Abstract
Epiphytism is responsible for a significant part of the diversity that makes tropical rainforests one of the most complex ecosystems in the biosphere. Approximately 9% of all vascular plants are epiphytes, found almost exclusively in tropical and subtropical forests. The objective of this study was to analyze the distribution of epiphytic species in different environments in a toposequence of a subtropical forest in southern Brazil. The species were sampled in six areas established within the forest: two areas with anthropic interference and four conserved areas, two near the river and two areas distant from the river. In each area, five point-centered quarters were demarcated, considering the nearest trees, with DBH ≥10 cm, as a sample unit. As a result, the correspondence analysis identified three distinct groups, influenced by different microclimates variable. Different environments favored the development of communities with characteristic species, where some have preference for sites near watercourses and others for more open or closed forests. Moisture and light incidence were some of the environmental factors that are linked to the preference of the species, contributing to the diversity and development of groups of species in the forest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epiphytism enables the enhancement of biodiversity in forests, providing the occupation of the different layers, creating environments susceptible for supporting life not dependent solely or directly from the soil (Madison 1977; Benzing 1990; Waechter 1992), becoming responsible for a significant part of diversity that makes the tropical rainforests one of the most complex ecosystems in the biosphere (Park 2003). It is estimated that 9% of all vascular plants are epiphytes (Zotz 2016), found almost exclusively in tropical and subtropical forests, and may represent in some countries more than 25% of flora species.
The sensitivity of epiphytic flora to moisture, associated with dependence on arboreal substrate, makes it a good environmental indicator for both successional stages of forest ecosystems and natural environmental variations (Triana-Moreno et al. 2003; Zotz and Bader 2009). For this reason, the communities of vascular epiphytes have been used as bioindicators of climate change, pollution and damage to ecosystems (Richter 1991; Lugo and Scatena 1992; Barthlott et al. 2001). This sensitivity becomes important in the context of conservation, given that many of the epiphytic species have a high degree of specialization with their phorophyte, thus becoming vulnerable to extinction by habitat change, climate change and, in the case of phorophyte-species specialists, by the phenomenon of coextinction (Clavel et al. 2011; Colwell et al. 2012). Nevertheless, the resilience of tropical epiphytes to environmental changes is still poorly understood (Larrea and Werner 2010).
The standards of richness, diversity and coexistence of vascular epiphytes have been studied in multiple approaches. The preference for phorophyte, interspecific interactions, soil conditions, microclimate variation, niche partitioning and dispersal limitation (Benzing 1990; Burns and Zotz 2010; Boelter et al. 2014; Zotz 2016) has been shown to be prominent in explaining these patterns found. Some studies associate high diversity observed in neotropical forests with attributes such as the age of the phorophyte and its physical characteristics such as branching and bark roughness, favoring water retention. These attributes tend to harbor a greater abundance of lichens and bryophytes, which results in positive interactions with many taxa of vascular epiphytes (Callaway et al. 2001, 2002; Hietz 2005).
The altitudinal and topographic variations observed in tropical forests generate different environmental conditions, featuring different microclimates for the vascular epiphytic guild (Woods et al. 2015). This environmental heterogeneity generates luminosity gradients (Benzing 1990; Clark et al. 1998; Cardelús et al. 2009) as well as moisture gradients (Cardelús and Chazdon 2005; De Le Rosa-Manzano et al. 2014). However, structural and successional aspects of arboreal vegetation are also important in the structuring of the epiphytic community, as these may be strongly influenced by the composition of the local arboreal assembly (Hietz and Hietz-Seifert 1995; Burns and Zotz 2010).
Our hypothesis is that the variation of microhabitats affects the richness of the vascular epiphytic guild; thus, this study aimed to analyze the distribution of vascular epiphytes in different environments of a subtropical forest in southern Brazil.
Materials and methods
Study area
– The study was conducted between 2010 and 2012 at the Serra Furada State Park (PAESF—Parque Estadual da Serra Furada), an Conservation Unit, located in the municipalities of Grão-Pará and Orleans, in the state of Santa Catarina, Brazil. The climate according to Köppen is Cfa (Alvares et al. 2013). PAESF has a vegetation formation characterized as subtropical rainforest, involving montane and upper montane formations, and the present study is performed on montane typology, according to IBGE (2012), at 400–1000 m altitudes.
