Abstract
We examined the structural and distribution patterns of woody species in the understory community of a Brazilian tropical rain forest in relation to two different soil types having distinct textures and chemical compositions. Plots were installed in five areas with clayey and in five with sandy soils, representing the main soil types in the region of the Una Biological Reserve, BA—Brazil (15°00′–15°20′S and 39°00′–39°15′W). Plant assemblages growing on those two soil types were sampled using the RAPELD methodology. A total of 1233 individuals were found on clayey soils (belonging to 303 species) and 1293 individuals on sandy soils (belonging to 320 species). No significant differences were observed among these two sets of plants in relation to their species compositions, abundances, species richness, heights, densities, or maximum diameter. Both plant communities had high alpha and beta diversity levels. Despite the apparent unstructured organizations of the communities and the lack of strong niche differentiation, we did not find evidence of ecological drift through processes such as random differentiation or increased dissimilarity with increasing distances. A combination of high species richness and high turnover rates as part of a much larger community may explain the results. The four most abundant species were likewise the most abundant on both soil types. This study contributed in identifying sets of species that respond well to local edaphic conditions and can be considered for restoration projects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Edaphic variables are key factors that can ultimately determine differences among plant communities at regional and local scales (Clark et al. 1998; Botrel et al. 2002; Castilho et al. 2006; John et al. 2007; Quesada et al. 2009; Guillherme et al. 2011; Saporetti Junior et al. 2012), and pedogenesis is known to be intimately associated with fertility and nutrient availability—especially in relation to nitrogen and phosphorus availability (Walker and Syers 1976). In addition to the composition of the parent rock (which largely defines nutrient availability), the physical characteristics of the soils themselves constitute important environmental filters and can drive differences in species distributions (Quesada et al. 2009; Saporetti Junior et al. 2012).
A number of studies have shown that in addition to influencing plant community composition, soil fertility can affect richness and diversity (Walker and Syers 1976; Braakhekke and Hooftman 1999; Laliberté et al. 2013). In this sense, researchers have associated the richest and most diverse communities with intermediate levels of soil fertility, with nutrients being available in sufficient quantities to allow plant growth but sufficiently limited to diminish the performance of the best competitors (Braakhekke and Hooftman 1999). It is also known, however, that the richest and most diverse plant communities on the planet grow on the oldest and most strongly weathered soils, where nitrogen and phosphorus are scarce (Laliberté et al. 2013).
In addition to being restricted to specific soil types (niches), or kept safe from competitive exclusion in soils of intermediate fertility, species that occur in heterogenic edaphic conditions can show distributions dominated by dispersal or recruitment limitations (Condit et al. 1992; Hubbell et al. 1999). Dispersal and recruitment limitations originate not only from dispersal failures (in failing to encounter appropriate sites), but also from heavy seedling and young plant mortality due to predators or pathogens (Hubbell 2006). In these cases, local species distributions and dynamics due to local extinction and immigration from metacommunities will not be governed by niche rules, but rather by chance and, as a consequence, composition differences between sites will be correlated with geographic distances (Hubbell 2001; Condit et al. 2002; Tuomisto et al. 2003).
Recent studies have shown the importance of both niche and chance processes to plant community distributions and to maintaining coexistence and high species richness (Hubbell 2001; Wright 2002; Tuomisto et al. 2003; Chave 2008; Andersen et al. 2010; Vellend 2010). Community simulations performed by Adler et al. (2013) indicated that combinations of environmental filters and variations in functional attributes linked to species dispersal would favor the coexistence of large numbers of taxa.
Niches or chance can produce communities that show compositional and abundance differences between sites—“beta diversity” (Whittaker 1972; Tuomisto and Ruokolainen 2006)—which is expressed as the dissimilarity between pairs of sites and can be calculated in several ways and with several indexes (Veech et al. 2002; Tuomisto and Ruokolainen 2006; Jost 2007; Anderson et al. 2011). There are essentially two ways in which a pair of sites can differ in composition: by turnover or species substitutions, or by nestedness (in which there is a reduction of richness in one site as compared to the other, with the poorest having only a subset of the richest composition) (Baselga and Orme 2012). Environmental filters can produce mixed patterns of nestedness and turnover between pairs of sites that will be related to natural or anthropogenic drivers, but strong stressors generally produce nestedness (Barros et al. 2014; Brendonck et al. 2014) while environmental gradients usually produce turnover (Fernandez-Going et al. 2013; Barros et al. 2014).
