Abstract
Purpose
Sodium iodide symporter (NIS), as a broadly exploited cancer theranostic molecule, has attracted considerable interest in non-thyroidal tumors. Several groups have investigated the significant potential of the NIS gene to induce radioiodine accumulation across various tumor types. In this review, we discuss the role of NIS as a theranostic gene in pre-clinical extra-thyroidal cancer therapy and highlight the remarkable progress in the viral and non-viral transgene transfer systems including oncolytic viruses, engineered mesenchymal stem cells (MSCs) and synthetic polyplexes.
Methods
A literature search was conducted using PubMed, Scopus, and Google Scholar databases. In addition, the keywords "sodium iodide symporter", "gene transfer", "extra-thyroidal malignancies", "viral transfer system", "non-viral transfer system", and "131I" were used.
Results
Following the exclusion of letters, editorials, commentaries, and duplicate publications, this review summarized preclinical studies that used viral and nonviral NIS delivery methods in conjunction with 131I to describe the effective role of combination therapy.
Conclusion
NIS-mediated expression in combination with 131I can be considered a promising approach in the preclinical treatment of extra-thyroidal malignancies and metastases. Malignant cells expressing NIS are thought to accumulate radionuclides intracellularly, which contributes to the remarkable therapeutic effects observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sodium iodide symporter (NIS), a transmembrane glycoprotein on the basolateral membrane of thyroid cells that actively participates in sodium and ion co-transports, is one of the oldest targets for the diagnostic and therapeutic application of radioiodide [1, 2]. The cloning of the NIS gene, in 1996 by Carrasco et al., has provided a powerful antitumor tool via the development of robust therapeutic application along with a sensitive reporter gene into non-thyroidal tumors [3]. NIS greatly facilitate in vivo real-time tracking performance of biological process through the use of whole-body imaging modalities such as positron emission tomography (PET) and single photon emission computed tomography (SPECT) [4]. To date numerous viral and non-viral delivery systems including viral vectors, oncolytic viruses, engineered MSCs, and polyplexes are represented as a promising platform for facilitating gene delivery into target cells.
The NIS-expressing cells via absorbing β-emitting radioisotopes like 131I and 188Re induce cell death [5, 6]. The radiopharmaceutical 131I, a theranostic agent emitting both 90% beta and 10% gamma particles, destroys target cells through direct and indirect mechanisms [7]. The potential destroying capability of 131I in the direct route imposes through cell proliferation inhibition, increasing apoptosis by downregulation of the BCL2 gene and inducing cell cycle arrest. Moreover, bystander or non-targeted effects is an indirect way that drives cell death via β-particulate radiation that occurs in the surrounding cells with path length penetration of 2.4 mm in tissue [8,9,10]. Currently, 131I is mostly utilized in thyroid malfunction as the high expression of NIS on the thyroid gland. Studies have shown other tissue including the mammary gland, the choroid plexus, the salivary gland, and stomach parietal cells expressing significant levels of NIS that can be used as a therapeutic target [11]. In this context, one of the early efforts was conducted by Spitzweg et al. who showed that prostate cancer cells expressing NIS induce cell death in androgen-sensitive human prostate adenocarcinomas after administration of 131I [12]. Further, another study found that the NIS protein plays a role in the accumulation of ions in breast cancer metastatic sites [13]. As a result, evaluating 131I for the treatment of other cancers has become more critical.
In this review, molecular events involved in the NIS-mediated treatment of extra-thyroidal malignancies, NIS regulatory mechanisms, and preclinical studies conducted in this field will be discussed. In addition, novel strategies like combinatorial therapeutic approaches to improve the efficiency of NIS-mediated iodine therapy in extra-thyroidal malignancies will be evaluated.
NIS functional characterization and regulation
The human NIS (hNIS) gene, localized on chromosome 19p12–13.2 and containing 1929 nucleotides comprising 15 exons and 14 introns, encodes a 643-amino acid protein with 13 transmembranes helix and molecular weight approximately 90–100 kDa. Several sorting motifs actively participate in the regulation of NIS localization, but the underlying molecular mechanisms remain poorly understood [2]. Moreover, post-translational modifications, including phosphorylation, glycosylation, and ubiquitination, have critically affected NIS interactions, localization, and activity. Studies have demonstrated three crucial N-linked glycosylation sites in the NIS protein structure lead to maturation and acquisition of appropriate weight for the NIS migration. A survey by Levy et al. showed NIS glycosylation has a critical role in protein stabilization and folding and does not require correct targeting of the protein on the plasma membrane. In addition, western blot analysis revealed the migration of NIS protein (at lower molecular weight) in other tissues was affected due to incomplete glycosylation [14].
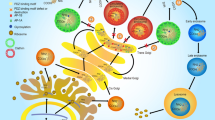
NIS protein regulates by both transcriptional (epigenetic) and post-translational mechanisms (subcellular localization) [6]. Depending on the species and tissue context, different regulatory elements act on the NIS gene with varying degrees of enhancer and promoter activities to modulate the transcription of NIS. In addition, NIS gene is regulated by three major epigenetic mechanisms, including DNA methylation, histone modification, and nucleosome positioning [15]. For example, histone deacetylase inhibitors (HDACi), which are known to exert epigenetic control by affecting non-histone proteins such as transcription factors and molecular chaperones, regulate chromatin structure and gene expression. In this context, Rathod et al. have well demonstrated that the use of bHDACi together with 131I significantly increases the expression of NIS and reduces the survival of MCF-7 cells (Fig. 1) [16].
