of tomato phytochrome mutants.
Mutations of distinct phytochromes and the influence of its light-dependent activation, simulated in this work by the shade conditions, led to an overall reduction in the vegetative biomass accumulation due to differences regarding plant growth and metabolic processes.
During the tomato vegetative phase, there is an allocation of biomass primarily on leaves, which, associated with the plant architecture, results in higher carbon gain to be reallocated into the fruits during the reproductive stage. The loss of phytochromes, however, leads into a different allocation pattern, accumulating biomass preferably in the roots and stems. Under shade conditions, WT allocation pattern is the same as the phytochrome mutants, indicating the role of light intensity over the modulation of active phytochromes in the control of biomass allocation.
PHYB2 overexpression in tomato leads to less biomass allocation into the stems and roots, while PHYB1 overexpression results in the accumulation of biomass in the roots in detriment of the stems (Husaineid et al. 2007). Other photomorphogenic tomato mutants such as the light-hyperresponsive high pigment 1 (hp1) increases biomass accumulation in the roots and leaves, while the phytochromobilin-deficient mutant aurea prioritizes allocation into the fruits (Melo et al. 2014). PHY overexpression effects and light-dependent exaggerated responses were, therefore, found to be opposed to the ones registered for the PHY mutations in this work concerning biomass allocation. The effectiveness of the phytochromes in the photosynthate partitioning regulation can also be assessed for other species in the works of Boccalandro et al. (2003), Schittenhelm et al. (2004) and Foreman et al. (2011).
Lesser total biomass accumulation in the end of the reproductive stage was registered for the phyA and phyB1 mutants (Fig. 1e). A wide plethora of factors may explain this response. For phyA, we registered lower height (Fig. 2a), lesser lateral branches (Fig. 2b), leaf (Fig. 2c) and xylem vessels area (Fig. 3, Table 1) and lower stomata conductance (Fig. 4b) and transpiration (Fig. 4c). Likewise, phyB1 mutant presented lesser leaf area and stomata density and index (Fig. 2c, Table 1) as well as lower chlorophyll a and b content (Fig. 5a, b) and lower stomata conductance and transpiration. For the phyB2 mutant, the registered impairments in the chlorophyll content seemed to be balanced by the higher leaf area, resulting in a total biomass accumulation comparable to the WT.
The lower height, branching and leaf area are directly related to the lower biomass accumulation. Leaf area growth is a critical parameter of plant productivity once dry weight growth of field crops is linearly related to the amount of intercepted light by leaves (Gifford et al. 1984).
As regulators of gas exchange in the leaves, stomata play important roles in determining plant productivity through carbon gain and therefore impacting biomass accumulation (Lawson and Blatt 2014; Qu et al. 2017). The lower stomata conductance and transpiration presented by the tomato phytochrome mutants can be associated with the corresponding lower stomata density and index in both leaf surfaces, especially for phyB1. In Arabidopsis, PHYB is required for a light-mediated stomata development (Casson and Hetherington 2014) and phyB mutants display lower stomata density and index (Boccalandro et al. 2009). Our findings suggest that in tomato, this role is mainly performed by PHYB1. As for the phyA mutant, the lower stomata conductance and transpiration registered are explained by a reduced stem water conductivity associated with the smaller xylem vessels area (Fig. 3, Table 1) and this limited sap flow may also have consequences for the water distribution through the plant (Auge et al. 2012).
Higher net photosynthesis rates due to higher availability of PAR radiation, as observed in this work, are a very common physiological response (Markesteijn et al. 2007; Ulqodry et al. 2014), and it was expected to occur once the shading conditions were simulated by a reduction of approximately 50% of the sun radiation. Light intensity is one of the main factors that influence stomata conductance affecting the efficiency of carboxylation (Costa and Marenco 2007). Despite lower levels of stomata conductance and transpiration of the tomato phytochrome mutants, net CO2 uptake remained unchanged under sun conditions. Limitations of photosynthesis rate by stomata conductance are more prominent under stressful conditions (Farquhar and Sharkey 1982), and in our experiment, plants were well watered and regularly fertilized and specific measurements were taken only in the morning. Phytochromes are also related to other mechanisms that influence CO2 plant balance such as biosynthesis of Rubisco subunits (Nishimura et al. 2008) and respiration and photorespiration enzymes (Igamberdiev et al. 2014), requiring further investigations to elucidate their influences over the net CO2 uptake.