Sampling method
– To sample the vascular epiphytic flora, each tree (phorophyte) was considered a sample unit, defined by the method of quadrants (Cottam and Curtis 1956). Six areas were established, and for each one, five point-centered quarters were determined, separated 20 m apart, totaling 120 sample units. The areas A–F were allocated in order to include different microhabitats (Fig. 1), with area A located at an altitude of 480 m and area F at 660 m. Areas A and B suffered anthropic interference, especially due to grazing in its surroundings and easy access, and an abundance of climbing plants was found by Oliveira (2016), in middle stage of natural regeneration. The other areas (C–F) are in an advanced stage of natural regeneration. Areas C and D are located parallel to watercourses, near rivers with waterfalls, with humid environments due to evapotranspiration; E and F are less humid, away from streams.
The criteria for including the phorophyte in the sample were to have a diameter at breast height (DBH) ≥10 cm. The epiphytes were sampled in two regions of the phorophyte, stem and crown, following the zonation proposed by Johansson (1974). The occurrence of epiphytic species in the stem and crown was recorded, by binoculars and photographic camera, for the presence (1) or absence (0) of the species. To estimate the distribution of each epiphytic species, procedures used by Waechter (1992) were adopted, based on the occurrence of individual phorophyte species.
The identification of species was based on Hoehne (1942, 1945, 1949, 1953), Pabst and Dungs (1975, 1977) Reitz (1983), Tamashiro and Zickel (1991), Guimarães (1998), Coelho (2000), Wanderley and Martins (2007), Azeredo and Citadini-Zanette (2012) and specialists. For the botanical families of angiosperms, the classification system adopted was APG IV (2016) and Smith et al. (2006) for ferns. The collected source material was deposited in the Herbarium Pe. Dr. Raulino Reitz (CRI) of Universidade do Extremo Sul Catarinense (UNESC), Criciúma, Santa Catarina.
Data analysis
– To assess the relation between the taxa and microhabitats, samples were submitted to correspondence analysis (Kent and Coker 1992), that may be applied to any data table that is dimensionally homogeneous and only contains positive integers or zeros (Legendre and Legendre 2003). The matrices of species and families were constituted by the number of occurrences in the area; therefore, we adopted the exclusion of the species under three sampled individuals and families with less than three species, preventing accidental species. A category called “no species” was created for those phorophytes who did not have vascular epiphytes (Oliveira et al. 2013; Padilha et al. 2015). By previous successful analysis, Bromeliaceae family was separated, based on their nutrient absorption mechanisms: tank bromeliads and atmospheric bromeliads (Benzing et al. 1978). Venn diagrams were made for species and families, considering the category “no species” in the analysis.
Results
A total of 46 species, distributed in 12 families, were recorded. The most representative families were Bromeliaceae (23.9%), Orchidaceae (17.4%), Polypodiaceae (15.2%) and Cactaceae (10.9%) (Table 1).
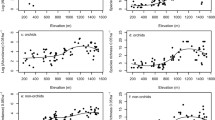
The areas A, B showed lower richness of vascular epiphytes in comparison with the areas C, D and E, F (Fig. 2) and the correspondence analysis formed three groups, based on frequency of families and species in each area. It was possible to see that in the cluster, most species tended to approach areas C, D.
The Venn diagram shows overlapping families and species (Fig. 2), and 42% of the species are common among all areas (Fig. 2a) and areas C, D had the greatest number of unique species (19%). Regarding families (Fig. 2b), 62% are common among all areas, and areas C, D and E, F showed the highest numbers of common families (31%). No family was exclusive in the areas.
