Abstract
Purpose
The purpose of this study was to evaluate the impact of surface sealants on the stain resistance of restorative materials exposed to iron syrups.
Methods
Sixty specimens were prepared from each of three restorative materials—compomer (Dyract XP), a microhybrid composite (Filtek Z250), and a nanohybrid composite (G-aenial Anterior). Specimens were divided into three solution groups (n = 20) and immersed in two iron syrups (Ferrum and Ferro Sanol B) and distilled water. Two subgroups, sealed (BisCover ( +)) and unsealed (BisCover (–)), were established for each group (n = 10). Color changes between baseline and measurements at 4, 8, 24, 48, and 72 h were calculated with CIEDE2000 (ΔE00) metrics. Data were analyzed with 4-factor mixed-design ANOVA.
Results
The first null hypothesis of this study that the use of surface sealant would not mitigate the restorative materials’ susceptibility to staining was rejected: significant differences were found between BisCover ( +) and BisCover (−) groups in ΔE00 values for all restorative materials tested in Ferro Sanol B (p < 0.001) and Ferrum (p = 0.002) solutions. The ΔE00 values in the Ferro Sanol B/BisCover ( +) groups were significantly lower than in Ferrum/BisCover ( +) groups (p = 0.002), the second null hypothesis that different forms of iron syrups would not impact the staining resistance of restorative materials was rejected. ΔE00 values were different for each restorative materials tested, the third null hypothesis that the type of restorative material would not affect staining resistance was rejected.
Conclusions
The application of surface sealant significantly improved the color stability of restorative materials. The content of iron syrups was also an important factor affecting color change. Nanohybrid composites seem to be more resistant to the staining effects of iron syrups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron is a substantial nutrient for the human body which plays a significant role in many metabolic processes, such as electron transport, oxygen transport and DNA synthesis (Bhattacharya et al. 2016). Iron deficiency is a major and global public health problem and is a commonly seen nutritional deficiency in the world (Hoffbrand and Herbert 1999). The main principles of treatment include nutritional improvement, iron supplementation and enhancing awareness of the patient and family (WHO 2016).
Children consume iron supplements usually as drops or syrups. Black discoloration on teeth is one of the basic drawbacks regarding to consume these supplements in addition to its undesired metallic taste (Talebi et al. 2012; Young et al. 2018). Black discoloration could be due to an insoluble ferric compound generated by a mutual effect between iron ions or gingival fluid composition and hydrogen sulfide caused by bacteria (Dayan et al. 1983). Pani et al. (2015) investigated the staining effect of iron in the ferric form, ferrous form and combination of these syrups on primary teeth. They stated that both the iron syrup groups exhibited significantly higher clinically visible staining in comparison to the combination solution group at the end of 72 h. Thus, it is important to consider the long-term results when using these formulations.
In the literature, there are few studies in which the staining effects of pediatric drugs including iron syrup were tested on restorative materials (Tuzuner et al. 2017; Yildirim and Uslu 2020). In view of the results of these studies, the highest color change values were reported in iron syrups which is the acceptability thresholds were exceeded. In our previous study, it was suggested that surface sealants can be used to minimize the color change related to pediatric liquid drugs on restorative materials (Yildirim and Uslu 2020). Surface sealants are used to saturate the material surface, as well as to correct irregularities, increasing stain-resistance, and thus enhancing the esthetic qualities (Brooksbank et al. 2018).
There have been several studies indicating that the use of surface sealants did not change the color stability of composite resins (Aguilar et al. 2012; Catelan et al. 2011; Khalaj et al. 2018; Lee and Powers 2007; Lepri and Palma-Dibb 2014; Zimmerli et al. 2012), whereas some of the studies have reported that the surface sealants caused less discoloration (Catelan et al. 2017; Dede et al. 2016; Miotti et al. 2016; Pedroso et al. 2016; Saygi et al. 2015). No study was found that investigated the impact of surface sealants on the staining resistance of composite resins related to common pediatric iron syrups.
In view of the above, the current research aimed to investigate the effects of sealant agent on color changes by measuring the discoloration of restorative materials after 72 h’ exposure to two different forms of iron syrups.
