Abstract
Montane environments create unique conditions that favour the development of remarkably rich and endemic biotas. The Serra da Mantiqueira is an important Brazilian mountain range and is recognised globally for certain groups of living organisms, whereas gaps in knowledge exist for other groups, such as epiphytes, which have been poorly studied in the region. The Serra da Mantiqueira contains rare fragments of a vegetation formation, the mixed ombrophilous forest (MOF), which is typical of the Southern Region of Brazil and is very threatened. The present study aimed to analyse the epiphytic communities occurring on individuals of Podocarpus lambertii Klotzsch ex Endl. (Podocarpaceae) in two biotopes: natural patches and continuous alluvial forest. We sampled 60 phorophytes, equally divided between the sites, and established five strata according to stem ramification. This phorophyte species is ecologically relevant, since it harbours 92 species and 19 families, and a single individual may support up to 30 % of the total richness of the studied community. The Shannon diversity index was 3.86, which is comparable to that found in other studies dealing with several phorophyte species and is the highest found to date for the MOF. The biotopes and strata did not show significant differences in the calculated diversity indices, except in richness (80 species in the alluvial forest and 60 in the patches). The most representative ecological categories were characteristic holoepiphytes (73 species) and accidental holoepiphytes (nine species). The present study shows the importance that a single species can have in an ecosystem and contributes to our knowledge concerning epiphytic synusia in mountainous environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mountainous regions possess features that make them environments with high indices of richness and endemism, and also represent vegetation islands that harbour important forest remnants, although these ecosystems are especially sensitive to anthropogenic disturbances (Martinelli 2007). The Serra da Mantiqueira, together with the Serra do Mar, represent the main highlands in the Atlantic Forest (AF), a geographic domain that contains 46 % of Brazilian flora (Stehmann et al. 2009; Forzza et al. 2012) and is therefore considered a world hotspot of biodiversity (Mittermeier et al. 2004). This mountainous range has a high biodiversity due to the occurrence of rare, endemic and threatened species of animals and plants (Drummond et al. 2005). However, its location in the Southeastern Region of Brazil, where 70 % of the Brazilian population is concentrated (Lino et al. 2007), places its astonishing biodiversity at risk, reinforcing the importance of enhancing knowledge that will support the conservation of this region (Drummond et al. 2009).
Mixed ombrophilous forest (MOF) is one of the most threatened forest ecosystems of the Brazilian Atlantic Forest. It is estimated that only approximately 3 % of the original cover remains, including regenerating areas (Bauermann and Behling 2009). This forest formation occupies the highest areas of the Serra da Mantiqueira. One of the few surviving remnants lies within the Parque Estadual da Serra do Papagaio (PESP) (Ab’saber 2003; Backes 2009). In this protected area, the MOF comprises two gymnosperm species as the most common arboreal plants: Araucaria angustifolia (Bertol.) Kuntze (Araucariaceae) and Podocarpus lambertii Klotzsch ex Endl. (Podocarpaceae). The latter species occurs in two different biotopes [concept that combines the physical environment and the assemblage of conspicuous species (Olenin and Ducrotoy 2006)]: the continuous alluvial forest below the canopy composed of A. angustifolia, and the natural forest patches (locally named as “capões”) composed predominantly of one or several individuals of P. lambertii and/or with the presence of other tree species in the “campo de altitude” (which is a vegetation predominantly composed of open fields with grasses, sometimes with rocky outcrops, also named by Safford (1999) as “Brazilian páramos”).
Vascular epiphytes represent a functional group with high ecological importance that provides resources to a large number of animal and plant species and performs an essential role in maintaining biodiversity (Gentry and Dodson 1987; Benzing 1990). This synusia is highly sensitive to environmental variations such as temperature, humidity, luminosity and the substratum (Benzing 1990), and is continuously threatened due to habitat loss, especially in cloud forests, an environment where epiphytes are highly diverse but has been rapidly degraded in the past three decades (Scatena et al. 2010). It has also suffered from predatory collection due to the ornamental features of families such as Orchidaceae, Bromeliaceae, Gesneriaceae, Araceae and Cactaceae, which contain a great number of epiphytic species (Benzing 1990).
Brazil possesses an astonishing floristic richness, notably of epiphytes, especially due to the forest physiognomies of the Atlantic Domain. Studies regarding epiphytic communities have been conducted more intensively in the past 20 years and have concentrated in the Southern Region, of which several were done in MOF (e.g., Kersten and Silva 2002; Kersten et al. 2009; Waechter 2009; Bernardi and Budke 2010; Geraldino et al. 2010) but are scarce in other regions of the country (Kersten 2010). The present study is the first that aims to analyse the structure of the epiphytic community in the Serra da Mantiqueira, and the first conducted above 1600 m a.s.l. in Brazil.
The main objective of this study was to broaden knowledge concerning the flora of the AF, especially the montane environments and the MOF. Thus, we evaluated the importance of P. lambertii as a phorophyte through analyses of richness, indices of diversity and the vertical structure of the epiphytic community. Furthermore, since epiphytes are sensitive to habitat variations, we also tested the hypothesis of differences existing in this community between the two biotopes, the continuous alluvial MOF and natural patches of forest vegetation in the “campo de altitude”.