Discussion
The number of species found in this study is consistent with the expected pattern for tropical and subtropical forests of southern Brazil (Kersten 2006; Blum et al. 2011), as well as the taxonomic distribution of species in a few families, mainly in Orchidaceae, Bromeliaceae and Polypodiaceae (Zotz and Bader 2009; Freitas et al. 2016). The lower richness presented in areas A-B can be attributed to the conditions of microhabitat (availability of water, light, humidity, temperature, substrate stability, mineral nutrition and toxicity) (Wagner et al. 2015) provided by phorophytes present in the area. This condition can be associated with the degree of conservation in the areas, as vascular epiphytes respond differently to microclimate variations within the forest, which is considered crucial to the pattern of distribution of vascular epiphytes (Wagner et al. 2015). Studies point to the pattern of epiphytic colonization related to low diametric distribution of phorophytes (Zotz and Bader 2009; Wagner et al. 2015), as found in the areas A, B for this study. As the phorophyte diameter increases, the colonization by epiphytes rises proportionally (Ribeiro 2009). According to our results, we accepted the hypothesis that the variation of microhabitats affects the richness of the vascular epiphytic guild.
The great ecological amplitude of Bromeliaceae in this study, demonstrated by the distribution of species in ordination axes (Fig. 3), shows the relation between the degree of conservation (successional stage) and the presence of epiphytes. Bromeliaceae stands out in the secondary vegetation, as it is associated with propagule dispersal strategies (Cascante-Marin et al. 2006) or physiological adaptations, especially in atmospheric-habit species (Hietz and Hietz-Seifert 1995; Hietz et al. 2006).
Reyes-García et al. (2008) studying the differentiation of niches in epiphytic bromeliads, showed an association with areas that suffered some disturbance, justifying the pattern found in this study for the areas A, B and for heliophytic species: Tillandsia aeranthos, T. stricta, T. usneoides, Vriesea carinata, V. flammea and V. vagans (Reitz 1983).
The ecological amplitude observed in Microgramma squamulosa in southern Brazil has granted to this plant the ability to survive in a range of environmental conditions and achieve high abundance in secondary vegetation (Borgo and Silva 2003), showing its high ability to support microclimate variations, found also in open areas, as in this study, and fragment edges.
Thus, the microclimate homogeneity observed in areas of average natural regeneration stage, when compared to areas at an advanced stage, shows a direct function of low specific richness found in areas A, B. Cascante-Marin et al. (2006) when studying communities of epiphytic bromeliads in Costa Rica found that the structure of the community cannot be taken only by physiological and/or morphological attributes, and emphasizes the aspects limiting the dispersal in successional stages caused by seed dispersal and availability. In this perspective, the history of life, the differences affecting the production and the location of seed sources, as well as local wind, may lead to a non-random dispersal. This justifies, in the present study, the number of “no species” (n = 12) phorophytes in areas A, B, and according to Cascante-Marin et al. (2006), the change in diversity and composition of species is expected during forest succession, according to factors specific to the location, affecting seed dispersal and availability, added to the performance and survival of the individual. Geraldino et al. (2010) reiterates that forest disturbances result in significant impacts on the community of vascular epiphytes, which can reflect on the ecological processes where they participate.
The separation of areas C, D in the ordination diagram (Fig. 3) reinforces the hypothesis (Larrea and Werner 2010) that heterogeneous environments are essential in the coexistence of vascular epiphytes, resuming the discussion that species exhibit different coexistence strategies, which somehow contributes to the community structure, indicating the influence of deterministic factors (separation of niches by environmental gradients), and stochastic events (colonization processes) (Hiura 2001), in structuring communities without establishing the relative importance of each one (Leibold and McPeek 2006). Thus, it is assumed that the higher the microclimatic heterogeneity within the forest habitats, the greater will be the epiphytes coexistence, which supports the richness obtained of 40 and 34 species for C and D areas, respectively, and also by the high frequency values obtained (187 and 147), as well as 11 and 12 of the 13 families used in correspondence analysis (Fig. 3). The greatest richness in these environments (C, D) may be related because of the proximity to rivers, which have many waterfalls and obstacles, generating the sprinkling that increases the availability of water for these plants (Bonnet et al. 2010) or because it is an area with low human impact and then there are older trees (Woods et al. 2015).