Three null hypotheses were considered: First, that the use of surface sealant would not mitigate the restorative materials’ susceptibility to staining; second, that exposure to different forms of iron syrups and the duration of exposure would not impact the staining resistance of restorative materials; and third, that the type of restorative material would not affect staining resistance.
Materials and methods
Table 1 presents the characteristics of the materials evaluated in this study. The composite resins were a nanohybrid anterior composite resin G-aenial Anterior (AC) and a microhybrid posterior composite resin Filtek Z250 (PC). The compomer was a Dyract XP. The surface sealant was a low-viscosity, light-cured resin surface sealant BisCover LV (BC).
Specimen preparation
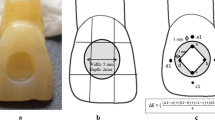
Using a Teflon ring, 60 disk-shaped specimens (8 mm in diameter × 2 mm thick) were obtained from each of the materials. A cellulose acetate matrix strip was placed over the ring, and it was held between two glass slides, with 1 mm thickness to eliminate air entrapment and voids. The manufacturer’s instructions were followed in preparing a total of 180 samples of restorative materials. To ensure standardization, A2 color was used in all materials. Specimens were prepared by the same operator (SY) to eliminate the operator-dependent variables.
The specimens of compomer and composite resins were polymerized by applying a light-emitting diode (LED) polymerization light (Elipar Free light 2, 1200 mW/cm2, 3 M ESPE, Ireland) for 20 s to each surface, with the tip of the light on the glass slide (1 mm from the specimen) for 40 s.
After completing the polymerization process, the specimens were polished using aluminum oxide disks (Sof-Lex, 3 M ESPE, St. Paul, MN, USA) with an electric handpiece, at 15,000 rpm for 10 s on each disk (coarse, medium, fine, and superfine). All specimens were kept in distilled water (Multiplus, ALG, Istanbul, Turkey) at 37 °C for 24 h to complete the polymerization process (Xing et al. 2014).
Specimens in sealed with surface sealant Biscover LV were called BisCover ( +) groups. Biscover (−) group was unsealed for control. No surface sealant was applied in the Biscover (−) groups. Specimens in BisCover + groups, one surface of the specimens was conditioned for 15 s with 37% phosphoric acid gel (Etchant Gel, Prime Dent, Chicago, IL, USA), followed by rinsing with an air/water syringe and the specimens were gently air-dried. One thin layer of BisCover LV was applied over the surface with a micro brush. Then it was allowed 15 s dwelling time for evaporation of solvent after application and light-cured for 30 s according to manufacturer’s instructions.
Solutions preparation
Ferrum solution (pH: 4.4, ferric polymaltose, Fe +3): 4 ml syrup containing of 40 mg Fe +3 was diluated with distilled water to make up 250 ml solution.
Ferro Sanol B solution (pH: 2.9, ferrous sulfate, Fe +2): 10 ml syrup (maximum therapeutic daily dose) containing of 40 mg Fe +2 was diluated with distilled water to make up 250 ml solution (Pani et al. 2015).
Color change measurement
After polishing, the specimens were rinsed and dried with tissue paper, and first color measurements were carried out. They were randomly divided into three solution groups (n = 20). Distilled water (pH 6.47) was used as the control solution. Two subgroups (sealed (BisCover +) and unsealed (BisCover −) groups) were established for each group (n = 10). Based on data from a previous study (Pani et al. 2015), a minimum sample size of 10 specimens per group was calculated using the G*Power software program (version 3.1.9.2; power 0.95, α = 0.05, β = 0.05).
The spectrophotometer was calibrated with its own calibration instrument, and measuring was performed at the center of each specimen. Whole color measurements were performed with the CIEDE2000 color system relative to D65 standard illumination against a standard white background using a clinical spectrophotometer (Vita EasyShade Advance 4.0, Ivoclar Vivadent, Liechtenstein). Each specimen’s measurement was done three times and the average was used. Prior to color measurement, any liquid on the specimens was removed, and they were lightly rinsed with distilled water and dried with tissue paper (ISO 2016).