TSH and iodide are two essential factors that control NIS expression and localization under physiological conditions [17, 18]. In addition, thyroglobulin, protein–protein interactions, through potassium voltage-gated channel subfamily Q member 1 and potassium voltage-gated channel subfamily H member 2 (KCNQ1–KCNH2) K+ channel are other mechanisms that are involved in the NIS regulation [4]. Several studies have confirmed intracellular expression of NIS in the thyroid and breast cancerous tissues [19]. In a study Peyrottes et al. showed that the expression level of NIS in thyroid and breast cancer is low and intracellular tracking was correlated with non-specific signals. These discouraging results allowed scientists to focus on NIS post-translational regulation to increase the uptake of iodide in tumor cells [20]. In addition, signaling pathways are shown to interfere with the expression and regulation of NIS in human cancers. In this regard, Knostman and colleagues revealed activation of PI3K significantly increases the expression of NIS in the MCF-7 cell line [21]. Furthermore, the MEK signaling pathway regulates NIS expression in thyroidal and breast tissue. Recently, Zhang et al. demonstrated MEK inhibition linked to NIS lysosomal degradation [22]. Also, the role of the MEK signaling pathway is highlighted in breast cancer via NIS protein stability and oncogenic transformation.
NIS expression in extra-thyroidal and therapeutic applications
As an essential iodide transporter for the biosynthesis of thyroid hormone, NIS protein is distributed in some extra-thyroidal organs, including salivary glands, renal tubules, the stomach, the lactating breast, etc. Studies have found that the NIS expression, regulation, function, subcellular localization, and glycosylation at extra-thyroidal organs depend on individual tissues and I− requirement/content [23].
In many cases, NIS is involved in multiple functions, including: (1) recycling and retaining as much I− as possible, either from food, recycling of secondary metabolism, or reabsorption from urine, thereby releasing I− into the blood and reaching the thyroid gland; (2) supply the fetus and the newborn with I− and (3) provides I−, which exerts an antioxidant role by reducing the levels of ROS, and finally ensure that I− exerts a potent antiviral and antimicrobial protective function when converting to hypoiodite (IO−) [23]. In addition, several advantages have been reported for the use of NIS compared with other reporter genes, including the human origin of NIS, no need for prior radiolabeling, which reduces the corresponding costs, the possibility of using different imaging systems such as PET, SPECT, combination of SPECT or PET with computerized tomography (CT) to provide anatomical information about cells expressing NIS, and improvement of detection sensitivity due to signal amplification by the accumulation of intracellular substrate.
As mentioned previously, the NIS protein is expressed in the normal breast tissues during pregnancy and lactation. Studies have shown treatment of nonlactating mice with oxytocin alone, consequently increased levels of NIS expression and accumulation of radioiodide [19]. Interestingly, tumor expression of NIS has been detected in animal models of breast cancer as well as in human samples. Analysis of human breast cancer by Tazebay et al. well-showed the NIS was expressed at more than 80% of samples when compared with normal nonlactating breast tissue [19]. Imaging studies with various substrates have opened an exciting chapter in the use of NIS as a theranostic tool in endogenous NIS-expressing tumors [13]. In contrast, NIS is not always localized on the surface of the cell membrane, which may explain the discrepancy between those breast tumors expressing NIS and those absorbing radioiodide. Therefore, comprehensive clinical trials are needed to characterize the results of radioiodide therapy in metastatic breast cancer.
However, a recent study in cancer and metastasis has confirmed the oncogenic effects of intracellularly retained NIS via its interaction with leukaemia-associated RhoA-guanine exchange factor (LARG) through the PI3K/AKT/mTOR pathway. This metastatic property could recruit intracellular NIS toward the plasma membrane, making tumors amenable to 131I radiotherapy, which has the additional beneficial effect of slowing tumor progression [48, 49].
The results of in vitro experiments illustrated that all-trans retinoic acid (atRA) alone or in combination with hydrocortisone, adenosine triphosphate (ATP), or dexamethasone triggered the expression of NIS in the MCF-7 cell line. Furthermore, in vivo experiments confirmed that atRA significantly increased radioiodide accumulation in NIS-mediated pathways compared to controls [6, 50,51,52,53,54]. In addition to the expression of endogenous NIS, experimental observations have also confirmed that transferring the hNIS gene to non-thyroid tumor cells would induce iodide accumulation. These results propose that exogenous hNIS expression in tissues of interest may play an essential role in noninvasive real-time molecular imaging using the intracellular accumulation of gamma-emitting radioisotopes such as (123I, 124I, 125I, and 131I), 99mTcO4-, 188Re, and 211At at site-specific gene expression.
One of the limitations of serial hNIS imaging is the likelihood that the radiotracer will be taken up by both malignant and normal tissue, as the hNIS protein is widely distributed in normal tissue, which may result in decreased accumulation of the radiotracer in the target structures of interest. Furthermore, the unfavorable biodistribution and accumulation of iodide in the thyroid gland is another disadvantage that must be considered. In treating extrathyroidal cancers, administration of THs to lower TSH levels is helpful to protect the thyroid gland from unwanted radiation. This strategy can be used both in treating cancers that endogenously express NIS (e.g., breast cancer) and in treating cancers induced to express NIS by gene transfer exogenously [55]. In addition, the administration of contrast media during radiological examinations is another way to notably reduce the uptake of radioiodide by NIS -transduced cells. Also, according to Dupertuis et al., potassium iodide administered 24–48 h before treatment suppressed thyroid iodide uptake after administration of a radiolabeled iodine analog [56].