It is important to elucidate the variables that promote beta diversity and the distributions of understory species, as those understory communities represent an important forest strata that provides food resources for the native fauna (Gentry and Emmons 1987), and the understory itself serves as a competitive filter for young arboreal individuals that can eventually attain canopy status (George and Bazzaz 1999; Harms et al. 2000). Additionally, studies focusing on the understory can identify a significant fraction of the diversity of woody forest species (Galeano et al. 1998) and aid in evaluating natural dynamics—information fundamental to management and/or restoration efforts (Guariguata et al. 1997).
The present study examined the patterns of woody understory species distributions and abundances in an edaphic mosaic within an extremely rich Brazilian Atlantic tropical forest (Thomas et al. 1998; Martini et al. 2007) to address the following questions: (1) Are there significant differences between the compositions, abundances, species richnesses, and structures (density, heights, and DBH) of understory plant communities growing on the two principal soil types in the Una Biological Reserve? (2) Are there indicator species for each soil type? (3) Are the observed dissimilarities correlated with geographical distance (indicating a random process of assemblage differentiation)? (4) Are the observed dissimilarities between these sites due to species reductions (nestedness) in any soil type or to substitution (turnover)? We expect to find differences in the structures, compositions, and species abundances of plants on the two soil types, with greater values of richness, height, DBH, and absolute density on the less restrictive (clay) soil type, but no correlation between geographical distances and dissimilarity. Additionally, we expected nestedness to be the preponderant component of beta diversity in sandy soil (more restrictive) and turnover component in clay soil (less restrictive).
Materials and methods
Study area
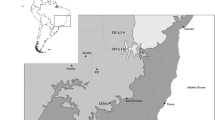
The present study was undertaken in the Una Biological Reserve (REBIO Una) in the municipality of Una, Bahia State, Brazil. The REBIO Una occupies an area of 17,704 ha (15°00′–15°20′S and 39°00′–39°15′W), and a Wildlife Refuge—REVIS Una (divided into two areas of 7456 and 15,948 ha) surrounds the REBIO Una, thus constituting a total conservation area of 41,108 ha (Decreto de 21 de Dezembro 2007) (Fig. 1).
The region has an annual precipitation between 1200 and 1800 mm (Mori et al. 1983). A meteorological station installed in the reserve in 2012 indicated that the minimum rainfall during the study period occurred in April/2012 (40.4 mm) and the maximum in January/2013 (260.2 mm) (França, S. personal communication 2013). The dominant vegetation in the REBIO Una is classified as Tropical Pluvial or Dense Ombrophilous forest (Thomas and Barbosa 2008).
While 13 soil types have been identified in the municipality of Una (Santana et al. 2009), the REBIO Una itself is dominated mainly by yellow latosols in the western sector and yellow argisols in the eastern sector. Both soil types were formed from sediments derived from the Barreiras formation, although they have different textures and drainage characteristics (Santana et al. 2009). The yellow latosols are deep and moderately well-drained, generally defining flat to slightly undulating landscapes with clay textures, with horizons having from 3 to 13 % silt and from 48 to 71 % clay; all horizons have minor proportions of fine and coarse sand (<40 %). These are acidic soils with pH varying from 4.5 to 5.2 (Santana et al. 2009). The yellow argisols are very deep, very well-drained and are found on rolling landscapes with sandy to sandy-clay textures, with horizons having from 8 to 17 % silt and from 13 to 35 % clay; all horizons have large proportions of sand (>60 %). These are acidic soils, with pH varying from 4.8 to 5.2 (Santana et al. 2009). The yellow argisols have comparatively higher concentrations of Mg and Ca (at least three times greater than the latosols), while the yellow latosols have higher concentrations of silicates (SiO2), aluminum oxide (Al2O3), and iron oxide (Fe2O3), the latter being four times greater than the argisols. In an ongoing study, Oliveira (in prep) found that yellow argisols near the study plots had significantly higher levels of potassium and phosphorus and higher pH levels as compared to the yellow latosols. We will identify the soils types here by referring to the yellow latosols as clayey soils (due to their greater clay contents) and the yellow argisols as sandy soils (due to their greater sand contents).
