Abstract
Aim
To evaluate the water storage degradation of resin–dentine bonds of different adhesive systems to primary and permanent human dentine.
Methods
Flat occlusal human dentine surfaces of 15 primary molars and 15 permanent molars were randomly assigned according to adhesive systems: Adper Single Bond 2; Clearfil SE Bond and One Up Bond F Plus. After bonding procedures, the adhesives were applied according to the manufacturers’ instructions and composite resin blocks were built. Restored teeth were sectioned rendering rectangular sticks (RS) (0.4 mm2). The RS were submitted to microtensile bond strength (µTBS) test according to the water storage time: 24 h, 1-year, and 2-years. Mean µTBS values were analysed by three-way analysis of variance (mixed design) and Tukey post hoc test (α = 0.05). The failure mode was analysed at 400× magnification.
Results
All three factors isolated showed significant influences on µTBS, as did the cross-product interactions between material vs. storage time (p = 0.01) and substrate vs. storage time (p = 0.002). Bond strength means to primary dentine were lower than to permanent dentine (34.7 ± 10.1 and 45.8 ± 12.9 mPa, respectively) after 2-years of water storage. The one-step self-etch adhesive (One Up Bond F Plus) showed less stable bond strength after 2-years of water storage.
Conclusion
The resin–dentine bond of primary teeth was more prone to degradation over time compared to permanent dentine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The longevity of resin–dentine interfaces can be compromised by several factors, including the hybrid layer degradation by water and by endogenous enzymes (matrix metalloproteinases and cysteine cathepsins (Carvalho et al. 2012; Feitosa et al. 2012; Montagner et al. 2014; Vidal et al. 2014). However, clinical data on adhesive restorations’ longevity are hard to achieve due to the long time required by in vivo studies, the high cost and ethical aspects involved. On that basis, in vitro studies have been extensively performed to evaluate the adhesive effectiveness and the resin–dentine bond degradation achieved, in order to predict the clinical longevity over time (De Munck et al. 2005, 2013; Bayne 2012; Tjäderhane et al. 2015).
Several studies have pointed out that permanent dentine differs from primary dentine in mineral content, density of dentinal tubules and area of intertubular dentine (Angker et al. 2004; Lenzi et al. 2013). These differences, however, are not enough to predict better or poorer bonding results for either substrate as there is no consensus in previous reports when comparing bond strength between primary and permanent dentine (da Costa et al. 2008; Ricci et al. 2010; Lenzi et al. 2012; Hosoya and Tay 2014).
It is of significant importance to identify if these differences do not influence immediate adhesive performance and play a role in bonding stability to primary dentine. Two main pathways explain the loss of bond strength after water storage for in vitro studies. First, the presence of hydrophilic components in adhesive systems became the interface susceptible to inherent plasticising degradation (Spencer et al. 2010; Feitosa et al. 2012). The hydrolysis of collagen fibrils is another pathway to adhesive interface degradation. Dentine proteases including metalloproteinases (MMPs) and cysteine cathepsins are related to resin–dentine bond degradation by exposing collagen fibril cleavage. These enzymes, primarily inactive as proenzymes in physiological states, can be activated under several conditions including the caries process and dentine hybridisation (Tjäderhane et al. 2015). Considering that primary dentine has a higher organic content than permanent dentine (Borges et al. 2009), and that MMPs are also related to physiological root resorption in the primary dentition (Linsuwanont-Santiwong et al. 2006), it can be expected that this substrate is more prone to collagen degradation. Although this is a relevant topic, the degradation of resin–dentine bonds of primary teeth has been poorly investigated (Sanabe et al. 2009; Lenzi et al. 2014) as have MMP inhibitors such as chlorhexidine on the durability of resin–dentine interfaces. Further, this research reported herein is the first study comparing the effect of long-term water storage on bond strength of different adhesive systems to human primary and permanent dentine. Therefore, the aim of this study was to evaluate the degradation of resin–dentine bonds of different adhesive systems to primary and permanent dentine. The null hypotheses tested were that (1) there is no difference in bond strength values of primary and permanent dentine; (2) the effect of water storage on bond strength degradation is similar for primary and permanent dentine and (3) adhesive systems present similar bond strength values, independently of the substrate and water storage time.
Materials and methods
Tooth selection and preparation
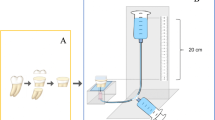
After approval of the study protocol by the Local Ethics Committee and obtaining patient’s informed consent, 30 extracted sound human molars were selected—15 third permanent molars and 15 first primary molars, with inclusion criteria of the absence of caries lesions, restorations, opacities or any kind of clinically detectable alterations on the occlusal or proximal surfaces. The teeth were disinfected in 0.5% chloramine-T solution and stored in distilled water at 4 °C for up to 3 months.