Approaches to NIS gene delivery; focusing on 131I combination therapies
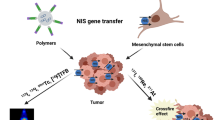
Over the past two decades, NIS has attracted more attention in the application and optimization of gene therapy due to its dual role as a reporter and therapeutic gene. In 1997, Shimura et al., who developed a stable Tc-rNIS cell line expressing NIS, showed that subcutaneous injection of Tc-rNIS induces I− accumulation and can be followed by the administration of 125I [57]. For the first time, this study suggested that NIS can be introduced as a theranostic agent. NIS has become the counterpart for research on human cancers, which have been extensively used in cells and other organisms (Table 1). The main areas of NIS-based gene transfer are reviewed, including (a) replicative-defective viral vectors/oncolytic virus-mediated gene therapy, (b) stem cell trafficking, and (c) regenerative medicine (Fig. 2) [58].
Schematic representation of pathways involved in apoptosis of tumor cells treated with 131I. Viral and non-viral vectors coupled with sodium iodide (Na+/I−) symporter increase the expression of NIS (A), and the level of NIS expression determined by SPECT—CT imaging after 123I injection (B), then tumor cells are irradiated with 131-iodine, iodine irradiation causes the release of reactive oxygen species (ROS) and induces apoptosis (C)
NIS-expressing viruses approach
In cancer therapy, viral vectors are considered one of the most promising tools that allow the insertion of therapeutic genes. NIS is one of the most amazing genes because, according to the translation, it increases the accumulation of theranostic radioisotopes for imaging and therapeutic purposes.
In this context, Trujillo et al. investigated the efficacy of an hNIS-expressing adenovirus (Ad5PB_ RSV-NIS) in a prostate cancer model. Interestingly, the result showed administration of 1 mCi of 131I along with Ad5PB_RSV-NIS, slowed the rates of tumor progression and increased survival through the accumulation of radioiodine into the tumor cells [34]. In addition, a study by Oneal and colleagues found that the administration of two novel conditionally replicating adenoviral vectors in combination with 131I specifically induced radioisotope uptake, cytopathic effects, and viral replication in an in vitro and in vivo prostate cancer model [59].
Oncolytic viruses are another platform increasingly used in cancer therapy trials because they can preferentially infect and kill tumor cells [60]. These viruses are divided into two main groups: (1) naturally oncolytic viruses, which have an innate ability to infect and kill tumor cells like Newcastle disease virus, and (2) genetically engineered oncolytic viruses, which have been genetically engineered to replicate and destroy cancer cells such as vaccinia, Herpes simplex, adenovirus and measles virus. Oncolytic viruses typically induce antitumor responses through two distinct strategies, including direct lysis of cancer cells after induction of immunogenic cell death, the release of tumor-associated antigens (TAAs), damage-associated molecular patterns, pathogen‐associated molecular patterns, and subsequently activation and recruitment of an additive immune response to the tumor microenvironment [61].
Increasing the sensitivity of tumor cells to ionizing radiation or chemotherapy, inducing immunogenic cell death, selective replication in tumor cells, and the ability to infect resistant tumor cells are some of the main advantages of oncolytic viruses. However, some issues that need to be considered in virotherapy include variability in preclinical and clinical response, limited ability to transduce tumors, and limited distribution within the solid tumor due to physical properties [62]. Further, virus immunogenicity must also be optimized to modulate the organism's immune response to be able to replicate and spread. To address these issues, the trajectory of viral particles, the number of infected cells, and the duration and spread of viral infection must be effectively monitored [63]. Functional expression of NIS correlates with the longevity of viral replication and allows tracking of radioisotope uptake during the viral infection cycle in the tumor. After infection of the target cells, the NIS gene is translated and the protein is translocated to the plasma membrane, allowing the detection of the accumulated radiotracer. At the peak of virus replication, radiotracer absorption reaches its maximum. Finally, cell lysis releases new viral particles, causing the infection to spread and NIS to be expressed in neighboring cells.
The first experiments were performed by Peerlinck and colleagues, which demonstrated that the radiotracer accumulation was positively correlated with cell viability in mice with colorectal carcinoma. Confirmation of the results by nanoSPECT/CT and immunohistochemistry (IHC) revealed accumulation of 131I in the first 48 h after infection. Thereafter, I accumulation decreased, NIS could no longer be detected at the plasma membrane, and the cells began to die [64]. In addition, the efficacy of intravenous administration of Ad5/3-hTERT-hNIS (the hTERT promoter controls an oncolytic adenovirus) in combination with 131I was investigated. SPECT Analysis showed that Ad5/3-hTERT-hNIS significantly prolonged survival in a prostate cancer model compared with mock or radioiodine-only groups [65].
The remission of metastatic cancer is another critical aspect that needs to be considered in new therapies. To address this issue, systemic delivery of oncolytic viruses encoding NIS has been developed to enable imaging and treatment of metastatic lesions and detection of positive surgical margins after tumor resection [66]. Recently, Naik et al. evaluated intravenous administration of VSV-IFNβ- NIS, a novel recombinant oncolytic vesicular stomatitis virus expressing NIS and interferon β, in spontaneous canine cancer. The results showed that VSV-IFNβ- NIS is well tolerated and safe in treating advanced or metastatic disease [67]. In addition, oncolytic measles viruses expressing NIS (MV-NIS) have been successfully used as an effective theranostic modality in various cancers. Considering this perspective, Russell et al. reported an impressive response to intravenous infusion of MV-NIS for the treatment of two measles seronegative patients suffering from relapsed drug-refractory myeloma [66].