Materials and methods
Study area
The Parque Estadual da Serra do Papagaio (PESP) is located in the southern region of Minas Gerais, in the Serra da Mantiqueira, neighbouring the Parque Nacional do Itatiaia (Fig. 1). It has an area of 22,917 ha, and lies between the municipalities of Aiuruoca, Alagoa, Baependi, Itamonte and Pouso Alto (22.1420S, 44.7328W). An important remnant of the AF is preserved within this protected area, which harbours a mosaic of cloud forests (high montane dense ombrophilous forest (DOF) and high montane MOF), and “campo de altitude”. In the studied area, the MOF occurs predominantly on humic and histic cambisols at altitudes between 1600 and 1700 m a.s.l., forming a transition area with the DOF at about 1900–2000 m a.s.l. in the southeast of the PESP, in the municipality of Baependi. The climate is Cwb (Köppen’s classification), a temperate highland tropical climate with dry winters (Silva et al. 2008).
The main fragments of the MOF are alluvial (biotope 1) and are found along the Santo Agostinho brook, forming a continuous vegetation that is composed of three strata: a canopy of A. angustifolia (about 30 m high), a second stratum composed predominantly of P. lambertii (between 10 and 15 m high) and a third stratum (up to about 8–10 m high) composed of shrubs and treelets of the families Lauraceae, Myrtaceae, Primulaceae and Winteraceae, among others. Podocarpus lambertii also occurs in patches interspersed within the “campo de altitude” (biotope 2), adjacent to the alluvial forest. The patches comprise one to four individuals of P. lambertii (between 3 and 5 m high), sometimes surrounded by shrubs and treelets of several families (Asteraceae, Lauraceae, Melastomataceae, Myrtaceae, Primulaceae, etc.), but naturally without A. angustifolia (Figs. 1–13). Hereafter, these biotopes will be referred to as alluvial forest and patches.
Environments of the studied area in the Parque Estadual da Serra do Papagaio, Minas Gerais, Brazil. 2. General view of the vegetation mosaic formed by the “campo de altitude” and mixed ombrophilous forest; 3, 4. General view of the alluvial forest canopy, with the presence of Araucaria angustifolia; 5–7. Views of the alluvial forest. 8–10. Interior of the alluvial forest; 11, 12. Patches of Podocarpus lambertii
Sampling
The sampling of phorophytes was conducted following a wide monthly survey of the vascular epiphytic flora of the MOF and the transition area with the DOF in the protected area, between April 2012 and September 2013, concerning its composition and, subsequently, a more precise identification of the observed specimens, even if they were sterile. The specimens were deposited in the CESJ herbarium of the Universidade Federal de Juiz de Fora (acronym according to Thiers 2014).
We sampled 60 individuals of P. lambertii equally divided between the two biotopes, and initially sampled 30 patches in an area of about 300 ha, between 1650 and 1750 m a.s.l. In each patch, we analysed only one individual of P. lambertii [when the patch contained two or more individuals, we chose the specimen with the largest diameter at breast height (1.3 m above the ground)]. In the alluvial forest, within the same area, we traversed existing tracks or opened new ones along the brook bank and sampled the largest phorophyte close to the trail. Starting from this individual, we then sampled the nearest and largest phorophyte at a distance of at least 50 m, covering the forest physiognomy in this systematic way until 30 individuals had been sampled (Fig. 1). Each phorophyte was divided into five strata: bole, first, second and third ramifications and external crown (the remainder of the crown). The epiphytes in each stratum were recorded and the ecological categories were classified according to Benzing (1990). The epiphytes were observed by the use of binoculars and through climbing the trees without ropes.
The evolutionary lineages of vascular plants were assigned according to Christenhusz et al. (2011) (lycophytes and monilophytes) and APG III (2009) (magnoliids, monocotyledons and eudicotyledons) (Table 2).
Data analyses
The richness of the biotopes was compared through rarefaction curves with 95 % confidence intervals according to Colwell et al. (2012). The obtained data were used to calculate the relative and absolute frequencies as well as the diversity indices of Shannon (H’) and Pielou (J) for each strata, each biotope and in total. To calculate the Shannon’s diversity index, we used the modification proposed by Waechter (1998), substituting abundance for frequency, due to the difficulty of identifying individuals in the epiphytic synusia. The J index is based on H’ and estimates the uniformity of the community, estimating the obtained diversity (H’) in relation to the maximum hypothetical diversity (Magurran 2004). The Hutcheson t test was used to compare the diversity between the biotopes as well as the diversity between the strata (α > 0.05) (Magurran 1988). The parameters of richness, H’ and J in other studies about vascular epiphytes conducted in the Southern and Southeastern regions of Brazil are listed in Table 3 in order to compare them with the values obtained in the studied community.
We also calculated the indices of taxonomic distinctness (Δ), taxonomic diversity (Δ*), average taxonomic distinctness (Δ+) and variation in taxonomic distinctness (Λ+), according to Clarke and Warwick (1998, 2001) for both biotopes (Table 4). In addition, the five most common species in this study were compared with those obtained in other studies performed in the MOF (Table 5) in order to evaluate if the most important species are similar in such physiognomy occurring in geographically distant sites.