Data collection
The present study was undertaken within a 5 × 5 km study area established during the Long Duration Ecological Studies Program (PELD Una) at the REBIO Una. This area is composed of a nearly continuous forest mosaic of different successional stages, “cabrucas” (forest/cocoa bean plantations), fields, and permanently swampy areas. Thirty 2 × 100 m plots were delimited inside the 5 × 5 km study area, with minimum distances of 1 km between them (17 in clay soil and 13 in sand soil) (Fig. 1).
The plots were located along isolines, as established by the RAPELD randomization method (Magnusson et al. 2005), as adapted to 2 × 100 m plots. Ten plots in forests without signs of recent disturbance were selected, five (P1.1, P1.2, P1.3, P1.4, and P1.5) on clay soils and five (P2.1, P2.2, P2.3, P2.4, and P2.5) on sandy soil, avoiding plots in contact regions; none of these 10 sites were in wetlands. The nearest plots were separated by 1 km and the most distant plots by 5.83 km (Fig. 1).
All woody individuals within these plots with minimum heights of 1.30 m and diameters at breast height (DBH) ≤10 cm were sampled. The diameters at breast height of all individuals were measured, their heights estimated, and branches were collected for identification. All shoots of highly subdivided individuals were measured and their individual areas calculated; the summed areas were used to calculate their pooled DBH. The individuals collected were separated into morphospecies and identified to the species level whenever possible. Testimonial specimens of each species/morphospecies were incorporated into the CEPEC herbarium.
Data analysis
In order to answer the first question regarding community structure (heights, DBH, and densities), species composition, and richness, the following analyses were used:
The understory communities on the two soil categories were compared in terms of their heights, DBH, and densities using a mixed linear model (Pinheiro and Bates 2000), considering the plots as random variables. The data were log-transformed due to the natural heteroscedasticity observed in the residuals of mixed models with raw data. The mean densities of individuals per transect in each of the two soil categories were compared using a permutation test for mean differences, with 10,000 randomizations to construct a confidence interval.
The species richnesses on the two soil types were compared using rarefaction curves constructed using incidence data (sample based rarefaction); 95 % confidence intervals were determined based on 10,000 randomizations. Richnesses between two sites were considered significantly different when their confidence intervals did not overlap.
Permutational multivariate analysis of variance (PERMANOVA) was used to evaluate differences between sampled assemblages on the different soil types (Anderson 2001), utilizing the Bray–Curtis distance and a 5 % level of significance. The confidence intervals for the differences between the soil categories were constructed using 10,000 randomizations.
In order to answer the second question, indicator species analyses (Dufrene and Legendre 1997) were performed, using the relative frequency data to generate indicator values for the species in relation to the soil types; the confidence intervals were defined using 10,000 randomizations. The species were also ordered in terms of their abundances on each soil type.
To answer the third question, the Mantel test (Mantel 1967), using Bray Curtis distances, were performed. This test examines the correlation between distance and the dissimilarities of the samples, with random differentiation. The confidence interval was obtained through 10,000 randomizations, considering a 5 % level of significance.
To better visualize the results of the PERMANOVA and Mantel tests, we performed Cluster analyses that could identify similarity relationships among the plots using species composition and abundance data and the Bray–Curtis index. These analyses allowed us to evaluate the degrees of similarity between plots within the same soil type in relation to their geographical proximity. The bootstrap method was used to generate the significance of each node through 10,000 randomizations. We perform this analysis to graphically illustrate the dissimilarities between the sites.
To answer the fourth question, an additive partitioning of beta diversity as proposed by Baselga and Orme (2012) was preformed that evaluated to what degree the differences in the compositions of plots on the same soil types were due to species substitutions (turnover) or to species reductions (nestedness). This analysis is based on the properties of the dissimilarity indexes commonly used in community and genetic analyses using incidence data, in which total variations between single plots or among multiple sites can be measured by the Sorensen index; the “pure” turnover component can be measured by the Simpson index; the “pure” nestedness component can then be measured by the difference between them (Baselga and Orme 2012). This method additively splits the two components of beta diversity (turnover and nestedness), which can then be compared in terms of the importance of their contributions to the total difference between the sites or to the entire dataset. Random variability in the components can be estimated using permutation methods.