NIS gene delivery by non-viral delivery systems
A critical step in the clinical implementation of NIS gene therapy is the development and evaluation of effective, safe, and highly efficacious delivery systems not only after local but also after systemic vector application. In addition to the ability to monitor and target primary tumors, the ability to target tumor metastases is another crucial aspect to be addressed by improved targeted delivery methods. Synthetic polyplexes and mesenchymal stem cells are two promising non-viral platforms that are currently being investigated and have the potential for clinical application.
Polyplex‑mediated NIS gene delivery
Synthetic vectors, as a promising delivery method inspired by viral biology, cover some of the current limitations of viral vectors, such as immunogenicity, difficulties in synthesis and upscaling, and limited nucleic acid transport. Recently, several generations of synthetic polycationic polymers have been investigated by Munich researchers at the LMU as delivery systems for systemic gene delivery medicine NIS [68]. Linear polyethylenimine (LPEI), a polycationic polymer, is a "gold standard" for synthetic gene delivery systems with improved targeting and higher efficiency through the incorporation of polyethylene glycol (PEG) and targeting ligands. PEG cover reduces self-aggregation by lowering the positive surface charge and provides prolonged blood circulation by evading immune recognition. Moreover, this positive surface charge has been shown to lead to high intrinsic tumor affinity. Furthermore, due to its biodegradability, no toxic side effects have been reported [69]. Up to now, several studies have evaluated tumor selectivity, biodistribution, transduction efficiency, and duration of transgene expression of these polyplexes after systemic administration using NIS-based imaging.
In a study, Urnauer and coworkers investigated the receptor specificity, transduction efficiency, and therapeutic efficacy of polymers composed of PEG and cationic amide nuclei complexed with NIS-DNA targeting sequence-defined cMET/HGFR (a dual-targeting approach) in a hepatocellular cancer model. Results showed that three cycles of polyplexes (cMBP2-PEG-Stp/NIS) and 131I resulted in high tumor-selective iodide accumulation and significant tumor growth delay and prolonged survival [70]. Effective control of metastases has become an important issue in novel cancer therapy, as they are one of the main causes of disease recurrence and patient death. Liver metastases are one of the major challenges in advanced colorectal cancer, of which less than 5% are cured. Urnauer et al. designed and studied a novel polymer containing polyethylenimine, PEG, the epidermal growth factor receptor (EGFR) targeting GE11 complexed with NIS (LPEI-PEG -GE11/NIS) in liver metastases from colorectal cancer. Analysis of PET confirmed that administration of three cycles of intravenous LPEI-PEG-GE11/NIS together with 55.5 MBq 131I significantly slowed the spread of liver metastases and was associated with animal survival [41].
Nonviral MSCs- NIS gene transfer
Mesenchymal stem cells (MSCs) are a diverse subset of multipotent progenitor cells that are characterized by a panel of specific cell surface antigens and play a fundamental role in tissue regeneration [71]. The high potential of MSCs to migrate to the site of various tumor types (in vivo) makes them a useful vehicle for the targeted delivery of therapeutic agents in different cancer types. Currently, MSCs expressing NIS have emerged as a platform for the selective delivery of radionuclides for better visualization and effective treatment of metastases [72].
Dwyer et al. investigated the application of MSC-NIS along with intraperitoneal injection of 131I for breast cancer imaging and therapy. Promising data support the feasibility of this approach as a novel therapy for breast cancer [38]. In addition, a study showed that mice transduced with the MSC-NIS/poly-l-lactic acid (PLLA) complex had higher iodide uptake than MSC-NIS, which was associated with the long-term survival of the mice [73].
External beam radiotherapy (EBRT) was shown to enhance the recruitment of NIS-expressing MSCs into human hepatocellular carcinoma (HuH7). Moreover, the expression of the cytokine TGFβ was strongly upregulated in HuH7 tumors after EBRT irradiation. Schug et al. investigated the combination of MSC-based NIS -mediated 131I therapy under the control of a synthetic TGFB1-inducible small mothers against decapentaplegic (SMAD)-responsive promoter (SMAD-NIS-MSCs) and 5 Gy focused EBRT, resulting in growth delay up to complete tumor remission and longer survival compared with CMV-NIS-MSCs treated mice [29]. In another study, the human HSP70B promoter was selected as a candidate gene promoter for MSC-mediated NIS gene therapy (HSP70B-NIS-MSCs). The results showed that the combination of HSP70B-NIS-MSC-mediated 131I with hyperthermia resulted in a significant reduction in tumor growth and prolonged survival of the animals [5].
Early growth response protein1 (Egr1) is a zinc finger transcription factor that regulates cell growth and differentiation. Egr1 expression is induced in the presence of 131I. Recently, Zhang et al. constructed MSCs from bone marrow bearing both ultrasmall gold nanoclusters (AuNCs) (to improve the efficacy of radiotherapy) and hNIS under the Egr1 promoter. Based on an in vivo result, the combination of BMSC-Egr1-hNIS + AuNCs with 131I reduced tumor volume by up to 56%, compared with the BMSC-Egr1-hNIS group alone (36%) [37].