We conducted a Kruskal–Wallis test to evaluate difference between the species richness in each stratum (Magurran 2004). A frequent close co-occurrence of Aechmea distichantha (Bromeliaceae) and Pecluma pectinatiformis (Polypodiaceae) was observed in the field and the potential correlation was tested through the Pearson coefficient.
The analyses were conducted using the software Past v. 3.01 (Hammer et al. 2001), EstimateS (Colwell 2013) and R (R Core Team 2014).
Results
Flora of vascular epiphytes
We found 92 species of vascular epiphytes on P. lambertii, distributed among 46 genera and 19 families. However, only 89 species were used in the analyses, since Hadrolaelia coccinea and H. mantiqueirae are considered as one morphospecies, as well as the taxon identified as Octomeria sp. (composed of three unidentified species with cylindrical leaves), due to the difficulty in identifying it when sterile (Table 1).
The evolutionary lineages of the vascular plants were represented by monocotyledons (45 spp., 50.5 %), followed by monilophytes (25 spp., 28.1 %), eudicotyledons (13 spp., 14.6 %), magnoliids (6 spp., 6.7 %) and lycophytes (3 spp., 3.4 %) (Table 2). The richest families were Orchidaceae (34 spp., 38 %), Polypodiaceae (17 spp., 19 %), Bromeliaceae (10 spp., 11 %) and Piperaceae (6 spp., 7 %), containing 75 % of the surveyed species (Table 1).
Structure of the community and biotopes
The alluvial forest demonstrated a higher richness than the patches (80 vs. 60 species) (Fig. 14). The two biotopes presented 29 and nine exclusive species, respectively. The diversity index (H’) for the whole area was 3.86 and the uniformity index (J) was 0.86 (Table 3).
There was no difference between the diversity of the biotopes (t = −0.17; df = 139.91; P = 0.86), and the higher value of J compared to the total value which showed an enhancement of uniformity within each environment separately. Similarly, the biotopes showed no difference in their indices of taxonomic diversity (P > 0.05), although the forest showed slightly higher values. The variation in taxonomic distinction was higher in the patches, representing unevenness in the taxonomic composition of this biotope due to lower taxonomic spread (Table 4).
Pleopeltis macrocarpa, Aechmea distichantha, Serpocaulon catharinae, Pecluma pectinatiformis and Rhipsalis floccosa were the most frequent species in the whole community (above 80 %). The first three species were slightly more frequent in patches, and the latter two species were more frequent in the alluvial forest (Table 1). In general, these species were not found among the most frequent epiphytic vascular flora in the MOF as presented in Table 5.
The maximum number of occurrences of a single phorophyte was 64, and the richness ranged from 10 to 28 species. The phorophytes of the patches had greater amplitude between the minimum and maximum number of observed species, with a median of 18 species per phorophyte, whereas the richness of the phorophytes in the alluvial forest was less dispersed, with a lower median (17 spp.) (Fig. 15).
The richness between the strata ranged from 52 (stratum A) to 63 species (stratum B) and the Kruskal–Wallis test showed no difference between the strata (H = 2.357, H c = 2.483, P = 0.6476). Similarly, the Hutchenson t test showed no significant difference between the diversity indices of the strata (P > 0.05 in all cases) (Fig. 16). However, some species were more frequent in only one stratum, such as Hymenophyllum polyanthos, with 62 % occurrence in stratum A, and Octomeria species, which in general were found more often in stratum E (O. crassifolia 51 %, O. geraensis 95 %, O. ochroleuca 94 %, O. wawrae 100 % and Octomeria sp. 41 %). Accidental holoepiphytes occurred with a frequency of 68 % in strata A and B and 45 % in stratum A. The Pearson coefficient showed a strong positive correlation with the occurrence of Aechmea distichantha and Pecluma pectinatiformis in each stratum (r = 0.98, P = 0.004).
The ecological categories of the species associated with the phorophytes were represented by 73 characteristic holoepiphytes (CHL), nine accidental holoepiphytes (AHL), six facultative holoepiphytes (FHL) and only one hemiepiphyte (HEM) (Table 1). Notably, all recorded AHL were found in the alluvial forest and were dominated by eudicotyledons: Asteraceae, Melastomataceae, Myrtaceae, Poaceae, Primulaceae and Ranunculaceae, in addition to specimens of two unidentified families. The only exception was Hapalorchis micranthus, an Orchidaceae. These AHL species tended occur in strata A and B (except for Myrsine umbellata and unidentified Eudicot 2).
Discussion
The richness and diversity found in this study are remarkable, even when compared to studies performed with more than one species of phorophyte. The values obtained are higher than those recorded in the MOF of the Southern Region, where equal or greater numbers of sampled phorophytes were analysed (Kersten and Silva 2002; Kersten et al. 2009; Waechter 2009; Bernardi and Budke 2010; Geraldino et al. 2010), or studies that evaluated only one species of phorophyte in different vegetation formations (Werneck and Espírito-Santo 2002; Gonçalves and Waechter 2003; Silva et al. 2008; Ferreira 2011). In general, only some areas of the DOF (Schütz-Gatti 2000; Petean 2009; Kersten and Waechter 2009) and the “Restinga” (Waechter 1992; Kersten and Silva 2006) showed higher values of richness and/or diversity. These data to some extent contradict the compilation of Kersten (2010), which asserts that the MOF is much more species-poor than other physiognomies, since Table 3 shows several areas poorer than we found in the present study. A reason for the observed richness could be the fact that the MOF in the PESP is probably much more conserved than those in the Southern Region, although the small extension of the surveyed area must also be highlighted.