The teeth had flat mid-coronal dentine surfaces exposed using a low-speed diamond saw on a cutting machine under water irrigation (Labcut 1010, Extec Co., Enfield, CT, USA) to remove occlusal enamel. The surrounding enamel was removed using a diamond bur in a high-speed handpiece (#3146, KG Sorensen, São Paulo, Brazil) under water irrigation. The surfaces were manually polished with #600-grit silicon carbide under water for 30 s, in order to obtain standardised smear layers (Burrow et al. 2002).
Experimental design and bonding procedures
Permanent and primary teeth were randomly allocated to three groups according to the adhesive system (n = 5): Adper Single Bond 2, Clearfil SE Bond and One Up Bond F Plus, which were applied strictly following the manufacturer´s instructions (Table 1). The same protocol was used on both substrates and a single trained operator performed all bonding procedures.
Resin composite blocks (Z100, 3 M ESPE, St Paul, MN, USA) were incrementally built on the hybridised surfaces, each increment (2 mm thickness) was individually light cured (Demetron LC Light Curing, Kerr, Orange, CA, USA; at an intensity of 500 mW/cm2) until reaching approximately 6 mm height.
Microtensile bond strength (µTBS) testing
After 24 h of storage in distilled water at 37 °C, the teeth were sectioned (Labcut 1010, Extec Co., Enfield, CT, USA) in two longitudinal axes rendering beam shaped specimens (RS) composed of resin and dentine, with a cross-sectional bonded area of around 0.4 mm2. The RS thus obtained were randomly allocated to three subgroups according to the storage time: 24 h, 1-year and 2-years. In the 24 h group, specimens were immediately submitted to µTBS testing. For this, they were fixed to Geraldelli´s devices using cyanoacrylate and tested under tension on a universal testing machine (Emic DL 1000, Equipment and Systems Ltda., São Jose dos Pinhais, PR, Brazil), with a crosshead speed of 1 mm/min. Specimens of other subgroups were tested after storage under the same conditions (distilled water, 37 °C) for an additional 1 and 2-years. During these periods, the storage media were replaced weekly.
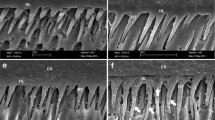
The failure mode was analysed using a stereomicroscope from a microdurometer (HMV 2, Shimadzu, Tokyo, Japan), at 400× magnification, classifying in cohesive (exclusively within the dentine or composite resin) or interfacial, that included failures at the resin–dentine interface or mixed with cohesive failure of the neighbouring substrate (De Munck et al. 2013). Cohesive failures were not considered in the statistical analysis.
Statistical analysis
The sample size of five teeth per group was estimated considering data from a previous pilot study, a coefficient of variation of 20% and a significant level of 5%, resulting in a power of 82%. The tooth was the experimental unit and thus, the RS from each tooth were allocated to the three subgroups according to the evaluation times (24 h, 1-year and 2-years). The mean of the µTBS values of the RS from the same tooth at each evaluation time were averaged for statistical analysis. Premature failures were not considered in the statistical analysis since the frequency of this kind of failure was low and homogeneous among groups. The Kolmogorov–Smirnov and Levene’s tests were used to confirm the normal distribution of the data and equally of variances, respectively. Bond strength values were subjected to mixed-design repeated measures analysis of variance (ANOVA) considering the main factors of adhesive system, substrate (permanent or primary dentine) and storage time (immediate, 1-year and 2-years of water storage) as well as the cross-interactions among the factors. The post hoc Tukey’s test was used for multiple comparisons. The significance level for all analyses was set at 5%. The analysis of the failure pattern was only descriptive (as a percentage).
Results
Statistically significant differences were found for the main factors—adhesive systems, substrate and storage time, each one separately (Table 2) as well as for cross-product interactions of adhesive system vs. storage time (p = 0.01) regardless of the substrate; and substrate vs. storage time (p = 0.002), regardless of the adhesive systems. The interaction among the three factors (adhesive system vs. substrate vs. storage time as a nested factor) was not significant (p = 0.06). This way, it was considered that storage time differently affected the bond strength values of each adhesive system and, interestingly, for each substrate (Table 3).