The color values (L*, c*, h*) of each specimen for each immersion period at 4, 8, 24, 48 and 72 h were measured three times by placing each specimen onto the measuring head of the spectrophotometer using the protocol developed by Lee et al. (2008). After measuring each specimen three times, the mean values were calculated and recorded. Color changes between baseline and measurements made at 4, 8, 24, 48 and 72 h were calculated. The measurements were performed in accordance with the CIEDE2000 (ΔE00) system. ΔE00 was calculated using the following formula (Alberton Da Silva et al. 2018):
Color differences were evaluated ultimately via comparison with 50:50% perceptibility (PT) and 50:50% acceptability (AT) thresholds. A color change value that can be visually perceived by 50% of the observers is defined as 50:50% PT. The color change value that is clinically acceptable for 50% of observers is defined as 50:50% AT. The PT (0.81 units) and AT (1.77 units) values for CIEDE2000 (1:1:1) were reported from a current study (Paravina et al. 2015). 50:50% AT as 1.8 ΔE00, meaning that ΔE00 > 1.8 values are considered clinically unacceptable color changes.
One specimen for each group was imaged on a scanning electron microscope (SEM; Tescan Vega-3 LMU). The acceleration voltage of the cathode was set to 10 kV and the working distance to 10 mm.
Statistical analysis
Descriptive statistics were calculated for each variable and expressed as “mean ± standard error of mean (SEM).” The data were subjected to 4-factor mixed-design ANOVA (analysis of variance) using the general linear model procedure for repeated measurements. The model included "iron syrup" (between-subjects), “restorative material” (between-subjects), “Biscover” (between-subjects) and “time” (within-subjects) as the main effects and all possible interaction terms. In cases where Mauchley's test revealed that the assumption of sphericity violated, the Greenhouse–Geisser procedure was applied to adjust for the degrees of freedom. Simple-effect analysis with Bonferroni adjustment was used to eliminate any significant interaction of effect terms as post hoc analysis. Statistical significance was set to p < 0.05, unless otherwise noted. SPSS version 14.01 software was used for the statistical analyses.
Results
The mean color changes (ΔE00) and standard deviations of all groups are demonstrated in Table 1. The highest change was observed in the Ferrum–compomer/biscover (–) group at 72 h (4.607 ± 0.414), while the minimum was found in the Ferro Sanol B– AC/biscover ( +) (0.263 ± 0.127) combination at 4 h.
Table 2 shows the effects of time, iron syrups, restorative materials and biscover on color change. There are statistically significant differences between the compomer and AC specimens for Ferro Sanol B solution at 24, 48, 72 h. In addition, a statistically significant differences were found between the PC and AC specimens for Ferro Sanol B solution at 48, 72 h. There are statistically significant differences between the compomer and AC specimens for Ferrum solution at 8, 48, 72 h (Table 3).
Figure 1 illustrates the color changes of all specimens exposed to iron syrups and distilled water over time. For the compomer and PC specimens, there were statistically significant differences between the distilled water and Ferrum, Ferro Sanol B solutions at 24,48,72 h. Among AC specimens, a statistically significant differences were found between the distilled water and Ferrum at 72 h. There were statistically significant differences between 4 and 72 h interval in ΔE00 values for all restorative materials tested in all solutions (p < 0.001).
There were statistically significant differences between biscover ( +) and biscover (–) groups in ΔE00 values for all restorative materials tested in Ferro Sanol B (p < 0.001) and Ferrum (p = 0.002) solutions. A statistically significant differences were observed between distilled water and Ferrum, Ferro Sanol B solutions in biscover ( +) groups in all specimens (p < 0.001). Between Ferrum and Ferro Sanol B solutions in biscover ( +) groups in all specimens, a statistically significant differences were found (p = 0.002).
Evaluating the rate of color change for iron syrups, distilled water and all restorative materials in over time, it was determined that, for some groups, the ΔE00 values were lower than 1.8 (50:50% acceptability threshold value for CIEDE2000 (1:1:1) obtained in a recent study carried out by Paravina et al. (2015). All the specimens showed acceptable color change values for distilled water across time intervals except in biscover (–) group at 72 h. At the 4 h-72 h intervals, the AC specimens biscover ( +) groups showed acceptable color change values for Ferro Sanol B solution.
SEM images of the specimens are shown in Figs. 2, 3, 4. SEM images of the specimens revealed that smoother surfaces could be obtained by applying a sealant agent especially in AC specimens than with the unsealed specimens.