However, the studied MOF areas were at altitudes below 1200 m. a.s.l.; thus, to date, the PESP is the studied area with the highest elevation (higher than 1600 m a.s.l.). This is one factor that is responsible for the higher richness and diversity values than those in the MOF, since the mountainous ranges act as refugia for the regional flora, as well as hosting endemic species (Chaverri-Polini 1998; Safford 1999; Martinelli 2007), due to their discontinuous geographic distribution, which is responsible for the isolation of species and restriction of gene flow between populations (Barbará et al. 2007). Also, the cloud forests, like the studied area, have continuous or intermittent cloud cover, which is responsible for the horizontal precipitation, providing high humidity that is important for the occurrence of epiphytes (Hamilton et al. 1995; Nieder et al. 2001).
In addition, these observations suggest the contribution of altitude to the diversity of vascular epiphytes in montane cloud forests of the Neotropical Region, which frequently attain peaks of richness at similar altitudes (Hietz and Hietz-Seifert 1995; Krömer et al. 2005; Cardelús et al. 2006). Regarding the AF, Tonhasca Jr. (2005) highlights the occurrence of high richness and abundance of vascular and avascular epiphytes in cloud forests above 700 and 1500 m a.s.l. in the Southern and Southeastern regions, respectively. On the other hand, Blum et al. (2011) found the highest richness at altitudes between 400 and 500 m a.s.l. in the DOF of Serra da Prata (Paraná), and the lowest richness at 1000–1100 m.s.m., which were the highest altitudes of their study. However, it is possible that the high latitude and consequently low temperature of Serra da Prata, including the occurrence of frosts in winter, have restrained the development of vascular epiphytes, reducing their richness (Nieder et al. 2001). Another factor, suggested by Blum et al. (2011), is that the reduced size of phorophytes at highest elevations results in a reduced availability of substratum for epiphytes. Since this is the only study to deal with the elevation gradient of vascular epiphytes in the AF, more data are necessary to draw other conclusions about the existence in this phytogeographic domain of the same pattern observed in the Neotropical Region. Because large knowledge gaps exist concerning the flora of the extensive Serra da Mantiqueira mountain range that contains the study area (Stehmann and Sobral 2009), this diversity could potentially increase as more locations are studied.
The high richness of monocotyledons was expected, although the proportion was lower than that found in the AF by Kersten (2010) (50.5 vs. 65 %) due to the increased richness of other lineages, especially monilophytes (with 28.1 % of the recorded species in the PESP, compared with 15.4 % in the AF). Regarding the composition of the families, the observed result agrees to some extent with the pattern found for the Neotropical region (Gentry and Dodson 1987) and the AF (Kersten 2010), with the Orchidaceae, Polypodiaceae and Bromeliaceae being among the richest families. Two major differences were observed: the first concerns the ranking of the families, since the Bromeliaceae contains more epiphytic species than the Polypodiaceae, both in the AF and in the Neotropical region (Gentry and Dodson 1987; Kersten 2010), and the second concerns the absence of the Araceae among the richest families in the PESP, since no species were recorded in the present study. The reduced richness of Bromeliaceae and the absence of Araceae contributed to the reduction in monocotyledons among the lineages.
The first difference consists of a substitution in the second and third positions among the Bromeliaceae and Polypodiaceae between areas, as highlighted by Alves and Menini Neto (2014), whereas Piperaceae and Araceae commonly alternate as the fourth and fifth richest families. The second difference, the absence of the Araceae, is due to its low representation in the MOF, as highlighted by Kersten (2010), which positions it as the tenth-largest family in this physiognomy of the AF. The reduced richness of the Bromeliaceae in both biotopes and the absence of the Araceae also contributed to the smaller number of monocotyledon species.
The absence of the Araceae also implied a lower representation of the ecological category of HEM, since only Dyssochroma viridiflorum (Solanaceae) was recorded. The AHL were more prominent and, as demonstrated by Kersten (2006), this result is consistent with those from more detailed surveys. Species without epiphytic adaptations establish in environments with a high humidity with relative ease (Benzing 1990), which might explain the representation of this category in the present study. Furthermore, all the AHL species were found in the alluvial forest, which is moister than the patches, because in addition to the occurrence of fog, it is located nearer to the brook.
The high value of J indicated a great uniformity in the analysed community, and 86 % of the hypothetical maximum diversity (H’) was obtained. Additionally, the 92 recorded species represented about 67 % of the diversity found in the floristic survey (138 spp.) (Furtado and Menini Neto, unpublished data) that was conducted on all phorophyte species. To date, no single phorophyte species that was studied in the Atlantic domain (Gonçalves and Waechter 2003; Ferreira 2011) has revealed such diversity and richness as P. lambertii, which highlights the ecological importance of this species.