Mixed linear models were developed using nlme packages (Pinheiro et al. 2013). Permutation tests to detect differences between the means of the densities per plot were developed in an R environment (R Development Core Team 2012). The rarefaction curves, PERMANOVA, and the Mantel tests were conducted using Vegan packages (Oksaken et al. 2010); analyses of indicator species were performed using labdsv packages (Roberts 2012); the partitioning of beta diversity was performed using betapart packages (Baselga and Orme 2012)—all in R environments (R Development Core Team 2012). Cluster analysis was performed using PAST software (Hammer et al. 2001).
Results
The heights of the individuals sampled varied from 1.3 to 20 m, and their DBH varied from 0.47 to 15.9 cm (Fig. 2). Four individuals had calculated diameters greater than 10 cm due to pooled DBH values. The numbers of individuals per plot varied from 151 to 327. The physical structures of the woody understory communities were similar on the two different soil types, with no significant differences between their heights (P = 0.50), DBH (P = 0.83), or densities (P = 0.24).
A total of 2526 live individuals were sampled, distributed among 449 species and 61 families. A total of 1233 individuals belonging to 303 species were encountered on clay soils, and 1293 individuals belonging to 320 species were encountered on sandy soils. The rarefaction curves showed overlapping of their confidence intervals, indicating similar values of species richness on both soil types (Fig. 3).
One hundred and thirty species were only found on clay soils, although 62.3 % of them were represented by only a single individual. One hundred and forty-six species were found only on sandy soils, with 60.3 % of them being represented by only a single individual. As a result of this strong representation of singletons, which incorporated noise into the analyses, PERMANOVA values were not significant (P = 0.13). One sandy soil plot had the lowest percentage of exclusive species (7.5 %) and one had the highest (26 %), with 50 and 65.6 % of their species being represented by just a single individual, respectively. All of the other plots demonstrated more than 16 % exclusive species.
Inga capitata Desv (80 %, P = 0.0469), Randia calycina Cham. (80 %, P = 0.0478), and Faramea coerulea Nees and Mart. (80 %, P = 0.0483) were identified as indicators of clay soils. The four most abundant species were the same on both soil sites, although with some alterations in their ordering (Table 1). Guapira opposita, Chamaecrista duartei, and Pouteria reticulata were also among the most abundant species growing on both soil types.
In evaluating species distributions, it was observed that only 1 % of the species occurred in all of the plots; 10.2 % were found in five plots (50 %); 43.9 % occurred in only a single plot. No significant spatial correlations were found in Mantel test (P = 0.61; r = −0.04).
Cluster analysis (Fig. 4) indicated low similarities between the plots (generally less than 0.4); only plots 1.4 and 1.1 demonstrated a similarity of 0.43. Cluster analysis indicated the separation of a single plot that was significantly distinct from the others (1.5), followed by the formation of two groups, although this grouping was not statistically significant (P > 95 %). The first group was composed of plots 1.1, 1.4, 2.1, 1.3, 2.5, 1.2, and 2.3 and the second group by plots 2.2 and 2.4. These groups were not influenced by the soil type or by spatial relationships between the samples, as neither plots on the same soil type, nor those close to each other, were grouped.
Grouping analyses of the plots located on the different soil types, considering the juvenile arboreal/shrub communities in the Una Biological Reserve, Bahia State, Brazil: clayey soils (P1.1, P1.2, P1.3, P1.4, and P1.5) and sandy soils (P2.1, P2.2, P2.3, P2.4, and P2.5). The values in the dendrogram represent the robustness of the traits evaluated using the bootstrap randomization method
The value of total beta diversity, considering all of the sampling plots, and independent of the soil type, was 0.825 (the maximum being 1.0); of this diversity, 0.802 was due to component turnover and only 0.023 was due to component nestedness (Fig. 5). In terms of each soil type, clay soil sites demonstrated a total beta diversity of 0.730, of which 0.704 was due to turnover and 0.026 was due to nestedness. The sandy soils sites demonstrated a total beta diversity of 0.734, with 0.690 due to turnover and 0.044 due to nestedness.