Discussion
In the current study, the impact of sealant agent was evaluated on the staining resistance of nanohybrid anterior composites, microhybrid composite resins and compomers, after 72 h’ exposure to iron syrups. According to these results, the first null hypothesis of this study was rejected: significant differences were found between sealed and unsealed groups in ΔE00 values for all restorative materials tested in iron syrups. Because ΔE00 values over time were significantly different for each iron syrup, the second null hypothesis that different forms of iron syrups would not impact the staining resistance of restorative materials was rejected. ΔE00 values were different for each restorative materials tested, the third null hypothesis that the type of restorative material would not affect staining resistance was rejected. Within our knowledge, this is the first study to investigate the effectiveness of surface sealant, which is applied to prevent discoloration related to iron syrups on restorative materials.
The rise in aesthetic expectations has caused the use of various restorative materials, resulting in an expanding diversity of dental materials used in clinical practice. Although there have been improvements in chemical formulations of the composite resins, the discoloration is still a commonly seen reason for the replacement of resin-based restorations (Eltahlah et al. 2018). It was reported that the extrinsic color change in deciduous teeth may negatively affect the social development of children in the pre-school period (Kumar et al. 2012). Other problems may arise as well, such as increased frequency of dental visits due to the need to replace restorations, increased cost of replacing restorations, and worsening behavior management/dental anxiety (Babu et al. 2008; Tupalli et al. 2014). Since dental treatments are costly and time-consuming processes, they should ideally last a long time. The crucial step in overcoming problems associated with exposure to medications may be surface sealants. The results of this study revealed significant differences between BC ( +) and BC (–) groups in ΔE00 values for all restorative materials tested in Ferro Sanol B and Ferrum solutions. ΔE00 was found to be consistently lower in BC ( +) groups. Parallel with our results, some of the studies have shown that the sealant agents positively influenced the color stability of composite resin specimens (Catelan et al. 2017; Dede et al. 2016; Miotti et al. 2016; Pedroso et al. 2016; Saygi et al. 2015). Discoloration of composite resins is affected notably by surface roughness (Lu et al. 2005). Applying sealant agent might improve stain resistance by reducing surface irregularities and ensuring surface smoothness.
The CIELAB color difference system is most commonly used in dentistry, but since 2001, CIEDE2000 (ΔE00), a new color difference system, has been suggested by the International Commission on Illumination (CIE). This system is benefitted from the concepts of hue and chroma, emphasizing the original concepts exhibited by Munsell (CIE 2004). In 2013, this formula was accepted as the standard for detecting color differences. In this formula, the number of parameters used was increased, and calculations became more complicated when compared to the CIELAB formula. Since color perception varies according to backgrounds with different brightness levels, this change in color perception was incorporated into the formula. The previous formula basically measured the distance between two points in the space, whereas the addition of SL to the formula of CIE2000 had the effect of including brightness in the calculation and seems to propose developments in the CIELAB formula, referring to improved clinical relevance (Lindon et al. 2000). Therefore, in the present study, ΔE00 was used to evaluate the color stability of restorative materials.
The detection of color change is based mainly on visibly perceptible changes in color values of an object and assessing the amount of color change that affects the aesthetic appearance (Khashayar et al. 2014). Perceptibility threshold (PT) and acceptability threshold (AT) define the extent of differences and serve as a control to assess the success of dental materials and to interpret visual and instrumental findings, as reported by Paravina et al. (2015). A color change value that can be visually perceived by 50% of the observers is defined as 50:50% PT. The color change value that is clinically acceptable for 50% of observers is defined as 50:50% AT (Khashayar et al. 2014; Paravina et al. 2015). As a result, ΔE which is less than or equal to AT is an agreeable match in dentistry. CIEDE2000 reported 50:50% AT as 1.8 ΔE00, meaning that ΔE00 > 1.8 values are considered clinically unacceptable color changes (Paravina et al. 2015). When the investigation of the color change rates for the solutions and materials for the whole periods, ΔE00 values were lower than 1.8 for distilled water across time intervals except in BC (−) group at 72 h. At the 4 h-72 h intervals, the AC specimens in the BC ( +) groups showed acceptable color change values for Ferro Sanol B solution. In this aspect, AC specimens yielded the highest color stability; this result can be explained by the material’s composition, as it includes urethane dimethacrylate (UDMA), dimethacrylate comonomers and is Bis-GMA free. However, compomer and PC specimens includes Bis-GMA (bisphenol-A-glycidylmethacrylate). Khokhar et al. ( 1991) reported that staining resistance was directly related to the resin phase of materials and that UDMA was more stain resistant than Bis-GMA or TEGDMA (tri- ethylene glycol dimethacrylate). Furthermore, restorative materials without TEGDMA have been stated to have a lower color stability in comparison to those containing this monomer. Water absorption is increased by TEGDMA which could move pigments causing material discoloration. TEGDMA enhances water absorption, which could move pigments causing material discoloration (Barutcigil and Yildiz 2012; Catelan et al. 2011). These arguments could explain high ΔE00 values exhibited by compomer specimens containing TEGDMA. Additionally, Sarac et al. (Sarac et al. 2006) stated that the nanohybrid composite resins with surface sealant (BC) exhibited significantly lower color change values than microhybrid composites. This outcome was referred not only to high density nanohybrid composites resulting in smoother surfaces but also to capability of the surface sealant filling out the defects and fissures on composite materials via capillary action.