The number of vascular epiphytes that a single specimen of P. lambertii can host is striking. The maximum value (28 spp.) represents 30 % of all species recorded in the present study and 20 % of the total found in the floristic survey (Furtado and Menini Neto, unpublished data). The extreme values of minimum and maximum richness found on a single phorophyte specimen were higher than those recorded by Kersten and Silva (2001) and Kersten (2006) in Ilha do Mel, Paraná, in DOF, “Restinga” and mangrove vegetation, by Kersten et al. (2009) in the Rio Iguaçu basin, Paraná in the MOF, and by Ferreira (2011), in the Serra da Brígida, Minas Gerais in a seasonal semideciduous forest.
Despite the absence of significant differences among the calculated diversity indices in both biotopes, variations in the microenvironment (for example, the higher humidity in the alluvial forest) probably influence the composition of epiphytic flora, since 38 out of 89 species occur exclusively in one of the biotopes, even on a single phorophyte species. In addition, several species that occur in both biotopes were recorded asymmetrically (with a much greater frequency in one biotope), indicating some sensitivity to microsite variations between the environments. Therefore, the biotopes are structurally similar, although they contain differences at the species level, but no major differences at other taxonomic levels, except in families, since the alluvial forest presented 18 families versus 10 families occurring in patches. This difference is to a large extent responsible for the higher value of variation in taxonomic distinction due to the concentration of species in two families, Orchidaceae and Polypodiaceae.
The most frequently occurring species often showed a higher frequency in one biotope, even when they were present in both biotopes. One example is Oncidium gardneri, an Orchidaceae species with well-developed pseudobulbs that can store large quantities of water and was present in the patches with a frequency of 89.5 %. These data reinforce the influence of variations in the microclimate on the richness (Cox and Moore 2011) and, consequently, on species conservation, by applying knowledge that can help to conserve species that show some habitat preferences. Another example is the morphotype Hadrolaelia coccinea/H. mantiqueirae, which suffers collection pressure due to its ornamental flowers. H. coccinea is threatened at the state level (Drummond et al. 2008) but showed a frequency of 75 % in the patches, which are isolated and more exposed to anthropic disturbances such as cattle and fire.
In general, the reason for differences among strata is the humidity and light gradient between the ground and the canopy (Benzing 1990). The analysis showed no difference in richness or diversity among the strata of the sampled phorophytes. Two reasons for this might be: (1) light is not limiting in either biotope, since the density of the canopy in the alluvial forest is reduced and only slightly hinders the entrance of light; however, in the patches, which possess no canopy, the only barrier to light is the crown of the phorophyte; (2) there is no marked humidity gradient between the ground and canopy due to the presence of watercourses within the alluvial forest as well as the fog that results from the high altitude, which also provides humidity from the horizontal precipitation to the patches in the “campo de altitude”. The epiphytic community itself contributes to the maintenance of such humidity, according to Benzing (1990), especially tank bromeliads such as Aechmea distichantha and Vriesea sceptrum, two of the most common species in the present study.
With the exception of Serpocaulon catharinae (Polypodiaceae), the most frequent species differed from those found in other areas with the same physiognomy. This was also observed in the composition of genera, highlighting the uniqueness of the studied area regarding the epiphytic community. The presence of Microgramma squamulosa (Polypodiaceae) is notable, because it is among the three most frequent species in all other studies performed in the MOF and is also common in seasonal forests (Gonçalves and Waechter 2002; Giongo and Waechter 2004; Ferreira 2011). In the studied area, M. squamulosa occupies the thirteenth position in terms of frequency, although it occurs in all strata, and represents only 25 % of occurrences within the alluvial forest. It is widely distributed in South America (Almeida 2014) and is often present in several communities of vascular epiphytes, regardless of their degree of conservation. The high richness and abundance of other species can influence and limit the occurrence of M. squamulosa, especially in the forest environment, and maintain this species at a lower frequency than that usually observed. Furthermore, Microgramma shows a lower richness and abundance at higher altitudes and is more frequent at intermediate altitude sites with high moisture content (T.E. Almeida, personal communication). Thus, although the humidity is high in the PESP, the high altitude limits the frequency of M. squamulosa as well as the size of the population.
Pecluma pectinatiformis (Polypodiaceae) and Aechmea distichantha (Bromeliaceae) were among the most frequent species and showed a close relationship, since almost all observed specimens of either species were found close to one another. The correlation for the occurrence of both species suggests that this relationship appears to be one of ecological facilitation. It is possible that A. distichantha may be responsible, playing a role commonly attributed to species of tank bromeliad, the so-called nurse plants (Benzing 1990), through retention of humidity or nutrients in the site of their fixation on the bark of phorophyte. A more specific study can elucidate this possibility.