In the immediate evaluation time, higher bond strength values were obtained with Adper Single Bond 2 compared with One Up Bond F Plus. All tested adhesive systems presented a significant drop in bond strength values after 1-year of water storage, without differences among adhesive systems. After 2-years, a trend of lower values was still observed for all materials, but Adper Single Bond 2 and Clearfil SE Bond seemed to have been equally affected by long-term water storage as the values were similar to 1-year for both adhesives. Conversely, One Up Bond F Plus showed significantly lower values after 2-years compared to all other conditions.
When looking at the influence of the storage time on bond strength values of each substrate, despite the material, at 24 h, primary and permanent dentine produced similar values. After 1-year, both substrates showed a significant reduction in bond strength values, similar to each other. However, after 2-years of water storage, bond strength degradation was more pronounced in primary than in permanent dentine. In Table 4, it can be observed that, as expected, all experimental groups had a predominance of inter-facial failures.
Discussion
The water storage of microtensile specimens is a classical form of accelerated aging (Deng et al. 2014; De Munck et al. 2015; Gotti et al. 2015) and this effect on bonding degradation could be seen in several previous studies (Feitosa et al. 2012; Lenzi et al. 2012; Muñoz et al. 2015; Reis et al. 2015). Therefore, long-term water storage is a meaningful way of ranking adhesive systems and discriminating factors associated with resin–dentine degradation. The results of the present study clearly showed the effect of direct exposure of the adhesive interface to water on the decreasing values of µTBS, independent of the adhesive systems or dentine substrates.
Despite the differences in composition and bonding strategy among the adhesive systems tested, after 1-year of water storage, bond strength values declined between 18 and 25% for the three materials. Water sorption seemed to be similar to Adper Single Bond 2 and Clearfil SE Bond (Feitosa et al. 2012) and this could explain the significant µTBS drop after 1-year of water storage. Nevertheless, a longer storage period (2-years) did not significantly affect the µTBS values obtained for etch-and-rinse (Adper Single Bond 2) and to two-step self-etch (Clearfil SE Bond) systems. Although the reduction on µTBS appeared to have been continued for up to 2-years of Adper Single Bond 2, no significant differences were found after 1-year of water storage. Clearfil SE Bond displayed a trend to no degradation after 1-year that can be explained by an extra layer of hydrophobic resin applied over primer rendering an adhesive interface less prone to hydrolytic degradation. Moreover, the presence of 10-MDP also enhanced bond stability to water degradation (Zhang et al. 2016).
The same findings were not valid for the one-step self-etch system (One Up Bond F Plus) that presented further reduction in µTBS values after 2-years of water storage. As expected, the one-step self-etch system presented lower immediate µTBS values compared to the etch-and-rinse system (Montagner et al. 2014). Similarly, a reduction of bonding effectiveness was more pronounced for this system, especially after 2-years of water storage. Poor bonding performance has been observed in previous studies (Osorio et al. 2010; Reis et al. 2010; Pinto et al. 2015) and it could be partially explained by the composition of this adhesive system. High HEMA concentration (30–60%) due to its hydrophilicity could reduce the water evaporation (Ye et al. 2009) interfering with adhesive polymerisation (Werle et al. 2015). Besides, the presence of HEMA allowed water uptake to continue from dentine into a polymer (Wang and Spencer 2005) rendering the adhesive-dentine interface more prone to hydrolytic degradation effects over time. For the One Up Bond F Plus, it seemed that even with the presence of MAC-10 in its composition, that conferred some hydrolytic stability, the adhesive system was the most sensitive material to water storage (Osorio et al. 2010).
In line with other studies, immediate bond strength values were similar for permanent and primary dentine (Ricci et al. 2010; Spencer et al. 2010) regardless of the adhesive system. The effect of differences in mineral content, even as tubular density and other physical and structural variations between primary and permanent dentine seemed to be insufficient to significantly influence the immediate bond strength values. However, the effect of water storage was more pronounced on adhesion to primary dentine. While µTBS values were similar for both substrates at baseline and, even with a decrease at the 1-year evaluation, water storage degradation was significantly higher for primary dentine. As stated above, collagen fibril degradation by host-derived proteases seemed to reasonably explain the results reported here. The effects of proteolytic enzymes could be associated with higher resin–dentine bond degradation in primary teeth. The higher organic matrix content in primary than in permanent dentine could be associated with higher collagen degradation in this substrate (Osorio et al. 2013). Therefore, the higher resin–dentine bond degradation might be explained by increased collagenolytic activity in primary dentine.