In the present study, ferrous sulfate (Ferro Sanol B) and ferric polymaltose (Ferrum) were preferred, because they are among the most frequently prescribed iron syrups, according to data obtained from the Turkish Medicines and Medical Devices Agency. It has been stated that low dose iron in the ferrous fumarate generates less tooth discoloration than syrups (Christofides et al. 2006), whereas this low-dose formula do not contain enough iron to combat anemia. (Griffin et al. 1999) This results in syrups with high-dose iron, such as ferrous sulfate, to be prescribed more often to help preventing anemia. (Rao and Georgieff 2009) Pani et al. (2015) showed the ferric polymaltose had significantly higher ΔE than ferrrous fumarate on primary teeth at the end of 72 h in their study. Similarly, it was observed that between Ferrum and Ferro Sanol B solutions in BC ( +) groups, the ferrous sulfate had significantly lower ΔE00 than ferric polymaltose in all restorative materials. This might be caused by the rapid chemical interaction of the insoluble ferric form in comparison to the ferrous form of iron (Christofides et al. 2006).
The level of discoloration is directly related to the duration of its contact with restorative materials and teeth. Droplets or oral liquid could cause discoloration of teeth. Therefore, combining iron syrups with liquids such as water, fruit juice and drinking with a straw or dropping on posterior parts of the tongue could prevent discoloration. Toothbrushing has a positive impact on reducing staining rate before consuming the mentioning supplements (Miguel et al. 1997).
Specific limitations of this study should be taken into consideration when interpreting our results. In the oral environment, restorative materials are constantly exposed to coloring ingredients from food and beverages, and they are immersed in saliva. This study attempted to mimic the oral environment, and iron solutions were diluted in distilled water. Clinically, this dilution occurs in saliva, whose special properties include the presence of enzymes, specific proteins, and ions that may affect the color stability of restorative materials. Additionally, toothbrushing should also be taken into consideration. Further studies need to be supported by in vitro study designs investigating the effect of different types of sealant agents on restorative materials and enamel topography to prevent staining related to iron syrups.
Conclusions
-
Application of surface sealant significantly improved the staining resistance of aesthetic restorative materials
-
Nanohybrid AC seem to be more resistant to the staining effects of iron syrups formulations.
-
Compomers yielded significant color change values when exposed to commonly used iron syrups.
-
The content of iron syrup is important to color change. The discoloration effect of drug solutions on restorative materials depends on the composition of the material, iron syrups and exposure time.
-
Further studies should be supported with in vivo study designs to evaluate the effects of iron syrups and surface sealants on restorative materials used in pediatric dentistry.
References
Aguilar FG, Roberti Garcia Lda F, Cruvinel DR, Sousa AB, de Carvalho F, Panzeri Pires-de-Souza (2012) Color and opacity of composites protected with surface sealants and submitted to artificial accelerated aging, Eur J Dent, 6: 24-33
Alberton Da Silva V, Alberton Da Silva S, Pecho OE, Bacchi A. Influence of composite type and light irradiance on color stability after immersion in different beverages. J Esthet Restor Dent. 2018;30:390–6.