Under shading, the registered differences indicate that the limiting factor to the photosynthesis is more related to the gas exchange capacity than the carboxylation efficiency as both can hinder net photosynthesis rate (Tenhunen et al. 1984). Corroborating these observations, no variation in the maximum potential quantum yield of the photosystem II was registered, except for a slight decrease registered in the phyA and phyB2 mutants under shade conditions.
Along with stomata resistance and photosystem quantum efficiency, the content of photosynthetic pigments is also an important factor of photosynthetic performance and consequent biomass accumulation. As ratified in this work by the lower contents of chlorophyll a of the tomato phytochrome mutants grown under sun, PHYA and PHYB have a regulatory role in the control of chlorophyll biosynthesis (Castillon et al. 2007; Brouwer et al. 2014).
The overall biomass reduction of phytochrome-deficient mutants is also registered for Arabidopsis thaliana, and it is associated with the lower levels of proteins, chlorophyll content and lower expression of genes responsible for the control of cell wall synthesis (Yang et al. 2016). Brassica rapa L. phyB mutants also presented lower weight associated with lower chlorophyll levels and stomata index (Arsovski et al. 2018). For tomato, reduced growth for phyA mutant was associated with alterations in the photosynthetic electron transport rates, resulting in changed starch regulation (Kharshiing and Sinha 2016). It is important to note that phytochromes may act redundantly in the control of some physiological responses (Franklin et al. 2003). For example, due to duplication of PHYB in tomato (Pratt et al. 1995), specific alterations are only noticeable in the phyB1phyB2 double mutant (Weller et al. 2000). Therefore, the determination of the roles of each phytochrome in the mechanisms underlying tomato plant growth and harvest index must be further investigated by employing double and triple mutants.
We conclude that, in tomato, despite the differences in biomass accumulation due to morphoanatomical and physiological changes related to the loss of phytochromes and distinct light regimes, the overall plant harvest index is not affected under sun.
References
Abdel-Mawgoud AMR, El-Abd SO, Singer SM, Abou-Hadid AF, Hsiao TC (1996) Effect of shade on the growth and yield of tomato plants. Acta Hortic 434:313–320. https://doi.org/10.17660/ActaHortic.1996.434.38
Alba R, Kelmenson PM, Cordonnier-Pratt M-M, Pratt LH (2000) The phytochrome gene family in tomato and the rapid differential evolution of this family in angiosperms. Mol Biol Evol 17:362–373. https://doi.org/10.1093/oxfordjournals.molbev.a026316
Alves FRR, Melo HC, Crispim-Filho AJ, Costa AC, Nascimento KJT, Carvalho RF (2016) Physiological and biochemical responses of photomorphogenic tomato mutants (cv. Micro-Tom) under water withholding. Acta Physiol Plant 38:155. https://doi.org/10.1007/s11738-016-2169-8
Arsovski AA, Zemke JE, Haagen BD, Kim S-H, Nemhauser JL (2018) Phytochrome B regulates resource allocation in Brassica rapa. J Exp Bot 69:2837–2846. https://doi.org/10.1093/jxb/ery080
Auge GA, Rugnone ML, Cortés LE, González CV, Zarlavsky G, Boccalandro HE, Sánchez RA (2012) Phytochrome A increases tolerance to high evaporative demand. Physiol Plant 146:228–235. https://doi.org/10.1111/j.1399-3054.2012.01625.x
Bianchetti RE, Cruz AB, Oliveira BS, Demarco D, Purgatto E, Peres LEP, Rossi M, Freschi L (2017) Phytochromobilin deficiency impairs sugar metabolism through the regulation of cytokinin and auxin signaling in tomato fruits. Sci Rep 7:7822. https://doi.org/10.1038/s41598-017-08448-2
Boccalandro HE, Ploschuk EL, Yanovsky MJ, Sánchez RA, Gatz C, Casal JJ (2003) Increased phytochrome B alleviates density effects on tuber yield of field potato crops. Plant Physiol 133:1539–1546. https://doi.org/10.1104/pp.103.029579
Boccalandro HE, Rugnone ML, Moreno JE, Ploschuk EL, Serna L, Yanovsky MJ, Casal JJ (2009) Phytochrome B enhances photosynthesis at the expense of water-use efficiency in Arabidopsis. Plant Physiol 150:1083–1092. https://doi.org/10.1104/pp.109.135509