References
Ab’saber AN (2003) Os domínios de natureza no Brasil: potencialidades paisagísticas. Ateliê Editorial, São Paulo
Almeida TE (2014) Estudos sistemáticos e filogenéticos no gênero Microgramma C. Tese de doutorado. Universidade Federal de Minas Gerais, Presl (Polypodiaceae - Polypodiopsida)
Alves FE, Menini Neto L (2014) Vascular epiphytes in a forest fragment of Serra da Mantiqueira and floristic relationships with Atlantic high altitude areas in Minas Gerais. Braz J Bot 37:187–196
APG, III – The Angiosperm Phylogeny Group (2009) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc 161:105–121
Backes A (2009) Distribuição geográfica atual da Floresta com Araucária: condicionamento climático. In: Fonseca CR, Souza AF, Leal-Zanchet AM, Dutra T, Backes A, Ganado G (eds) Floresta com Araucária: Ecologia, conservação e desenvolvimento sustentável. Holos Editora, Ribeirão Preto, pp 137–148
Barbará T, Martinelli G, Fay MF, Mayo SJ, Lexer C (2007) Population differentiation and species cohesion in two closely related plants adapted to neotropical high altitude ‘inselbergs’, Alcantarea imperialis and Alcantarea geniculata (Bromeliaceae). Mol Ecol 16:1981–1992
Bataghin FA, Barros F, Pires JSR (2010) Distribuição da comunidade de epífitas vasculares em sítios sob diferentes graus de perturbação na Floresta Nacional de Ipanema, São Paulo, Brasil. Rev Bras Bot 33:531–542
Bauermann SG, Behling H (2009) Dinâmica paleovegetacional da Floresta com Araucária a partir do final do Pleistoceno: o que mostra a palinologia. In: Fonseca CR, Souza AF, Leal-Zanchet AM, Dutra TL, Backes A, Ganado G (eds) Floresta com Araucária: ecologia, conservação e desenvolvimento sustentável. Holos Editora, Ribeirão Preto, pp 35–38
Becker DFP, Cunha S, Marchioretto MS, Schmitt JL (2013) Riqueza, estrutura comunitária e distribuição vertical de epífitos vasculares do Parque Natural Municipal Tupancy, Arroio do Sal, RS, Brasil. Pesq Bot 64:127–139
Benzing DH (1990) Vascular epiphytes. Cambridge University Press, New York
Bernardi S, Budke JC (2010) Estrutura da sinúsia epifítica e efeito de borda em uma área de transição entre Floresta Estacional Semidecídua e Floresta Ombrófila Mista. Floresta 40:81–92
Blum CT, Roderjan CV, Galvão F (2011) Composição florística e distribuição altitudinal de epífitas vasculares da Floresta Ombrófila Densa na Serra da Prata, Morretes, Paraná, Brasil. Biota Neotrop 11:141–159
Cardelús CL, Colwell RK, Watkins JE Jr (2006) Vascular epiphyte distribution patterns: explaining the mid-elevation richness peak. J Ecol 94:144–156
Chaverri-Polini A (1998) Mountains, biodiversity and conservation. Unasylva 195:22–33
Christenhusz MJM, Zhang X-C, Schneider H (2011) A linear sequence of extant families and genera of lycophytes and ferns. Phytotaxa 19:7–54
Clarke KR, Warwick RM (1998) A taxonomic distinctness index and its statistical properties. J App Ecol 35:523–531
Clarke KR, Warwick RM (2001) A further biodiversity index applicable to species lists: variation in taxonomic distinctness. Mar Ecol Prog Ser 216:265–278
Colwell RK (2013) EstimateS: Statistical estimation of species richness and shared species from samples. Version 9 and earlier User’s Guide and application http://www.viceroyeebuconnedu/estimates/index html. Accessed 1 July 2014
Colwell RK, Chao A, Gotelli NJ, Lin S-Y, Mao CX, Chazdon RL, Longino JT (2012) Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J Pl Ecol 5:3–21
Cox CB, Moore PD (2011) Biogeografia: uma abordagem ecológica e evolucionária, 7th edn. Livros Técnicos e Científicos Editora, Rio de Janeiro
Dettke GA, Orfrini AC, Milaneze-Gutierre MA (2008) Composição florística e distribuição de epífitas vasculares em um remanescente alterado de Floresta Estacional Semidecidual no Paraná, Brasil. Rodriguésia 59:859–872
Drummond GM, Martins CS, Machado ABM, Sebaio FA, Antonini Y (orgs) (2005) Biodiversidade em Minas Gerais, um atlas para sua conservação. 2ª ed. Fundação Biodiversitas, Belo Horizonte
Drummond GM, Machado ABM, Martins CS, Mendonça MP, Stehmann JR (2008) Listas vermelhas das espécies da fauna e flora ameaçadas de extinção em Minas Gerais. Fundação Biodiversitas, Belo Horizonte
Drummond GM, Martins CS, Greco MB, Vieira F (eds) (2009) Biota Minas: diagnóstico do conhecimento sobre a biodiversidade do estado de Minas Gerais – subsídio ao Programa Biota Minas. Fundação Biodiversitas, Belo Horizonte
Ferreira MTM (2011) Composição florística e distribuição vertical de epífitas vasculares sobre indivíduos de Guapira opposita (Vell) Reitz (Nyctaginaceae) em um fragmento florestal na Serra da Brígida, Ouro Preto, MG. Dissertação de Mestrado, Universidade Federal de Ouro Preto