On a local scale, as in this study, the differentiation of microclimates is seen as one of the main mechanisms in the structure of vascular epiphytes communities (Hietz et al. 2006; Woods et al. 2015; Dislich and Mantovani 2016). The presence of watercourses at the sampling site of areas C, D promotes an increase in moisture and consequently the differentiation of habitats, which offers milder conditions for the establishment of vascular epiphytes, considering that water is the main factor related to vegetative growth (Bonnet et al. 2010; Boelter et al. 2014). The influence of moisture as a structural strength of the community in areas C, D can be evidenced by the number of unique species, these associated with Hymenophyllaceae, Orchidaceae, Dryopteridaceae and Araceae, the latter extending to the areas E, F (Figs. 2, 3). The pronounced effect of moisture on the richness and preference for habitat can be seen in the reduction of occurrence of taxa from Hymenophyllaceae and Selaginellaceae toward the extremes of the studied toposequence (areas A, B and E, F), which constitute hydrophilic taxa and extremely sensitive to habitat disturbance (Barthlott et al. 2001).
The continued toposequence separating areas E, F reveals an environment where the moisture factor ceases to be predominant, since the sample units, on average, are distanced 100 m from the watercourses. On the other hand, there was evidence of a significant increase in the mean diameter of the stems of phorophytes sampled for the areas E (42.0 cm) and F (31.3 cm) (Padilha et al. 2015). Woods et al. (2015) studying the rainforests canopies of Costa Rica noted that with increasing heterogeneity of microhabitat in the same phorophyte, there is an increase in richness and abundance of epiphytic species. The main reason for the heterogeneity is caused by the increasing height of the trees, in addition to the increase in diameter and the expansion and architecture of their canopies, where the ones presenting the highest number for these items had greater diversity of microhabitat and epiphytes (Woods and Dewalt 2013). This fact leads to the appearance of Araceae, Cactaceae, Gesneriaceae and Piperaceae. Larrea and Werner (2010), as they studied the response of vascular epiphytes to different intensities of land use, observed that Araceae was the only family that responded positively to the use of the landscape, not occurring in different areas of unmanaged forests. Barbosa (2005) points out in his study that Araceae family epiphytes have preference for certain characteristics of phorophytes such as larger diameters and rough rhytidome.
Woods and DeWalt (2013) showed preference in the colonization of habitats by species of Araceae, Gesneriaceae and Cactaceae in forests over 55 years old, which corroborates the data from this study and the hypothesis of the influence of height, diameter of phorophytes and water viability on the structure of an epiphytic community.
Faced with the different mechanisms that were observed acting in structuring communities, it is important to resume the discussion based on the data obtained, of which mechanisms are involved in vascular epiphytic communities, niche data (niche partitioning, regeneration niche) (sensu Silvertown 2004) or data related to dispersion (dispersal limitation, neutral model) (sensu Hubbell 2001). On the assumption that species occupy niches according to their ecophysiological characteristics, a prevalent issue is determining the environment characteristics, and consequently, the species that structure plant communities over time. It is assumed that species are not environmentally equivalent to justify the occurrence of complex patterns of vegetal diversity found in subtropical forests, as in this study, and therefore we believe the niche partitioning is one of the essential mechanisms in the structure of vascular epiphytic communities (Silvertown 2004; Silvertown et al. 2006; Dislich and Mantovani 2016).
It is shown that environmental and historical factors (Nakashizuka 2001) contribute to the structuring of epiphytic communities, especially (1) horizontal and vertical heterogeneity (branching and crown expansion, as well as height of the phorophyte) (Boelter et al. 2014; Woods et al. 2015; Dislich and Mantovani 2016), (2) disturbance regime (use of landscape, successional stages, anthropic disturbance) (Hietz et al. 2006; Larrea and Werner 2010; Woods and Dewalt 2013) (3) biotic interactions (Cascante-Marin et al. 2006; Clavel et al. 2011). Thus, our study shows that the differentiation of microhabitats caused by different successional stages and associated environmental variables, led to the coexistence of different species of vascular epiphytes. In this study, with the exception of biological interactions, these factors proved decisive in the coexistence of species in formations of subtropical forest in southern Brazil. The plants require the same environmental features: light, water, CO2, substrate and mineral nutrients; however, the ecological differences between vascular epiphytes are manifested in different ways to acquire these resources (Westoby et al. 2002).