Challenges and supportive strategies
Despite the suitable physical properties of α-emitting radioisotopes, it is not possible to use them routinely because of their relative unavailability. Therapeutic β-emitting radioiodide (131I) is the relevant radioisotope used in studies because of its unique ability as a therapeutic agent. The effective translocation of NIS protein into the cell membrane through the induction of membrane insertion of the gene NIS leads to high performance of 131I-mediated therapy for the destruction of solid tumors. Furthermore, successful iodine therapy depends on 131I retention in tumor cells and the biological half-life of 131I in the body. While the uptake of radioiodide into the thyroid gland is associated with organification and prolonged retention of iodide in this tissue, long-term radiation exposure of normal thyroid cells results in induced hypothyroidism [74].
Another critical issue following the administration of 131I for extra-thyroidal cancer is the optimization of the appropriate dose to avoid delivering a higher dosage of the radioisotope to other tissues, particularly the thyroid gland [75]. In extra-thyroidal tumors, iodide is not efficiently incorporated into proteins, resulting in shorter iodide retention, unlike in the thyroid glands. The longer retention time of radioiodine in tumor cells increases the duration of radiation exposure within the cell. The retention time of radioiodide in serum is almost three times longer in human extrathyroidal tumors than in rodents, which resulted in a higher radiation dose of 131I in humans with NIS -expressing tumors [76, 77]. However, it may be possible to enhance the biological effect of 131I by inhibiting the repair of DNA double-strand breaks (DSBs), which are considered the most lethal form of DNA damage [78]. This could potentially address some of the limitations of 131I therapy in the treatment of NIS-transduced extra-thyroidal cancer. Moreover, in exogenously NIS-transduced tumor tissue after thyroid sequestration, radioiodide absorption in thyroid tissue may be reduced, which in turn leads to a high therapeutic effect of radioiodide in the target tumor cell.
Although no evidence has yet been provided for the common use of external iodide to suppress NIS function in the normal thyroid, pretreatment supplementation KI appears to be a more pragmatic solution for blocking iodide uptake in the thyroid gland for NIS-mediated 131I therapy in extra-thyroidal cancer [13].
Another problem with the empirical activity strategy is the fact that the influence of radioiodine function does not depend directly on the absorbed dose, but depends on various factors such as the homogeneity of radioiodine uptake by the cells, the iodine deficiency of the body before treatment and the TSH level, the excretion rate of radioiodine, and finally the biology of the tumor.
According to the preclinical studies mentioned in this review, the inherent ability of viral vectors to insert their genetic material into host cells has led them to receive special attention, as have adenoviral vectors for NIS-mediated 131I therapy. However, cancer gene therapy with non-replicating adenoviral vectors, based on initial clinical trials, has its limitations. These include high potency immunogenicity, deprivation of non-transduced tumor cells from the therapeutic effects of the vectors, and high incidence of leukemia due to uncontrolled insertion of DNA into the host genome [29].
The use of HSP70B-NIS-MSCs has worked well in most animal studies, including Mariella Tutter's group. However, in some animal studies, the effect was lower due to the less homogeneous response of tumors to hyperthermia. Although this problem is less addressed in clinical studies, as hybrid magnetic resonance therapy with high-intensity focused ultrasound is now used for hyperthermia therapy of tumors. In addition to effective radioiodine therapy through NIS transgenes in non-viral delivery methods such as genetically modified MSCs in the treatment of breast cancer or extracellular vesicles in preclinical hepatocellular carcinoma [13], MSCs have also been shown to trigger or amplify tumors, as demonstrated by a number of studies with ovarian [79], melanoma [80], breast [81], and other tumor cells. In this regard, engineering and administration of immune cells could be an alternative to reduce unwanted side effects of MSCs.
Conclusion
The application of NIS-mediated radionuclide therapy is a rapidly developing field that has shown promising results in extra-thyroidal tissues. Currently, a variety of systemic NIS transfer methods are being investigated in preclinical studies, including viral vectors, engineered MSCs, and polyplex-mediated NIS. NIS as a theranostic gene facilitates spatial and temporal noninvasive imaging, dose optimization, and interpretation following therapeutic radionuclide application. Even though iodide accumulation and retention are constant arguments against effective NIS gene delivery, studies have shown that this level is sufficient to elicit significant antitumor responses. The levels of exogenous expression of NIS by different platforms in the quiescent and/or hypoxic tumor microenvironment is another aspect that needs to be thoroughly investigated in preclinical experiments. Meanwhile, non-invasive imaging techniques such as PET and SPECT have provided a high-resolution and highly sensitive tool to optimize therapeutic regimens.