Participation of total beta diversity (full dark line) in the nesting (full gray line) and substitution (dashed gray line) components. Data from the juvenile arboreal/shrub communities in the Una Biological Reserve, Bahia State, Brazil, on clay and sandy soils. The upper graph demonstrates the partitioning of beta diversity on both soil types, while the lower graphs show the partitioning of beta diversity in each soil type
Discussion
We found extremely rich communities growing on both soil types (449 species in 0.2 ha), with no differences in richness and physical structure (DBH, height, and density) between them. The absence of any significant differences suggests that the two soil types did not cause major distinctions between the plant communities, with soil restrictions apparently not being severe enough to impede plant development. Both soil types were probably within a low-medium fertility range that has high predictable richness (Braakhekke and Hooftman 1999; Laliberté et al. 2013). Plots showed very similar or low values of similarity within the same soil type or between soil types when using incidence or abundance data, the latter reflected in no significant differences in PERMANOVA. The low observed similarity was almost entirely due to turnover (considering each soil type or the entire set of plots). These results indicate that niche differentiation between soil types, if it occurs, is poorly represented in the bulk of the understory community.
Few species apparently responded to the soil types, and we found no evidence of random differentiation leading to greater dissimilarities in more distant plots (as observed in the Mantel test and cluster analysis)—which is contrary to the predictions of neutral dynamic colonization processes within the community (Hubbell 2001; Condit et al. 2002). This apparently contradictory evidence pointing to random and niche processes can be understood if we consider other findings as well as the regional context. In addition to extremely high richness, high turnover, and low similarity between any pair of plots, we found elevated numbers of exclusive species in each plot, which is consistent with the known mega-diversity patterns of tropical forests (Condit et al. 2002; Duivenvoorden et al. 2002) and this region (Thomas et al. 1998; Martini et al. 2007).
The low apparent levels of influence of edaphic conditions on species distributions may be related to very high-regional species richness as observed in the high heterogeneity within the plots, and it can also be inferred by the absence of any correlation between dissimilarity and geographical distance at the scale of this study (~1–6 km). The factors responsible for these high beta diversity values are still largely undefined; however, as different variables can act at different scales (Condit et al. 2002; Duivenvoorden et al. 2002; Tuomisto and Ruokolainen 2006; Davidar et al. 2007) and will reflect historical influences (Zobel et al. 2011).
There is evidence that local richness is strongly influenced by regional species pools and their evolutive histories (Zobel et al. 2011). It is therefore possible that the extremely high richness and diversity encountered on both soil types reflects rich historical communities, as this region is within an important center of endemism (Thomas et al. 1998) and is thought to represent a Pleistocene refuge (Carnaval and Moritz 2008)—although this still does not explain the lack of differentiation. Montgomery and Chazdon (2001) noted that the high-species diversity encountered in tropical forests makes it difficult to elaborate deductions about the distribution patterns of understory species in relation to light availability—for despite the fact that light is a severe filter, it also shows random and dynamic distribution patterns in mature forests (Brokaw and Busing 2000). This difficulty in identifying patterns related to light availability in extremely rich communities may also apply to other deterministic variables such as edaphic conditions—especially if those conditions are not very rigorous. Meira Neto et al. (2005) did observe, however, that soil type was the variable that best explained the distributions of herbaceous and shrub species in a seasonal Semideciduous forest (in an Atlantic Forest)—although these authors evaluated only 80 species, while the present study evaluated 449. Additionally, studies have shown the effects of anthropogenic impacts on the compositions (Martini et al. 2008) and structures (Faria et al. 2009) of understory communities in the same region as this study, and accidental fires (Martini et al. 2008) and logging (Faria et al. 2009) were found to be effective drivers of understory change. This suggests that recent anthropogenic activities can effectively drive understory community changes.
Fully 67.9 % of the species encountered were represented by less than five individuals, and this low species density made it difficult to identify indicator species (Phillips et al. 2003). In fact, of the 449 species identified, only three were considered indicators. Costa et al. (2005) and Meira Neto et al. (2005) reported that it was easier to detect significant preferences related to environmental variables in more abundant species. This was not observed in the present study; however, as the three indicator species were not among the most abundant (but did have higher frequencies on the clayey soils). More precise estimates of species densities might have been possible if the sampling intensity had been increased, but the relatively large number of specimens surveyed and identified (2526 individuals) indicates that a very large contingent of plants were capable of growing on both soil types. The environmental filters that have affected and currently affect those communities apparently act on different temporal and spatial scales than those considered here.
In spite of the fact that species distributions in the woody understory communities were only poorly explained by edaphic conditions, regional historical processes appear to have generated and maintained extremely rich and diverse sets of species with extreme spatial heterogeneity. These findings therefore present an opportunity (and at the same time a challenge) for current ecological restoration programs (Rodrigues et al. 2009) underway in the region following several infrastructure, and demand knowledge of species responses to edaphic conditions as a large subset of species apparently can be used in reforestation projects. Local plant communities, however, may be pre-adapted to conditions of enormous diversity, and it will be difficult to predict their responses to habitat reduction, the removal of large sets of the local fauna, or climate change.