We had evidence of processes based on the niche as a key mechanism in the structure of vascular epiphytes communities. The differentiation of niches is generated by microclimate gradients within the forest that may be associated with: the successional stage, in the case of areas with a history of land use by the heterogeneity of resources (e.g., water). Otherwise, the homogeneity of resources leads to the formation of structurally less complex communities, but no less important for conservation, since they keep the ability of evolution (successional replacement). According to our results, we accepted the hypothesis that the variation of microhabitats affects the richness of the vascular epiphytic guild.
References
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorol Z 22:711–728. doi:10.1127/0941-2948/2013/0507
Apg, IV (The Angiosperm Phylogeny Group) (2016) An update of the Angiosperm Phylogeny Group classification for the ordens and families of flowering plants: APG IV. Bot J Linn Soc 181:1–20. doi:10.1111/boj.12385
Azeredo TEV, Citadini-Zanette V (2012) Aspectos florísticos, taxonômicos e ecológicos de bromélias da Mata Atlântica do sul de Santa Catarina. REA 14:20–43. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1676-06032011000400015. Accessed 25 April 2016
Barbosa JC (2005) Especificidade de epífitas da família Araceae a diferentes substratos arbóreos na Amazônia central. Ecologia da Floresta Amazônica, Manaus. http://pdbff.inpa.gov.br/cursos/efa/livro/2005/efa2005top.html. Acessed 1 June 2016
Barthlott W, Schmit-Neuerburg V, Nieder J, Engwald S (2001) Diversity and abundance of vascular epiphytes: a comparison of secondary vegetation and primary montane rain forest in the Venezuelan Andes. Plant Ecol 152:145–156. doi:10.1023/A:1011483901452
Benzing DH (1990) Vascular epiphytes. Cambridge University Press, New York
Benzing DH, Seeman J, Renfrow A (1978) The foliar epidermis in Tillandsioideae (Bromeliaceae) and its role in habitat selection. Am J Bot 65:359–365. doi:10.2307/2442278
Blum CT, Roderjan CV, Galvão F (2011) Composição florística e distribuição altitudinal de epífitas vasculares da Floresta Ombrófila Densa na Serra da Prata, Morretes, Paraná, Brasil. Biota Neotrop 11:141–159. http://www.biotaneotropica.org.br/v11n4/en/abstract?inventory+bn00811042011. Accessed 25 April 2016
Boelter CR, Dambros CS, Nascimento HEM, Zartman CEA (2014) Tangled web in tropical tree-tops: effects of edaphic variation, neighbourhood phorophyte composition and bark characteristics on epiphytes in a central Amazonian forest. J Veg Sci 25:1090–1099. doi:10.1111/jvs.12154
Bonnet A, Curcio GR, Lavoranti OJ, Galvão F (2010) Relações de epífitos vasculares com fatores ambientais nas florestas do rio Tibagi, Paraná, Brasil. Biotemas 23:37–47. https://periodicos.ufsc.br/index.php/biotemas/article/view/15865. Accessed 25 April 2016
Borgo M, Silva SM (2003) Epífitos vasculares em fragmentos de Floresta Ombrófila Mista, Curitiba, Paraná, Brasil. Braz J Bot 26:391–401. doi:10.1590/S0100-84042003000300012
Burns KC, Zotz G (2010) A hierarchical framework for investigating epiphyte assemblages: networks, meta-communities, and scale. Ecology 91:377–385. doi:10.1890/08-2004.1
Callaway RM, Reinhart KO, Tucker SC, Pennings SC (2001) Effects of epiphytic lichens on host preference of the vascular epiphyte Tillandsia usneoides. Oikos 94:433–441. doi:10.1034/j.1600-0706.2001.940306.x