Babu KL, Rai K, Hedge AM. Pediatric liquid medicaments–do they erode the teeth surface? An in vitro study: part I. J Clin Pediatr Dent. 2008;32:189–94.
Barutcigil C, Yildiz M. Intrinsic and extrinsic discoloration of dimethacrylate and silorane based composites. J Dent. 2012;40(Suppl 1):e57-63.
Bhattacharya PT, Misra SR, Hussain M. Nutritional aspects of essential trace elements in oral health and disease: an extensive review. Scientifica (Cairo). 2016;2016:5464373.
Brooksbank A, Owens BM, Phebus JG, Blen BJ, Wasson W. 2018. Surface sealant effect on the color stability of a composite resin following ultraviolet light artificial aging, Oper Dent.
Catelan A, Briso AL, Sundfeld RH, Goiato MC, dos Santos PH. Color stability of sealed composite resin restorative materials after ultraviolet artificial aging and immersion in staining solutions. J Prosthet Dent. 2011;105:236–41.
Catelan A, Suzuki TYU, Becker F, Jr., Briso ALF, Dos Santos PH. 2017. Influence of surface sealing on color stability and roughness of composite submitted to ultraviolet-accelerated aging, J Investig Clin Dent, 8.
Christofides A, Asante KP, Schauer C, Sharieff W, Owusu-Agyei S, Zlotkin S. Multi-micronutrient sprinkles including a low dose of iron provided as microencapsulated ferrous fumarate improves haematologic indices in anaemic children: a randomized clinical trial. Matern Child Nutr. 2006;2:169–80.
CIE. 2004. Colorimetry, Technical Report. In. CIE Central Bureau, Vienna.
Dayan D, Heifferman A, Gorski M, Begleiter A. Tooth discoloration–extrinsic and intrinsic factors. Quintessence Int Dent Dig. 1983;14:195–9.
Dede DO, Sahin O, Koroglu A, Yilmaz B. Effect of sealant agents on the color stability and surface roughness of nanohybrid composite resins. J Prosthet Dent. 2016;116:119–28.
Eltahlah D, Lynch CD, Chadwick BL, Blum IR, Wilson NHF. An update on the reasons for placement and replacement of direct restorations. J Dent. 2018;72:1–7.
Griffin IJ, Cooke RJ, Reid MM, McCormick KP, Smith JS. Iron nutritional status in preterm infants fed formulas fortified with iron. Arch Dis Child Fetal Neonatal Ed. 1999;81:F45–9.
Hoffbrand AV, Herbert V. Nutritional anemias. Semin Hematol. 1999;36:13–23.
ISO. 2016. TechnicalReport (E): dentistry—guidance on colour measurements. Geneva, Switzerland: ISO. ISO/TR 28642:2016'.
Khalaj K, Soudi A, Tayefi-Nasrabadi M, Keshvad MA. The evaluation of surface sealants’ effect on the color stability of Nano-hybrid composite after polishing with One-Step system (in-vitro). J Clin Exp Dent. 2018;10:e927–32.
Khashayar G, Bain PA, Salari S, Dozic A, Kleverlaan CJ, Feilzer AJ. Perceptibility and acceptability thresholds for colour differences in dentistry. J Dent. 2014;42:637–44.
Khokhar ZA, Razzoog ME, Yaman P. Color stability of restorative resins. Quintessence Int. 1991;22:733–7.
Kumar A, Kumar V, Singh J, Hooda A, Dutta S. Drug-induced discoloration of teeth: an updated review. Clin Pediatr (Phila). 2012;51:181–5.
Lee YK, Powers JM. Combined effects of staining substances on resin composites before and after surface sealant application. J Mater Sci Mater Med. 2007;18:685–91.
Lee BS, Huang SH, Chiang YC, Chien YS, Mou CY, Lin CP. Development of in vitro tooth staining model and usage of catalysts to elevate the effectiveness of tooth bleaching. Dent Mater. 2008;24:57–66.
Lepri CP, Palma-Dibb RG. Influence of surface sealant on the color-stability of a composite resin immersed in different beverages. Oral Health Dent Manag. 2014;13:600–4.
Lindon JC, Tranter GE, Holmes JL. Encyclopedia of spectroscopy and spectrometry (Academic Press; 2000: San Diego, CA).