Abbreviations
- PET:
-
Positron emission tomography
- NIS:
-
Sodium iodide symporter
- hNIS:
-
Human sodium iodide symporter
- BCL2:
-
B-cell lymphoma 2
- MCF -7:
-
Michigan cancer foundation-7
- PI3K:
-
Phosphoinositide 3-kinases
- IO− :
-
Hypoiodite
- at-RA:
-
All-trans retinoic acid
- 99mTcO4 − :
-
99MTc pertechnetate
- 123I:
-
Iodine-123
- Tc:
-
Thyroid cancer
- IHC:
-
Immunohistochemistry
- IFNβ:
-
Interferon beta (IFNβ)
- MV:
-
Measles viruses
- PLLA:
-
Poly-l-lactic acid
- SMAD:
-
Small mothers against decapentaplegic
- DSBs:
-
Double-strand breaks
- KCNQ1:
-
Potassium voltage-gated channel subfamily Q member 1
- KCNH2:
-
Potassium voltage-gated channel subfamily H member 2
- LARG:
-
Leukaemia-associated RhoA-guanine exchange factor
- SPECT:
-
Single photon emission computed tomography
- 131I:
-
Iodine-131
- 188 Re:
-
Rhenium-188
- HDACi:
-
Histone deacetylase inhibitor
- TSH:
-
Thyroid stimulating hormone
- MEK:
-
Mitogen-activated protein kinase
- CT:
-
Computerized tomography
- ATP:
-
Adenosine triphosphate
- At-211:
-
Astatine-211
- 124I:
-
Iodine-124
- TAAs:
-
Tumor-associated antigens
- TERT:
-
Telomerase reverse transcriptase
- VSV:
-
Vesicular stomatitis virus
- LPEI:
-
Linear polyethylenimine
- HuH:
-
Human hepatocellular carcinoma
- AuNCs:
-
Ultrasmall gold nanoclusters
- PEG:
-
Polyethylene glycol
References
De la Vieja A et al (2000) Molecular analysis of the sodium/iodide symporter: impact on thyroid and extrathyroid pathophysiology. Physiol Rev 80(3):1083–1105
Hingorani M et al (2010) The biology of the sodium iodide symporter and its potential for targeted gene delivery. Curr Cancer Drug Targets 10(2):242–267
Dai G, Levy O, Carrasco N (1996) Cloning and characterization of the thyroid iodide transporter. Nature 379(6564):458–460
Ravera S et al (2017) The sodium/iodide symporter (NIS): molecular physiology and preclinical and clinical applications. Annu Rev Physiol 79:261
Tutter M et al (2020) Effective control of tumor growth through spatial and temporal control of theranostic sodium iodide symporter (NIS) gene expression using a heat-inducible gene promoter in engineered mesenchymal stem cells. Theranostics 10(10):4490
Kogai T, Brent GA (2012) The sodium iodide symporter (NIS): regulation and approaches to targeting for cancer therapeutics. Pharmacol Ther 135(3):355–370
Drude N, Tienken L, Mottaghy FM (2017) Theranostic and nanotheranostic probes in nuclear medicine. Methods 130:14–22
Zhao L, Pang A (2017) Iodine-131 treatment of thyroid cancer cells leads to suppression of cell proliferation followed by induction of cell apoptosis and cell cycle arrest by regulation of B-cell translocation gene 2-mediated JNK/NF-κB pathways. Braz J Med Biol Res 50
Lyckesvärd MN et al (2016) Linking loss of sodium-iodide symporter expression to DNA damage. Exp Cell Res 344(1):120–131
Pouget J-P, Georgakilas AG, Ravanat J-L (2018) Targeted and off-target (bystander and abscopal) effects of radiation therapy: redox mechanisms and risk/benefit analysis. Antioxid Redox Signal 29(15):1447–1487
Jadvar H et al (2018) Radiotheranostics in cancer diagnosis and management. Radiology 286(2):388
Marshall SK et al (2022) Anti-EpCAM functionalized I-131 radiolabeled biomimetic nanocarrier sodium/iodide-symporter-mediated breast-cancer treatment. Bioengineering 9(7):294
Wapnir IL et al (2004) The Na+/I− symporter mediates iodide uptake in breast cancer metastases and can be selectively down-regulated in the thyroid. Clin Cancer Res 10(13):4294–4302
Levy O et al (1998) N-linked glycosylation of the thyroid Na+/I− symporter (NIS): implications for its secondary structure model. J Biol Chem 273(35):22657–22663
Riesco-Eizaguirre G, Santisteban P, De la Vieja A (2021) The complex regulation of NIS expression and activity in thyroid and extrathyroidal tissues. Endocr Relat Cancer 28(10):T141–T165
Rathod M et al (2020) FOXA1 regulation turns benzamide HDACi treatment effect-specific in BC, promoting NIS gene-mediated targeted radioiodine therapy. Mol Ther Oncolytics 19:93–104
Riedel C, Levy O, Carrasco N (2001) Post-transcriptional regulation of the sodium/iodide symporter by thyrotropin. J Biol Chem 276(24):21458–21463
Eng PH et al (2001) Regulation of the sodium iodide symporter by iodide in FRTL-5 cells. Eur J Endocrinol 144(2):139–144
Tazebay UH et al (2000) The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med 6(8):871–878
Peyrottes I et al (2009) Immunoanalysis indicates that the sodium iodide symporter is not overexpressed in intracellular compartments in thyroid and breast cancers. Eur J Endocrinol 160(2):215–225
Knostman KA et al (2007) PI3K activation is associated with intracellular sodium/iodide symporter protein expression in breast cancer. BMC Cancer 7(1):1–10
Zhang Z, Beyer S, Jhiang SM (2013) MEK inhibition leads to lysosome-mediated Na+/I-symporter protein degradation in human breast cancer cells. Endocr Relat Cancer 20(2):241–250
De la Vieja A, Santisteban P (2018) Role of iodide metabolism in physiology and cancer. Endocr Relat Cancer 25(4):R225–R245