Callaway RM, Reinhart KO, Moore GW, Moore DJ, Pennings SC (2002) Epiphyte host preferences and host traits: mechanisms for species-specific interactions. Oecologia 132:221–230. doi:10.1007/s00442-002-0943-3
Cardelús CL, Chazdon RL (2005) Inner-crown microenvironments of two emergent tree species in a lowland wet forest. Biotropica 37:238–244. doi:10.1111/j.1744-7429.2005.00032.x
Cardelús CL, Mack MC, Woods CL, Demarco J, Treseder KK (2009) The influence of tree species on canopy soil nutrient status in a tropical lowland wet forest in Costa Rica. Plant Soil 318:47–61. doi:10.1007/s11104-008-9816-9
Cascante-Marin A, Wolf JHD, Oostermeijer JGB, Den-Nijs JCM, Sanahuja O, Duran-Apuy A (2006) Epiphytic bromeliad communities in secondary and mature forest in a tropical premontane area. Basic Appl Ecol 7:520–532. doi:10.1016/j.baae.2005.10.005
Clark KL, Nadkarni NM, Schaefer D, Gholz HL (1998) Atmospheric deposition and net retention of ions by the canopy in a tropical montane forest, Monteverde, Costa Rica. J Trop Ecol 14:27–45. doi:10.1017/S0266467498000030
Clavel J, Julliard R, Devictor V (2011) Worldwide decline of specialist species: Toward a global functional homogenization? Front Ecol Environ 9:222–228. doi:10.1890/080216
Coelho MAN (2000) Flora Fanerogâmica do Reserva Biológica do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família Araceae. Hoehnea 27:33–39
Colwell RK, Dunn RR, Harris NC (2012) Coextinction and persistence of dependent species in a changing world. Annu Rev Ecol Evol Syst 43:183–203. doi:10.1146/annurev-ecolsys-110411-160304
Cottam G, Curtis JT (1956) The use of distance measurements in phytosociological sampling. Ecology 37:451–460. doi:10.2307/1930167
De Le Rosa-Manzano E, Andrade JL, Zotz G, Reyes-Garcia C (2014) Epiphytic orchids in tropical dry forests of Yucatan, Mexico—species occurrence, abundance and correlations with host tree characteristics and environmental conditions. Flora 209:100–109. doi:10.1016/j.flora.2013.12.002
Dislich R, Mantovani W (2016) Vascular epiphyte assemblages in a Brazilian Atlantic Forest fragment: investigating the effect of host tree features. Plant Ecol 217:1–12. doi:10.1007/s11258-015-0553-x
Freitas L, Salino A, Neto LM, Almeida TE, Mortara SR, Stehmann JR, AMORIM AM, Guimarães EF, Coelho MN, Zanin A, Forzza RC (2016) A comprehensive checklist of vascular epiphytes of the Atlantic Forest reveals outstanding endemic rates. PhytoKeys 58:65–79. doi:10.3897/phytokeys.58.5643
Geraldino HCL, Caxambú MG, Souza DC (2010) Composição florística e estrutura da comunidade de epífitas vasculares em uma área de ecótono em Campo Mourão, PR, Brasil. Acta Bot Bras 24:469–482. doi:10.1590/S0102-33062010000200018
Guimarães EF (1998) Flora Fanerogâmica do Reserva Biológica do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família Piperaceae. Hoehnea 15:46–51
Hietz P (2005) Conservation of vascular epiphyte diversity in Mexican coffee plantations. Conserv Biol 19:391–399. doi:10.1111/j.1523-1739.2005.00145.x
Hietz P, Hietz-Seifert U (1995) Composition and ecology of vascular epiphyte communities along an altitudinal gradient in central Veracruz, México. J Veg Sci 6:487–498. doi:10.2307/3236347
Hietz P, Buchlerger G, Winkler P (2006) Effect of forest disturbance on abundance and distribution of epiphytic bromeliads and orchids. Ecotropica 27:156–164. http://www.soctropecol.eu/publications/pdf/12-2/Hietz%20et%20al.pdf. Accessed 25 April 2016