Brouwer B, Gardeström P, Keech O (2014) In response to partial plant shading, the lack of phytochrome A does not directly induce leaf senescence but alters the fine-tuning of chlorophyll biosynthesis. J Exp Bot 65:4037–4049
Cagnola JI, Ploschuk E, Benech-Arnold T, Finlayson SA, Casal JJ (2012) Stem transcriptome reveals mechanisms to reduce the energetic cost of shade-avoidance responses in tomato. Plant Physiol 160:1110–1119. https://doi.org/10.1104/pp.112.201921
Carlson KD, Bhogale S, Anderson D, Tomanek L, Madlung A (2019) Phytochrome A Regulates Carbon Flux in Dark Grown Tomato Seedlings. Front Plant Sci 10:152. https://doi.org/10.3389/fpls.2019.00152
Casson SA, Hetherington AM (2014) Phytochrome B is required for light-mediated systemic control of stomatal development. Curr Biol 24:1216–1221. https://doi.org/10.1016/j.cub.2014.03.074
Castillon A, Shen H, Huq E (2007) Phytochrome interacting factors: central players in phytochrome-mediated light signaling networks. Trends Plant Sci 12:514–521. https://doi.org/10.1016/j.tplants.2007.10.001
Chen M, Chory J (2011) Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol 21:664–671. https://doi.org/10.1016/j.tcb.2011.07.002
Chen M, Chory J, Fankhauser C (2004) Light signal transduction in higher plants. Annu Rev Gen 38:87–117. https://doi.org/10.1146/annurev.genet.38.072902.092259
Costa GC, Marenco RA (2007) Fotossíntese, condutância estomática e potencial hídrico foliar em árvores jovens de andiroba (Carapa guianensis). Acta Amaz 37:229–234. https://doi.org/10.1590/S0044-59672007000200008
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Ann Rev Plant Physiol 33:317–345. https://doi.org/10.1146/annurev.pp.33.060182.001533
Foreman J, Johansson H, Hornitschek P, Josse E-M, Fankhauser C, Halliday KJ (2011) Light receptor action is critical for maintaining plant biomass at warm ambient temperatures. Plant J 65:441–452. https://doi.org/10.1111/j.1365-313X.2010.04434.x
Franklin KA, Praekelt U, Stoddart WM, Billingham OE, Halliday KJ, Whitelam GC (2003) Phytochromes B, D and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol 131:1340–1346. https://doi.org/10.1104/pp.102.015487
Gifford RM, Thorne JH, Hitz WD, Giaquinta RT (1984) Crop productivity and photoassimilate partitioning. Science 225:801–808. https://doi.org/10.1126/science.225.4664.801
Han Y-J, Song P-S, Kim J-I (2007) Phytochrome-mediated photomorphogenesis in plants. J Plant Biol 50:230–240. https://doi.org/10.1007/BF03030650
Hauser BA, Pratt LH, Cordonnier-Pratt M-M (1997) Absolute quantification of five phytochrome transcripts in seedlings and mature plants of tomato (Solanum lycopersicum L.). Planta 201:379–387. https://doi.org/10.1007/s004250050080
Husaineid SSH, Kok RA, Schreuder MEL, Hanumappa M, Cordonnier-Pratt M-M, Pratt LH, Van Der Plas LHW, Van Der Krol AR (2007) Overexpression of homologous phytochrome genes in tomato: exploring the limits of photoperception. J Exp Bot 58:615–626. https://doi.org/10.1093/jxb/erl253
Igamberdiev AU, Eprintsev AT, Fedorin DN, Popov VN (2014) Phytochrome-mediated regulation of plant respiration and photorespiration. Plant, Cell Environ 37:290–299. https://doi.org/10.1111/pce.12155
Kalaitzoglou P, Van Ieperen W, Harbinson J, Van der Meer M, Martinakos S, Weerheim K, Nicole CCS, Marcelis LFM (2019) Effects of continuous or end-of-day far-red light on tomato plant growth, morphology, light absorption, and fruit production. Front Plant Sci 10:322. https://doi.org/10.3389/fpls.2019.00322
Kami C, Lorrain S, Hornitschek P, Fankhauser C (2010) Light-regulated plant growth and development. Curr Top Dev Biol 91:29–66. https://doi.org/10.1016/S0070-2153(10)91002-8
Kharshiing E, Sinha SP (2016) Deficiency in phytochrome A alters photosynthetic activity, leaf starch metabolism and shoot biomass production in tomato. J Photochem Photobiol B: Biol 165:157–162. https://doi.org/10.1016/j.jphotobiol.2016.10.026
Lawson T, Blatt MR (2014) Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol 164:1556–1570. https://doi.org/10.1104/pp.114.237107