Forzza RC, Baumgratz JFA, Bicudo CEM, Canhos DAL, Carvalho AA Jr, Coelho MAN, Costa AF, Costa DP, Hopkins MG, Leitman PM, Lohmann LG, Lughadha EM, Maia LC, Martinelli G, Menezes M, Morim MP, Peixoto AL, Pirani JR, Prado J, Queiroz LP, Souza S, Souza VC, Stehmann JR, Sylvestre LS, Walter BMT, Zappi DC (2012) New Brazilian floristic list highlights conservation challenges. Bioscience 62:39–45
Gentry AH, Dodson CH (1987) Diversity and biogeography of neotropical vascular epiphytes. Ann Miss Bot Gard 74:205–223
Geraldino HCL, Caxambu MG, Souza DC (2010) Composição florística e estrutura da comunidade de epífitas vasculares em uma área de ecótono em Campo Mourão, PR, Brasil. Acta Bot Bras 24:469–482
Giongo C, Waechter JL (2004) Composição florística e estrutura comunitária de epífitos vasculares em uma floresta de galeria na Depressão Central do Rio Grande do Sul. Rev Bras Bot 27:563–572
Gonçalves CN, Waechter JL (2002) Epífitos vasculares sobre espécimes de Ficus organensis isolados no norte da planície costeira do Rio Grande do Sul: Padrões de abundância e distribuição. Acta Bot Bras 16:429–441
Gonçalves CN, Waechter JL (2003) Aspectos florísticos e ecológicos de epífitos vasculares sobre figueiras isoladas no norte da planície costeira do Rio Grande do Sul. Acta Bot Bras 17:89–100
Hamilton LS, Juvik JO, Scatena FN (eds) (1995) Tropical montane cloud forests. Springer Verlag, New York
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological Statistics software package for education and data analysis. Pal Elect 4:1–9
Hietz P, Hietz-Seifert U (1995) Composition and ecology of vascular epiphyte communities along an altitudinal gradient in Central Veracruz. Mexico J Veg Sci 6:487–498
Kersten RA (2006) Epifitismo vascular na Bacia do Alto Iguaçu, Paraná. Tese de Doutorado, Universidade Federal do Paraná
Kersten RA (2010) Epífitas vasculares – histórico, participação taxonômica e aspectos relevantes com ênfase na Mata Atlântica. Hoehnea 37:9–38
Kersten RA, Kuniyoshi YS (2009) Conservação das florestas na Bacia do Alto Iguaçu, Paraná - Avaliação da comunidade de epífitas vasculares em diferentes estágios serais. Rev Floresta 3:51–66
Kersten RA, Silva SM (2001) Composição florística e distribuição espacial de epífitas vasculares em floresta da planície litorânea da Ilha do Mel, Paraná, Brasil. Rev Bras Bot 24:213–226
Kersten RA, Silva SM (2002) Florística e estrutura do componente epifítico vascular em Floresta Ombrófila Mista Aluvial do rio Barigüi, Paraná, Brasil. Rev Bras Bot 25:259–267
Kersten RA, Silva SM (2006) The floristic compositions of vascular epiphytes of a seasonally inundated forest on the coastal plain of Ilha do Mel Island, Brazil. Rev Biol Trop 54:935–942
Kersten RA, Waechter J (2009) Florística e estrutura de epífitas vasculares na transição entre as florestas ombrófila densa e mista da vertente oeste da Serra do Mar paranaense, Brasil. In: Felfili JM, Eisenlohr PV, Melo MMRF, Andrade LA, Meira Neto JAA (eds) Fitossociologia no Brasil – Métodos e estudos de casos. Editora UFV, Viçosa, pp 479–503
Kersten RA, Borgo M, Silva SM (2009) Diversity and distribution of vascular epiphytes in an insular Brazilian coastal forest. Int J Trop Biol 57:749–759
Krömer T, Kessler M, Gradstein R, Acebey A (2005) Diversity patterns of vascular epiphytes along an elevational gradient in the Andes. J Biogeogr 32:1799–1809
Lino CF, Albuquerque JL, Dias H (orgs) (2007) Cadernos da Reserva da Biosfera da Mata Atlântica nº 32 – Mosaicos de unidade de conservação no corredor da Serra do Mar. Conselho Nacional da Reserva da Biosfera da Mata Atlântica, São Paulo
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, Princeton
Magurran AE (2004) Measuring biological diversity. Blackwell Science, Oxford
Martinelli G (2007) Mountain biodiversity in Brazil. Rev Bras Bot 30:587–597
Mittermeier RA, Gil PR, Hoffmann M, Pilgrim J, Brooks J, Mittermeier CG, Lamourux J, Fonseca GAB (2004) Hotspots revisited: earth’s biologically richest and most endangered terrestrial ecoregions. Ceme, Washington
Nieder J, Prosperí J, Michaloud G (2001) Epiphytes and their contribution to canopy diversity. Pl Ecol 153:51–63
Olenin S, Ducrotoy JP (2006) The concept of biotope in marine ecology and coastal management. Mar Poll Bull 53:20–29
Oliveira LC, Padilha PT, Dalmolim EB, Azeredo TEV, Citadini-Zanette V (2013) Componente epifítico vascular de um fragmento florestal urbano, município de Criciúma, Santa Catarina, Brasil. Biotemas (UFSC) 26:33–44
Padilha PT (2014) Comunidade epifítica vascular do Parque Estadual da Serra Furada, Sul de Santa Catarina. Dissertação de Mestrado. Universidade do Extremo Sul Catarinense