References
Adler PB, Fajardo A, Kleinhesselink AR, Kraft NJB (2013) Trait-based tests of coexistence mechanisms. Ecol Lett 16:1294–1306
Andersen KM, Turner BL, Dalling JW (2010) Soil-based habitat partitioning in understorey palms in lower montane tropical forests. J Biogeogr 37:278–292
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson MJ, Crist TO, Chase JM, Vellend M, Inouye BD, Freestone AL, Sanders NJ, Cornell HV, Comita LS, Davies KF, Harrison SP, Kraft NJB, Stegen JC, Swenson NG (2011) Navigating the multiple meanings of beta diversity: a roadmap for the practicing ecologist. Ecol Lett 14:19–28
Barros F, Blanchet H, Hammerstrom K, Sauriau PG, Oliver J (2014) A framework for investigating general patterns of benthic β-diversity along estuaries. Estuar Coast Shelf Sci 149:223–231
Baselga A, Orme CDL (2012) Betapart: an R package for the study of beta diversity. Methods Ecol Evol 3:808–812
Botrel RT, Oliveira Filho AT, Rodrigues LA, Curi N (2002) Influência do solo e topografia sobre as variações da composição florística e estrutura da comunidade arbóreo-arbustiva em uma floresta estacional semidecidual em Ingaí, MG. Rev Bras Bot 25:195–213
Braakhekke WG, Hooftman DAP (1999) The resource balance hypothesis of plants species diversity in grassland. J Veg Sci 10:187–200
Brendonck L, Jocqué M, Tuytens K, Timms BV, Vanschoenwinkel B (2014) Hydrological stability drives both local and regional diversity patterns in rock pool metacommunities. Oikos 124:1–9
Brokaw NVL, Busing RT (2000) Niche versus change and tree diversity in forest gaps. Trends Ecol Evol 15:183–188
Carnaval AC, Moritz C (2008) Historical climate modeling predicts patterns of current biodiversity in the Brazilian Atlantic Forest. J Biogeogr 35:1187–1201
Castilho CV, Magnusson W, Araujo RNO, Luizão RCC, Luizão FJ, Lima AP, Higuchi N (2006) Variation in aboveground tree live biomass in a central Amazonian forest: effects of soil and topography. For Ecol Manag 234:85–96
Chave J (2008) Spatial variation in tree species composition across tropical forests: pattern and process. In: Carson WP, Schitzer SA (eds) Tropical forest community ecology. Wiley, Chichester, pp 11–30
Clark DB, Clark DA, Read JM (1998) Edaphic variation and the mesoscale distribution of tree species in a neotropical rain forest. J Ecol 86:101–112
Condit R, Hubbell SP, Foster RB (1992) Short term dynamics of a neotropical forest. Bioscience 42:822–828
Condit R, Pitman N, Leigh EG Jr, Chave J, Terborgh J, Foster RB, Nunez P, Aguilar VS, Valencia R, Villa G, Muller-Landau HC, Losos H, Hubbell SP (2002) Beta diversity in tropical forest trees. Science 295:666–669
Costa FRC, Magnusson WE, Luizão RC (2005) Mesoscale distribution patterns of Amazonian understorey herbs in relation to topography, soil and watersheds. J Ecol 93:863–878
Davidar P, Rajagopal B, Mohandass D, Puyravaud J, Condit R, Wright SJ, Leigh EG Jr (2007) The effect of climatic gradients, topographic variation and species traits on the beta diversity of rain forest trees. Global Ecol Biogeogr 16:510–518
Development Core Team R (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Decreto não numerado de 21 de dezembro de (2007) Criação da Reserva Biológica do Refúgio Silvestre de Una e Ampliação da Reserva Biológica de Una. Diário oficial da União, edição extra, pp 12–13
Dufrene M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Duivenvoorden JF, Svenning JC, Wright SJ (2002) Beta diversity in tropical forests. Science 295:636–637
Faria DM, Mariano-Neto E, Martini AMZ, Ortiz JV, Montingelli RG, Rosso S, Paciencia MLB, Baumgarten J (2009) Forest structure in a mosaic of rainforest sites: the effect of fragmentation and recovery after clear cut. For Ecol Manag 257:2226–2234
Fernandez-Going BM, Harrison SP, Anacker BL, Safford HD (2013) Climate interacts with soil to produce beta diversity in a Californian plant communities. Ecology 94:2007–2018