In this sense, it might be thought that the application of a MMP inhibitor, mainly chlorhexidine (Collares et al. 2013), would significantly alter these results because of the beneficial effect on dentine bonding stability. However, in a systematic review by Montagner et al. (2014) the effect of chlorhexidine on the decrease of loss of bond strength was not clearly shown after long-term in vitro storage. Conversely, primary dentine has been identified as being more reactive to acid-etching, producing a thicker hybrid layer as a consequence of deeper demineralisation (Nör et al. 1996). Therefore, it has been suggested as appropriate to reduce the acid-etching time on primary dentine (Scheffel et al. 2012; Lenzi et al. 2014). However, the results of the present study did not show an effect of water storage on the interaction of the adhesive system vs. substrate (primary or permanent dentine). Thus, the effect of the etching step on bond strength degradation in primary dentine could not be shown.
It is important to consider that in this study, only sound dentine was used and it would be expected that in caries-affected dentine the effects of resin–dentine bond degradation would be even more evident. MMP activity is higher in carious than in sound dentine (Scheffel et al. 2012; Toledano et al. 2010; Nascimento et al. 2011) and so it is reasonable to expect faster bonding degradation on caries-affected dentine. Nonetheless, additional research is required to confirm the influence of caries-affected dentine and enzymatic inhibitors on bonding stability over time. Furthermore, laboratory degradation seemed to be faster than for the real clinical situation (Carvalho et al. 2012).
Conclusions
Resin–dentine bonds of primary teeth were more prone to degradation with time compared with permanent resin-dentine bonds. A decrease in bond strength values was higher when using a one-step self-etch adhesive that contained 10-MAC over 2-years of water storage.
References
Angker L, Nockolds C, Swain MV, Kilpatrick N. Quantitative analysis of the mineral content of sound and carious primary dentine using BSE imaging. Arch Oral Biol. 2004;49:99–107.
Bayne SC. Correlation of clinical performance with ‘in vitro tests’ of restorative dental materials that use polymer-based matrices. Dent Mater. 2012;28:52–71.
Borges AF, Puppin-Rontani RM, Bittar RA, et al. Effects of acidic primer/adhesives on primary and permanent dentin. Am J Dent. 2009;22:30–6.
Burrow MF, Nopnakeepong U, Phrukkanon S. A comparison of microtensile bond strengths of several dentine bonding systems to primary and permanent dentine. Dent Mater. 2002;18:239–45.
Carvalho RM, Manso AP, Geraldeli S, Tay FR, Pashley DH. Durability of bonds and clinical success of adhesive restorations. Dent Mater. 2012;28:72–86.
Collares FM, Rodrigues SB, Leitune VC, et al. Chlorhexidine application in adhesive procedures: a meta-regression analysis. J Adhes Dent. 2013;15:11–8.
Da Costa CC, Oshima HM, Costa Filho LC. Evaluation of shear bond strength and interfacial micromorphology of direct restorations in primary and permanent teeth—an in vitro study. Gen Dent. 2008;56:85–93.
De Munck J, Van Landuyt K, Peumans M, et al. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005;84:118–32.
De Munck J, Luehrs AK, Poitevin A, Van Ende A, Van Meerbeek B. Fracture toughness versus micro-tensile bond strength testing of adhesive-dentine interfaces. Dent Mater. 2013;29:634–44.
De Munck J, Poitevin A, Lührs AK, et al. Interfacial fracture toughness of aged adhesive-dentine interfaces. Dent Mater. 2015;31:462–72.
Deng D, Yang H, Guo J, et al. Effects of different artificial ageing methods on the degradation of adhesive-dentine interfaces. J Dent. 2014;42:1577–85.
Feitosa VP, Leme AA, Sauro S, et al. Hydrolytic degradation of the resin–dentine interface induced by the simulated pulpal pressure, direct and indirect water ageing. J Dent. 2012;40:1134–43.
Gotti VB, Feitosa VP, Sauro S, et al. Effect of antioxidants on the dentine interface bond stability of adhesives exposed to hydrolytic degradation. J Adhes Dent. 2015;17:35–44.
Hosoya Y, Tay FR. Bonding ability of 4-META self-etching primer used with 4-META/MMA-TBB resin to enamel and dentine: primary vs permanent teeth. J Dent. 2014;42:425–31.
Lenzi TL, Soares FZM, Rocha RO. Degradation of resin–dentine bonds of etch-and-rinse adhesive system to primary and permanent teeth. Braz Oral Res. 2012;26:511–5.
Lenzi TL, Guglielmi Cde A, Arana-Chavez VE, Raggio DP. Tubule density and diameter in coronal dentine from primary and permanent human teeth. Microsc Microanal. 2013;19(6):1445–9.
Lenzi TL, Braga MM, Raggio DP. Shortening the etching time for etch-and-rinse adhesives increases the bond stability to simulated caries-affected primary dentine. J Adhes Dent. 2014;16:235–41.