Hiura T (2001) Stochasticity of species assemblage of canopy trees and understorey plants in a temperate secondary forest created by major disturbances. Ecol Res 16:887–893. doi:10.1046/j.1440-1703.2001.00449.x
Hoehne FC (1942) Orchidáceas In: Hoehne FC (ed) Fl Bras, Instituto de Botânica, São Paulo, 12:1–218
Hoehne FC (1945) Orchidáceas. In: Hoehne FC (ed) Fl Bras, Instituto de Botânica, São Paulo, 12:1–389
Hoehne FC (1949) Iconografia das Orchidaceas do Brasil. Lanzara, São Paulo
Hoehne FC (1953) Orchidáceas. In: Hoehne FC (ed) Fl Bras. Instituto de Botânica, São Paulo, 12:1–397
Hubbell SP (2001) The unified neutral theory of biodiversity and biogeography. Princeton University Press, Princeton. doi:10.1016/j.tree.2011.03.024
IBGE (2012) Fundação Instituto Brasileiro de Geografia e Estatística. Manuais Técnicos em Geociências 1: Manual técnico da vegetação brasileira. IBGE, Rio de Janeiro. http://biblioteca.ibge.gov.br/visualizacao/livros/liv63011.pdf. Accessed 25 April 2016
Johansson D (1974) Ecology of vascular epiphytes in West African rain forest. Acta Phytogeogr Suec 59:1–136
Kent M, Coker P (1992) Vegetation description and analysis, a pratical approach. Belhaven, London
Kersten RA (2006) Epifitismo vascular na bacia do Alto Iguaçu – Composição florística. Estud Biol 7:55–71
Larrea ML, Werner FA (2010) Response of vascular epiphyte diversity to different land-use intensities in a neotropical montane wet forest. Forest Ecol Manag 260:1950–1955. doi:10.1016/j.foreco.2010.08.029
Legendre P, Legendre L (2003) Numerical ecology. Elsevier, Amsterdam
Leibold MA, Mcpeek MA (2006) Coexistence of the niche and neutral perspectives in community ecology. Ecology 87:1399–1410. doi:10.1890/0012-9658(2006)87[1399:COTNAN]2.0.CO;2
Lugo AE, Scatena FN (1992) Epiphytes and climate change research in the Caribbean: a proposal. Selbyana 13:123–130. http://www.jstor.org/stable/41759801. Accessed 15 April 2016
Madison M (1977) Vascular epiphytes: The systematic occurrence and salient features. Selbyana 2:1–13. http://www.jstor.org/stable/41759613. Accessed 12 April 2016
Nakashizuka T (2001) Species coexistence in temperate, mixed deciduous forest. Trends Ecol Evol 16:205–210. doi:10.1016/S0169-5347(01)02117-6
Oliveira LC, Padilha PT, Dalmolin EB, Azeredo TEV, Citadini-Zanette V (2013) Componente epifítico vascular em um fragmento florestal urbano, município de Criciúma, Santa Catarina, Brasil. Biotemas 26:33–44. doi:10.5007/2175-7925.2013v26n2p33
Oliveira LC (2016) Distribuição de trepadeiras em diferentes ambientes de uma Floresta Atlântica Subtropical. Dissertation, Universidade do Extremo Sul Catarinense, Criciúma
Pabst GFJ, Dungs F (1975) Orchidaceae Brasilienses. Brucke-Verlag Kurt Schmersow, Hildesheim
Pabst GFJ, Dungs F (1977) Orchidaceae Brasilienses. Brucke-Verlag Kurt Schmersow, Hildesheim
Padilha PT, Santos Junior R, Custódio SZ, Oliveira LC, Santos R, Citadini-Zanette V (2015) Comunidade epifítica vascular do Parque Estadual da Serra Furada, sul de Santa Catarina, Brasil. Ciênc Nat 37:64–78. doi:10.5902/2179460X14368
Park CC (2003) Tropical rainforests. Routledge, London
Reitz R (1983) Bromeliáceas e a malária: bromélia endêmica. Flora Ilustrada Catarinense, Itajaí
Reyes-García C, Griffiths H, Rincon E, Huante P (2008) Niche differentiation in tank and atmospheric epiphytic bromeliads of a seasonally dry forest. Biotropica 40:168–175. doi:10.1111/j.1744-7429.2007.00359.x