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Markesteijn L, Poorter L, Bongers F (2007) Light-dependent leaf trait variation in 43 tropical dry forest tree species. Am J Bot 94:515–525. https://doi.org/10.3732/ajb.94.4.515
Melo HC, Constantino EJ, Cacho RC, Carvalho RF (2014) Photosynthate partitioning and morphoanatomical aspects of photomorphogenic mutants of tomato. Biosci J 30: 447-457. http://www.seer.ufu.br/index.php/biosciencejournal/article/view/18031
Nishimura K, Ogawa T, Ashida H, Yokota A (2008) Molecular mechanisms of RuBisCO biosynthesis in higher plants. Plant Biotechnol 25:285–290. https://doi.org/10.5511/plantbiotechnology.25.285
Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L (2012) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193:30–50. https://doi.org/10.1111/j.1469-8137.2011.03952.x
Pratt LH, Cordonnier-Pratt MM, Hauser B, Caboche M (1995) Tomato contains two differentially expressed genes encoding B-type phytochromes, neither of which can be considered an ortholog of Arabidopsis phytochrome B. Planta 197:203–206. https://doi.org/10.1007/BF00239958
Qu M, Zheng G, Hamdani S, Essemine J, Song Q, Wang H, Chu C, Sirault X, Zhu X-G (2017) Leaf photosynthetic parameters related to biomass accumulation in a global rice diversity survey. Plant Physiol 175:248–258. https://doi.org/10.1104/pp.17.00332
Sandri MA, Andriolo JL, Witter M, Ross TD (2003) Effect of shading on tomato plants grown under greenhouse. Hortic Bras 21:642–645. https://doi.org/10.1590/S0102-05362003000400013
Schittenhelm S, Menge-Hartmann U, Oldenburg E (2004) Photosynthesis, carbohydrate metabolism and yield of phytochrome-B-overexpressing potatoes under different light regimes. Crop Sci 44:131–143. https://doi.org/10.2135/cropsci2004.0131
Schrager-Lavelle A, Herrera LA, Maloof JN (2016) Tomato phyE Is Required for Shade Avoidance in the Absence of phyB1 and phyB2. Front Plant Sci 7:1275. https://doi.org/10.3389/fpls.2016.01275
Sharrock RA (2008) The phytochrome red/far-red photoreceptor superfamily. Genome Biol 9:230. https://doi.org/10.1186/gb-2008-9-8-230
Shinomura T, Uchida K, Furuya M (2000) Elementary process of photoperception by phytochrome A for high-irradiance response of hypocotyl elongation in Arabidopsis. Plant Physiol 122:147–156. https://doi.org/10.1104/pp.122.1.147
Strasser B, Sánchez-Lamas M, Yanovsky MJ, Casal JJ, Cerdán PD (2010) Arabidopsis thaliana life without phytochromes. Proc Natl Acad Sci USA 107:4776–4781. https://doi.org/10.1073/pnas.0910446107
Tenhunen JD, Lange OL, Gebel J, Beyschlag W, Weber JA (1984) Changes in photosynthetic capacity, carboxylation efficiency and CO2 compensation point associated with midday stomatal closure and midday depression of net CO2 exchange of leaves of Quercus suber. Planta 162:193–203. https://doi.org/10.1007/BF00397440
Ulqodry TZ, Matsumoto F, Okimoto Y, Nose A, Zheng SH (2014) Study on photosynthetic responses and chlorophyll fluorescence in Rhizophora mucronata seedlings under shade regimes. Acta Physiol Plant 36:1903. https://doi.org/10.1007/s11738-014-1566-0
Wang H, Wang H (2015) Phytochrome signaling: time to lighten up the loose ends. Mol Plant 8:540–551. https://doi.org/10.1016/j.molp.2014.11.021
Weller JL, Schreuder ME, Smith H, Koornnef M, Kendrick RE (2000) Physiological interactions of phytochromes A, B1 and B2 in the control of development in tomato. Plant Journal 24:345–356. https://doi.org/10.1046/j.1365-313x.2000.00879.x
Yang D, Seaton DD, Krahmer J, Halliday KJ (2016) Photoreceptor effects on plant biomass, resource allocation, and metabolic state. Proc Natl Acad Sci USA 113:7667–7672. https://doi.org/10.1073/pnas.160130911
Zhang L, Copini P, Weemstra M, Sterck F (2016) Functional ratios among leaf, xylem and phloem areas in branches change with shade tolerance, but not with local light conditions, across temperate tree species. New Phytol 209:1566–1575. https://doi.org/10.1111/nph.13731
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mereb, E.L., Alves, F.R.R., Rezende, M.H. et al. Morphophysiological responses of tomato phytochrome mutants under sun and shade conditions. Braz. J. Bot 43, 45–54 (2020). https://doi.org/10.1007/s40415-020-00584-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-020-00584-w