Gholami S et al (2013) Vaccinia virus GLV-1h153 is a novel agent for detection and effective local control of positive surgical margins for breast cancer. Breast Cancer Res 15(2):1–12
Penheiter AR et al (2010) Sodium iodide symporter (NIS)-mediated radiovirotherapy for pancreatic cancer. AJR Am J Roentgenol 195(2):341
Schmohl KA et al (2017) Imaging and targeted therapy of pancreatic ductal adenocarcinoma using the theranostic sodium iodide symporter (NIS) gene. Oncotarget 8(20):33393
Schipper ML et al (2003) Radioiodide treatment after sodium iodide symporter gene transfer is a highly effective therapy in neuroendocrine tumor cells. Can Res 63(6):1333–1338
Faivre J et al (2004) Long-term radioiodine retention and regression of liver cancer after sodium iodide symporter gene transfer in wistar rats. Can Res 64(21):8045–8051
Schug C et al (2019) Radiation-induced amplification of TGFB1-induced mesenchymal stem cell-mediated sodium iodide symporter (NIS) gene 131I therapyenhanced TGFB1-induced MSC-mediated NIS gene delivery. Clin Cancer Res 25(19):5997–6008
Sieger S et al (2003) Tumour-specific activation of the sodium/iodide symporter gene under control of the glucose transporter gene 1 promoter (GTI-13). Eur J Nucl Med Mol Imaging 30(5):748–756
Müller AM et al (2016) Hypoxia-targeted 131I therapy of hepatocellular cancer after systemic mesenchymal stem cell-mediated sodium iodide symporter gene delivery. Oncotarget 7(34):54795
Willhauck M et al (2008) α-Fetoprotein promoter-targeted sodium iodide symporter gene therapy of hepatocellular carcinoma. Gene Ther 15(3):214–223
Son SH, Gangadaran P, Ahn B-C (2019) A novel strategy of transferring NIS protein to cells using extracellular vesicles leads to increase in iodine uptake and cytotoxicity. Int J Nanomed 14:1779
Trujillo MA et al (2012) A steep radioiodine dose response scalable to humans in sodium-iodide symporter (NIS)-mediated radiovirotherapy for prostate cancer. Cancer Gene Ther 19(12):839–844
Gao X-F et al (2014) Radioiodine therapy for castration-resistant prostate cancer following prostate-specific membrane antigen promoter-mediated transfer of the human sodium iodide symporter. Asian J Androl 16(1):120
Kakinuma H et al (2003) Probasin promoter (ARR2PB)-driven, prostate-specific expression of the human sodium iodide symporter (h-NIS) for targeted radioiodine therapy of prostate cancer. Can Res 63(22):7840–7844
Zhang L et al (2021) Bone marrow mesenchymal stem cell-mediated ultrasmall gold nanoclusters and hNIS gene synergize radiotherapy for breast cancer. J Mater Chem B 9(12):2866–2876
Dwyer RM et al (2011) Mesenchymal stem cell-mediated delivery of the sodium iodide symporter supports radionuclide imaging and treatment of breast cancer. Stem Cells 29(7):1149–1157
Shi S et al (2021) Feasibility of bone marrow mesenchymal stem cell-mediated synthetic radiosensitive promoter-combined sodium iodide symporter for radiogenetic ovarian cancer therapy. Hum Gene Ther 32(15–16):828–838
Scholz I et al (2005) Radioiodine therapy of colon cancer following tissue-specific sodium iodide symporter gene transfer. Gene Ther 12(3):272–280
Urnauer S et al (2017) EGFR-targeted nonviral NIS gene transfer for bioimaging and therapy of disseminated colon cancer metastases. Oncotarget 8(54):92195
Knoop K et al (2015) Mesenchymal stem cell-mediated, tumor stroma–targeted radioiodine therapy of metastatic colon cancer using the sodium iodide symporter as theranostic gene. J Nucl Med 56(4):600–606
Warner SG et al (2019) A novel chimeric poxvirus encoding hNIS is tumor-tropic, imageable, and synergistic with radioiodine to sustain colon cancer regression. Mol Ther Oncol 13:82–92
Riesco-Eizaguirre G et al (2011) Telomerase-driven expression of the sodium iodide symporter (NIS) for in vivo radioiodide treatment of cancer: a new broad-spectrum NIS-mediated antitumor approach. J Clin Endocrinol Metab 96(9):E1435–E1443
Zhao Z et al (2019) Radionuclide imaging and therapy in malignant melanoma after survivin promoter-directed sodium iodide symporter gene transfer in vitro and in vivo. Int J Clin Exp Pathol 12(2):613
Klutz K et al (2009) Targeted radioiodine therapy of neuroblastoma tumors following systemic nonviral delivery of the sodium iodide symporter genesystemic nonviral sodium iodide symporter gene transfer. Clin Cancer Res 15(19):6079–6086
Spellerberg R et al (2021) Selective sodium iodide symporter (NIS) genetherapy of glioblastoma mediatedby EGFR-targeted lipopolyplexes. Mol Ther Oncolytics 23:432–446
Feng F et al (2018) A nonpump function of sodium iodide symporter in thyroid cancer via cross-talk with PTEN signaling. Can Res 78(21):6121–6133
Lacoste C et al (2012) Iodide transporter NIS regulates cancer cell motility and invasiveness by interacting with the rho guanine nucleotide exchange factor LARGNIS enhances cell migration and invasion. Can Res 72(21):5505–5515
Kogai T et al (2000) Induction of follicle formation in long-term cultured normal human thyroid cells treated with thyrotropin stimulates iodide uptake but not sodium/iodide symporter messenger RNA and protein expression. J Endocrinol 167(1):125–136