Linsuwanont-Santiwong B, Takagi Y, Ohya K, Shimokawa H. Expression of MT1-MMP during deciduous tooth resorption in odontoclasts. J Bone Miner Metab. 2006;24:447–53.
Montagner AF, Sarkis-Onofre R, Pereira-Cenci T, Cenci MS. MMP inhibitors on dentine stability: a systematic review and meta-analysis. J Dent Res. 2014;93:733–43.
Muñoz MA, Luque-Martinez I, Malaquias P, et al. In vitro longevity of bonding properties of universal adhesives to dentine. Oper Dent. 2015;40:282–92.
Nascimento FD, Minciotti CL, Geraldeli S, et al. Cysteine cathepsins in human carious dentine. J Dent Res. 2011;90:506–11.
Nör JE, Feigal RJ, Dennison JB, Edwards CA. Dentine bonding: SEM comparison of the resin–dentine interface in primary and permanent teeth. J Dent Res. 1996;75:1396–403.
Osorio R, Aguilera FS, Otero PR, et al. Primary dentine etching time, bond strength and ultra-structure characterization of dentine surfaces. J Dent. 2010;38:222–31.
Osorio R, Yamauti M, Ruiz-Requena ME, Toledano M. MMPs activity and bond strength in deciduous dentine–resin bonded interfaces. J Dent. 2013;41:549–55.
Pinto CF, Vermelho PM, Aguiar TR, et al. Enamel and dentine bond strength, interfacial ultramorphology and fluoride ion release of self-etching adhesives during a pH-cycling regime. J Adhes Dent. 2015;17:27–34.
Reis AF, Carrilho MR, Ghaname E, et al. Effects of water-storage on the physical and ultramorphological features of adhesives and primer/adhesive mixtures. Dent Mater J. 2010;29:697–705.
Reis A, Martins GC, de Paula EA, Sanchez AD, Loguercio AD. Alternative aging solutions to accelerate resin–dentine bond degradation. J Adhes Dent. 2015;17:321–8.
Ricci HA, Sanabe ME, Costa CA, Hebling J. Bond strength of two-step etch-and-rinse adhesive systems to the dentine of primary and permanent teeth. J Clin Pediatr Dent. 2010;35:163–8.
Sanabe ME, Kantovitz KR, Costa CA, Hebling J. Effect of acid etching time on the degradation of resin–dentine bonds in primary teeth. Am J Dent. 2009;22:37–42.
Scheffel DL, Tenuta LM, Cury JA, Hebling J. Effect of acid etching time on demineralization of primary and permanent coronal dentine. Am J Dent. 2012;25:235–8.
Spencer P, Ye Q, Park J, et al. Adhesive/Dentine interface: the weak link in the composite restoration. Ann Biomed Eng. 2010;38:1989–2003.
Tjäderhane L, Buzalaf MA, Carrilho M, Chaussain C. Matrix metalloproteinases and other matrix proteinases in relation to cariology: the era of ‘dentine degradomics’. Caries Res. 2015;49:193–208.
Toledano M, Nieto-Aguilar R, Osorio R, et al. Differential expression of matrix metalloproteinase-2 in human coronal and radicular sound and carious dentine. J Dent. 2010;38:635–40.
Vidal CM, Tjäderhane L, Scaffa PM, et al. Abundance of MMPs and cysteine cathepsins in caries-affected dentine. J Dent Res. 2014;93:269–74.
Wang Y, Spencer P. Interfacial chemistry of class II composite restoration: structure analysis. J Biomed Mater Res A. 2005;75:580–7.
Werle SB, Steglich A, Soares FZ, Rocha RO. Effect of prolonged air drying on the bond strength of adhesive systems to dentine. Gen Dent. 2015;63:68–72.
Ye Q, Wang Y, Spencer P. Nanophase separation of polymers exposed to simulated bonding conditions. J Biomed Mater Res B Appl Biomater. 2009;88:339–48.
Zhang ZY, Tian FC, Niu LN, et al. Defying ageing: an expectation for dentine bonding with universal adhesives? J Dent. 2016;45:43–52.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was previously approved by the Local Ethics Committee, Universidade Federal de Santa Maria, CAAE 367657 14.3.0000.5346
This study has no funding.
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Soares, F.Z.M., Lenzi, T.L. & de Oliveira Rocha, R. Degradation of resin–dentine bond of different adhesive systems to primary and permanent dentine. Eur Arch Paediatr Dent 18, 113–118 (2017). https://doi.org/10.1007/s40368-017-0282-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40368-017-0282-z