Galeano G, Suárez S, Balslev H (1998) Vascular plant species count in a wet forest in the Chocó area on the Pacific Coast of Colombia. Biodivers Conserv 7:1563–1575
Gentry AH, Emmons LH (1987) Geographical variation in fertility, phenology, and composition of the understory of neotropical forests. Biotropica 19:216–227
George LO, Bazzaz FA (1999) The Ferns understory as an ecological filter: growth and survival of Canopy-Tree seedlings. Ecology 80:846–856
Guariguata MR, Chazdon RL, Denslow JS, Dupuy JM, Anderson L (1997) Structure and floristics of secondary and old-growth forest stands in lowland Costa Rica. Plant Ecol 132:107–120
Guillherme FAG, Ferreira TO, Assis MA, Torrado PV, Morellato LPC (2011) Soil profile, relief features and their relation to structure and distribution on Brazilian Atlantic rain forest trees. Sci Agr 69:61–69
Hammer O, Harper DAT, Ryan PD (2001) PAST: paleontological statistics soft ware package for education and data analysis. Palaeontol Electron 4:1–9
Harms KE, Wright SJ, Calderón O, Hernández A, Herre EA (2000) Pervasive density-dependent recruitment enhances seedling diversity in a tropical forest. Nature 404:493–495
Hubbell SP (2001) The unified neutral theory of biodiversity and biogeography. Princeton University Press, Oxfordshire
Hubbell SP (2006) Neutral theory and the evolution of ecological equivalence. Ecology 87:387–1398
Hubbell SP, Foster RB, O’Brien ST, Harms KE, Condit R, Wechler B, Wright SJ, Loo De Lao S (1999) Light-gap disturbances, recruitment limitation, and tree diversity in a Neotropical forest. Science 283:554–557
John R, Dalling JW, Harms KE, Yavitt JV, Stallard RF, Mirabello M, Hubbell SP, Valencia R, Navarrete H, Vallejo M, Foster RB (2007) Soil nutrients influence spatial distributions of tropical tree species. Proc Natl Acad Sci USA 104:864–869
Jost L (2007) Partitioning diversity into independent alpha and beta components. Ecology 88:2427–2439
Laliberté E, Grace JB, Huston MA, Lambers H, Teste FP, Turner BL, Wardle DA (2013) How does pedogenesis drive plant diversity? Trends Ecol Evol 28:331–340
Magnusson WE, Lima AP, Luizão R, Luizão F, Costa FRC, Castilho CV, Kinupp VF (2005) Rapeld: a modification of the gentry method for biodiversity surveys in long-term ecological research sites. Biota Neotrop 5:1–6
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Martini AMZ, Fiaschi P, Amorim AM, Paixão JL (2007) A hot-point within a hot-spot: a high diversity site in Brazil´s Atlantic Forest. Biodivers Conserv 16:3111–3128
Martini AMZ, Jardim JG, Santos FAM (2008) Floristic composition and growth habits of plants in understory, natural treefall gaps and fire-disturbed areas of a tropical forest in southern Bahia State, Brazil. Mem New York Botan Gard 100:147–192
Meira Neto JAJ, Martins FR, Souza AL (2005) Influência da cobertura e do solo na composição florística do sub-bosque em uma floresta estacional semidecidual em Viçosa, MG, Brasil. Acta Bot Bras 19:473–486
Montgomery RA, Chazdon RL (2001) Forest structure, canopy architecture, and light transmittance in tropical wet forests. Ecology 82:2707–2718
Mori SA, Boom BM, Carvalho AM, Santos TS (1983) Southern Bahian moist forest. Bot Rev 49:155–232
Oksaken J, Blanchet FG, Kindt R, Legendre P, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2010) vegan: community ecology package. http://CRAN.R-project.org/package=vegan. Accessed Nov 2012
Phillips OL, Vargas N, Monteagudo AL, Cruz AP, Zans M-EC, Sánchez WG, Yli-Halla M, Rose S (2003) Habitat association among Amazonian tree species: a landscape-scale approach. J Ecol 91:757–775
Pinheiro JC, Bates DM (2000) Mixed effects models in S and S-Plus. Springer, New York