Kogai T et al (2005) Differential regulation of sodium/iodide symporter gene expression by nuclear receptor ligands in MCF-7 breast cancer cells. Endocrinology 146(7):3059–3069
Unterholzner S et al (2006) Dexamethasone stimulation of retinoic acid-induced sodium iodide symporter expression and cytotoxicity of 131-I in breast cancer cells. J Clin Endocrinol Metab 91(1):69–78
Dohán O, De la Vieja A, Carrasco N (2006) Hydrocortisone and purinergic signaling stimulate sodium/iodide symporter (NIS)-mediated iodide transport in breast cancer cells. Mol Endocrinol 20(5):1121–1137
Kogai T et al (2004) Systemic retinoic acid treatment induces sodium/iodide symporter expression and radioiodide uptake in mouse breast cancer models. Can Res 64(1):415–422
Джикия E et al (2018) Na+/I-cимпopтep (NIS): cтpyктypa, фyнкции, экcпpeccия в нopмe и oпyxoляx. Becтник Poccийcкoгo нayчнoгo цeнтpa peнтгeнopaдиoлoгии Mинздpaвa Poccии 18(1):3
Dupertuis YM et al (2002) Unlabelled iododeoxyuridine increases the rate of uptake of [125I] iododeoxyuridine in human xenografted glioblastomas. Eur J Nucl Med Mol Imaging 29(4):499–505
Shimura H et al (1997) Iodide uptake and experimental 131I therapy in transplanted undifferentiated thyroid cancer cells expressing the Na+/I− symporter gene. Endocrinology 138(10):4493–4496
Spitzweg C et al (2021) The sodium iodide symporter (NIS): novel applications for radionuclide imaging and treatment. Endocr Relat Cancer 28(10):T193–T213
Oneal MJ et al (2012) Characterization of infectivity-enhanced conditionally replicating adenovectors for prostate cancer radiovirotherapy. Hum Gene Ther 23(9):951–959
Keshavarz M et al (2022) Oncolytic virus delivery modulated immune responses toward cancer therapy: challenges and perspectives. Int Immunopharmacol 108:108882
Keshavarz M et al (2020) Virotheranostics, a double-barreled viral gun pointed toward cancer; ready to shoot? Cancer Cell Int 20(1):1–17
Bommareddy PK, Shettigar M, Kaufman HL (2018) Integrating oncolytic viruses in combination cancer immunotherapy. Nat Rev Immunol 18(8):498–513
Miller A, Russell SJ (2016) The use of the NIS reporter gene for optimizing oncolytic virotherapy. Expert Opin Biol Ther 16(1):15–32
Peerlinck I et al (2009) Targeted radionuclide therapy using a Wnt-targeted replicating adenovirus encoding the Na/I symporter. Clin Cancer Res 15(21):6595–6601
Rajecki M et al (2012) SPECT/CT imaging of hNIS-expression after intravenous delivery of an oncolytic adenovirus and 131I. PLoS ONE 7(3):e32871
Russell SJ et al (2014) Remission of disseminated cancer after systemic oncolytic virotherapy. In: Mayo clinic proceedings. Elsevier
Naik S et al (2018) Comparative oncology evaluation of intravenous recombinant oncolytic vesicular stomatitis virus therapy in spontaneous canine cancercomparative oncology informs development of oncolytic VSV. Mol Cancer Ther 17(1):316–326
Peng L, Wagner E (2019) Polymeric carriers for nucleic acid delivery: current designs and future directions. Biomacromol 20(10):3613–3626
Freitag F, Wagner E (2021) Optimizing synthetic nucleic acid and protein nanocarriers: the chemical evolution approach. Adv Drug Deliv Rev 168:30–54
Urnauer S et al (2016) Sequence-defined cMET/HGFR-targeted polymers as gene delivery vehicles for the theranostic sodium iodide symporter (NIS) gene. Mol Ther 24(8):1395–1404
Nombela-Arrieta C, Ritz J, Silberstein LE (2011) The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol 12(2):126–131
Zhang X et al (2022) The role of mesenchymal stem cells in the occurrence, development, and therapy of hepatocellular carcinoma. Cancer Med 11(4):931–943
Assadi M et al (2011) Nanotechnology and nuclear medicine; research and preclinical applications. Hell J Nucl Med 14(2):149–159
Tafreshi NK et al (2019) Development of targeted alpha particle therapy for solid tumors. Molecules 24(23):4314
Maxon HR et al (1983) Relation between effective radiation dose and outcome of radioiodine therapy for thyroid cancer. N Engl J Med 309(16):937–941
, M.D.e.r.N. (1975) Summary of current radiation dose estimates to humans from 123I, 124I, 125I, 126I, 130I, 131I, and 132I as sodium iodide. J Nucl Med 16: 857–860
Spitzweg C et al (2001) In vivo sodium iodide symporter gene therapy of prostate cancer. Gene Ther 8(20):1524–1531
Abd-Aziz N, Poh CL (2021) Development of oncolytic viruses for cancer therapy. Transl Res 237:98–123
Spaeth EL et al (2009) Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE 4(4):e4992
Petrie Aronin CE, Tuan RS (2010) Therapeutic potential of the immunomodulatory activities of adult mesenchymal stem cells. Birth Defects Res Part C Embryo Today Rev 90(1):67–74
Rhodes LV et al (2010) Adult human mesenchymal stem cells enhance breast tumorigenesis and promote hormone independence. Breast Cancer Res Treat 121(2):293–300
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Darabi, N., Keshavarz, M., Nabipour, I. et al. The sodium iodide symporter (NIS) as theranostic gene: potential role in pre-clinical therapy of extra-thyroidal malignancies. Clin Transl Imaging 11, 113–125 (2023). https://doi.org/10.1007/s40336-023-00540-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-023-00540-0