Ribeiro DCA (2009) Estrutura e composição de epífitas vasculares em duas formações vegetais na Ilha da Marambaia, Mangaratiba, Dissertation, Universidade Federal Rural do Rio de Janeiro, Rio de Janeiro
Richter M (1991) Methoden der Klimaindikation durch pflanzenmorphologische Merkmale in den Kordilleren der Neotropis. Erde 122:267–289. http://www.geographie.nat.uni-erlangen.de/personen/michael-richter/. Accessed 12 April 2016
Silvertown J (2004) Plant coexistence and the niche. Trends Ecol Evol 19:605–611. doi:10.1016/j.tree.2004.09.003
Silvertown J, Dodd M, Gowing D, Lawson C, Mcconway K (2006) Phylogeny and the hierarchical organization of plant diversity. Ecology 87:39–49. doi:10.1890/0012-9658(2006)87[39:PATHOO]2.0.CO;2
Smith AR, Pryer KM, Schuettpelz E, Korall P, Schneider H, Wolf PGA (2006) Classification for extant ferns. Taxon 55:705–731. doi:10.2307/25065646
Tamashiro JY, Zickel CS (1991) Flora Fanerogâmica do Reserva Biológica do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família Cactaceae. Hoehnea 181:37–141
Triana-Moreno LA, Garzón-Venegas NJ, Sánchez-Zambrano J, Vargas O (2003) Epífitas vasculares como indicadores de regeneración en bosques intervenidos de la amazônia Colombiana. Acta Biol Colomb 8:31–42. http://www.revistas.unal.edu.co/index.php/actabiol/article/view/26669. Accessed 12 April 2016
Waechter JL (1992) O epifitismo vascular na Planície Costeira do Rio Grande do Sul. Thesis, Universidade Federal de São Carlos, São Carlos
Wagner K, Mendieta-Leiva G, Zotz G (2015) Host specificity in vascular epiphytes: a review of methodology, empirical evidence and potential mechanisms. AoB Plants 7:1–58. doi:10.1093/aobpla/plu092
Wanderley MGL, Martins SE (2007) Bromeliaceae. In: Wanderley MGL, Shepherd GJ, Melhem TS, Giulietti AM (Coord). Flora fanerogâmica do Estado de São Paulo. Instituto de Botânica, São Paulo, pp 39–61
Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ (2002) Plant ecological strategies: some leading dimensions of variation between species. Annu Rev Ecol Syst 33:125–159. doi:10.1146/annurev.ecolsys.33.010802.150452
Woods CL, Dewalt SJ (2013) The conservation value of secondary forests for vascular epiphytes in central Panama. Biotropica 45:119–127. doi:10.1111/j.1744-7429.2012.00883.x
Woods CL, Cardelús CL, Dewalt SJ (2015) Microhabitat associations of vascular epiphytes in a wet tropical forest canopy. J Ecol 103:421–430. doi:10.1111/1365-2745.12357
Zotz G (2016) Plants on plants—the biology of vascular epiphytes. Springer, Switzerland. doi:10.1007/978-3-319-39237-0
Zotz G, Bader M (2009) Epiphytic plants in a changing world: global change effects on vascular and non-vascular epiphytes. Prog Bot 70:147–170. doi:10.1007/978-3-540-68421-3_7
Acknowledgements
To the Foundation for Support to Research and Innovation of the State of Santa Catarina (FAPESC—Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina), to CAPES for granting the PhD scholarship to the first author and the Environmental Foundation of Santa Catarina (FATMA—Fundação do Meio Ambiente de Santa Catarina) for allowing access and collection of samples, particularly the biologist Vanessa Matias Bernardo, chief of the State Park of Serra Furada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Padilha, P.T., Elias, G.A., dos Santos, R. et al. Vascular epiphytes respond to successional stages and microhabitat variations in a subtropical forest in southern Brazil. Braz. J. Bot 40, 897–905 (2017). https://doi.org/10.1007/s40415-017-0391-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-017-0391-2