Lu H, Roeder LB, Lei L, Powers JM. Effect of surface roughness on stain resistance of dental resin composites. J Esthet Restor Dent. 2005;17:102–8.
Miguel JC, Bowen WH, Pearson SK. Influence of iron alone or with fluoride on caries development in desalivated and intact rats. Caries Res. 1997;31:244–8.
Miotti LL, Nicoloso GF, Durand LB, Susin AH, Rocha RO. Color stability of a resin composite: effect of the immersion method and surface treatments. Indian J Dent Res. 2016;27:195–9.
Pani SC, Alenazi FM, Alotain AM, Alanazi HD, Alasmari AS. Extrinsic tooth staining potential of high dose and sustained release iron syrups on primary teeth. BMC Oral Health. 2015;15:90.
Paravina RD, Ghinea R, Herrera LJ, Bona AD, Igiel C, Linninger M, Sakai M, Takahashi H, Tashkandi E, Perez Mdel M. Color difference thresholds in dentistry. J Esthet Restor Dent. 2015;27(Suppl 1):S1-9.
Pedroso LB, Barreto LF, Miotti LL, Nicoloso GF, Durand LB. Effect of a surface sealant on the color stability of composite resins after immersion in staining solution. Gen Dent. 2016;64:e22–5.
Rao R, Georgieff MK. Iron therapy for preterm infants. Clin Perinatol. 2009;36:27–42.
Sarac D, Sarac YS, Kulunk S, Ural C, Kulunk T. The effect of polishing techniques on the surface roughness and color change of composite resins. J Prosthet Dent. 2006;96:33–40.
Saygi G, Karakoc P, Serbes I, Erdemir U, Yucel T. Effect of surface sealing on stain resistance of a nano-hybrid resin composite. J Istanb Univ Fac Dent. 2015;49:23–30.
Talebi M, Parisay I, Mokhtari N. The parents’ knowledge and behavior towards the effects of using iron supplements on tooth staining and dental caries in Mashhad, Iran. Dent Res J (Isfahan). 2012;9:715–8.
Tupalli AR, Satish B, Shetty BR, Battu S, Kumar JP, Nagaraju B. Evaluation of the erosive potential of various pediatric liquid medicaments: an in-vitro study. J Int Oral Health. 2014;6:59–65.
Tuzuner T, Turgut S, Baygin O, Yilmaz N, Tuna EB, Ozen B. Effects of different pediatric drugs on the color stability of various restorative materials applicable in pediatric dentistry. Biomed Res Int. 2017;2017:9684193.
WHO. 2016. Use of multiple micronutrient powders for point-of-use fortification of foods consumed by infants and young children aged 6–23 months and children aged 2–12 years (Geneva).
Xing W, Jiang T, Liang S, Sa Y, Wang Z, Chen X, Wang Y. Effect of in-office bleaching agents on the color changes of stained ceromers and direct composite resins. Acta Odontol Scand. 2014;72:1032–8.
Yildirim S, Uslu YS. Effects of different pediatric drugs and toothbrushing on color change of restorative materials used in pediatric dentistry. Niger J Clin Pract. 2020;23:610–8.
Young MF, Girard AW, Mehta R, Srikantiah S, Gosdin L, Menon P, Ramakrishnan U, Martorell R, Avula R. Acceptability of multiple micronutrient powders and iron syrup in Bihar, India. Matern Child Nutr. 2018;14:e12572.
Zimmerli B, Koch T, Flury S, Lussi A. The influence of toothbrushing and coffee staining on different composite surface coatings. Clin Oral Investig. 2012;16:469–79.
Acknowledgements
The authors do not have any financial interest in the companies whose materials are included in this article.
Funding
In this study, no fund was received.
Author information
Authors and Affiliations
Contributions
Hypothesis and experimental design: SY. Performed the experiments: SY, EK. Wrote and proofread the manuscript: SY, EK.
Corresponding author
Ethics declarations
Conflict of interest
Declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yıldırım, S., Kaya, E. Can the use of surface sealant on restorative materials prevent discoloration related to iron syrup supplements?. Eur Arch Paediatr Dent 22, 739–750 (2021). https://doi.org/10.1007/s40368-021-00614-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40368-021-00614-5