Petean MP (2009) O componente epifítico vascular em Floresta Ombrófila Densa no litoral paranaense: análise florística, estrutural e de biomassa. Tese de Doutorado. Universidade Federal do Paraná
R Core Team (2014) R: a language and environment for statistical computing R foundation for statistical computing Vienna. http://www.R-project.org. Accessed 15 May 2014
Rizzini CT (1997) Tratado de fitogeografia do Brasil, 2ª ed. Âmbito Cultural Edições Ltda, Rio de Janeiro
Safford HD (1999) Brazilian Páramos I - An introduction to the physical environment and vegetation of the campos de altitude. J Biog 26:693–712
Scatena FN, Bruijnzeel LA, Bubb P, Das S (2010) Chapter 1 - Setting the stage. In: Bruijnzeel LA, Scatena FN, Hamilton LS (eds) Tropical Montane cloud forests – science for conservation and management. International Hydrology Series. Cambridge University Press, Cambridge, Cambridge
Schütz-Gatti AL (2000) O componente epifítico vascular na Reserva Natural de Salto Morato, Guaraqueçaba - PR. Dissertação de mestrado, Universidade Federal do Paraná
Silva LVC, Viana PL, Mota NFO (2008) Plano de Manejo do Parque Estadual da Serra do Papagaio, Minas Gerais, Brasil. Instituto Estadual de Florestas, Belo Horizonte
Silva MAP, Barros LM, Alencar AL, Braga MR, Ferreira JKA, Santos ACB (2009) Epífitos vasculares sobre espécimes de Schinopsis brasiliensis Engl. (Baraúna) em uma área de caatinga-Alagoinha-PE. Cad Cult Ciênc 1:22–25
Stehmann JR, Sobral M (2009) Fanerógamas. In: Drummond GM, Martins CS, Greco MB, Vieira F (eds) Biota Minas – Diagnóstico do conhecimento sobre a biodiversidade no estado de Minas Gerais, subsídio ao programa Biota Minas. Fundação Biodiversitas, Belo Horizonte, pp 355–374
Stehmann JR, Forzza RC, Salino A, Sobral M, Costa DP, Kamino LHY (2009) Diversidade taxonômica na Floresta Atlântica. In: Stehmann JR, Forzza RC, Salino A, Sobral M, Costa DP, Kamino LHY (eds) Plantas da Floresta Atlântica. Jardim Botânico do Rio de Janeiro, Rio de Janeiro, pp 3–12
Thiers B (2014) Index herbariorum: a global directory of public herbaria and associated staff. New York botanical garden’s virtual herbarium. http://www.sweetgumnybgorg/ih. Accessed 1 July 2014
Tomazini V (2007) Estrutura de epífitas vasculares e de forófitos em formação florestal ripária do Parque Estadual do Rio Ivinhema, Estado de Mato Grosso do Sul, Brasil. Tese de Doutorado, Universidade Estadual de Maringá
Tonhasca A Jr (2005) Ecologia e história natural da Mata Atlântica. Editora Interciência, Rio de Janeiro
Waechter JL (1992) O epifitismo vascular na planície costeira do Rio Grande do Sul. Universidade Federal de São Carlos, Tese de Doutorado
Waechter JL (1998) Epifitismo vascular em uma floresta de restinga do Brasil Subtropical. Rev Ciênc Nat 20:43–66
Waechter JL (2009) Capítulo 11: epífitos vasculares da Floresta com Araucária do sul do Brasil. In: Fonseca CR, Souza AF, Leal-Zanchet AM, Dutra TL, Backes A, Ganade G (eds) Floresta com araucária: ecologia, conservação e desenvolvimento sustentável. Holos Editora, Ribeirão Preto, pp 127–136
Werneck M, Espírito-Santo MM (2002) Species diversity and abundance of vascular epiphytes on Vellozia piresiana in Brazil. Biotropica 34:51–57
Acknowledgments
We wish to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the scientific initiation scholarship provided to the first author, to the Programa de Pós-Graduação em Ecologia of the Universidade Federal de Juiz de Fora (PGECOL-UFJF) for logistic support, to the Instituto Estadual de Florestas de Minas Gerais (IEF-MG) for granting a licence and for logistical support, to Prof. Dr. Fátima Regina Gonçalves Salimena for general support and to the specialists that helped with plant identification (Alexandre Salino, Daniele Monteiro, Diego Gonzaga, Filipe Soares Luciana Carvalho, Rafaela Forzza and Vinicius Dittrich).
Author information
Authors and Affiliations
Corresponding author
Additional information
Part of the work course conclusion of the first author, at the Course of Biological Sciences of Universidade Federal de Juiz de Fora.
Rights and permissions
About this article
Cite this article
Furtado, S.G., Menini Neto, L. Diversity of vascular epiphytes in two high altitude biotopes of the Brazilian Atlantic Forest. Braz. J. Bot 38, 295–310 (2015). https://doi.org/10.1007/s40415-015-0138-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-015-0138-x




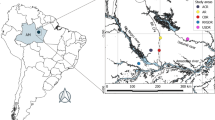

 Sampled individuals of Alluvial Forest;
Sampled individuals of Alluvial Forest;  Sampled individuals of Patches
Sampled individuals of Patches