Pinheiro J, Bates D, DebRoy S, Sarkar D (2013) nlme: linear and nonlinear mixed effects models. http://CRAN.R-project.org/package=nlme. Accessed Aug 2013
Quesada CA, Lloyd J, Schwarz M, Baker TR, Phillips OL, Patiño S, Czimczik C, Hodnett MG, Herrera R, Arneth A, Lloyd G, Malhi Y, Dezzeo N, Luizão FJ, Santos AJB, Schmerler J, Arroyo L, Silveira M, Priante Filho N, Jimenez EM, Paiva R, Vieira I, Neill DA, Silva N, Peñuela MC, Monteagudo A, Vásquez R, Prieto A, Rudas A, Almeida S, Higuchi N, Lezama AT, López-González G, Peacock J, Fyllas NM, Alvarez Dávila E, Erwin T, di Fiore A, Chao KJ, Honorio E, Killeen T, Peña Cruz A, Pitman N, Núñez Vargas P, Salomão R, Terborgh J, Ramírez H (2009) Regional and large-scale patterns in Amazon forest structure and function are mediated by variations in soil physical and chemical properties. Biogeosci Discuss 6:3993–4057
Roberts D (2012) Labdsv: ordination and multivariate analysis for ecology. http://CRAN.R-project.org/package=Labdsv. Accessed Dec 2012
Rodrigues RR, Lima RAF, Gandolfi S, Nave AG (2009) On the restoration of high diversity forest: 30 years of experience in the Brazilian Atlantic Forest. Biol Conserv 142:1242–1251
Santana SO, Mendonça JR, Faria Filho AF, Azevedo DG, Waldburger T (2009) Levantamento semidetalhado dos solos do município de Una, Bahia, Brasil. CEPEC-CEPLAC, Bahia
Saporetti Junior AW, Schaefer CEGR, Souza AL, Soares MP, Araujo DSD, Meira Neto JAA (2012) Influence of soil physical properties on plants of the Mussununga ecosystem, Brazil. Folia Geobot 47:29–39
Thomas WW, Barbosa MRV (2008) Natural vegetation types in the Atlantic coastal forest of northeastern Brazil. Mem New York Botan Gard 100:6–20
Thomas WMW, Carvalho AM, Amorim AM, Garrison J, Arbelàez AL (1998) Plant endemism in two forests in southern Bahia, Brazil. Biodivers Conserv 7:311–322
Tuomisto H, Ruokolainen K (2006) Analyzing or explaining beta diversity? Understanding the targets of different methods of analysis. Ecology 87:2697–2708
Tuomisto H, Ruokolainen K, Yli-Halla M (2003) Dispersal, environment, and floristic variation of western Amazonian forests. Science 299:241–244
Veech JA, Summerville KS, Crist TO, Gering JC (2002) The additive partitioning of species diversity: recent revival of an old idea. Oikos 99:3–9
Vellend M (2010) Conceptual synthesis in community ecology. Q Rev Biol 85:183–206
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19
Whittaker RH (1972) Evolution and measurement of species diversity. Taxon 21:213–251
Wright SJ (2002) Plant diversity in tropical forests: a review of mechanisms of species coexistence. Oecologia 130:1–14
Zobel M, Otto R, Laanisto L, Naranjo-Cigala A, Partel M, Fernández-Palacios JM (2011) The formation of species pool: historical habitat abundance affects current local diversity. Glob Ecol Biogeogr 20:251–259
Acknowledgments
We would like to thank all of the experts who have identified and/or confirmed parts of the collected material, especially Jomar G. Jardim and Charlotte Taylor (Rubiaceae), the Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq for resources provided for the implementation of research project (PELD, Grant # 558269/2009; PPBIO, Grant # 457483/2012-1) and the productivity scholarship awarded to Amorim AM (Produtividade, Grant # 306992/2012-4), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES for the scholarship awarded to Lopes LCM, and the Instituto Chico Mendes de Conservação da Biodiversidade—ICMBIO for the plant collection license and logistical support in the REBIO Una, Bahia, Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lopes, L.C.M., Mariano-Neto, E. & Amorim, A.M. Can soil types explain species distributions? Evaluating the woody understory component of a tropical forest in Brazil. Braz. J. Bot 39, 251–259 (2016). https://doi.org/10.1007/s40415-015-0235-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-015-0235-x