Abstract
Introduction
Despite the significant research activity in the design and validation of new PSMA-targeting agents, prostate cancer (PCa) remains the second most common cancer in men worldwide. PSMA-specific labeled monoclonal antibodies (mAbs) demonstrated a discrete effectiveness in the clinic, but with some drawbacks due to their large size. To circumvent these problems, mAbs-derived fragments have been investigated, since they retain the high affinity of the parent mAb for the target, being also endowed with a more favorable pharmacokinetics. This review focuses on the single-chain variable fragment D2B (scFvD2B) potentiality as a new prostate-specific membrane antigen (PSMA)-specific molecular vector in nuclear medicine (NM) applications for both diagnosis and treatment of PCa.
Methods
A critical review of PubMed and Web of Science (including MEDLINE) in the early 2019 was performed, searching for research articles focusing on the application of the fragment scFvD2B and the parent antibody IgGD2B in preclinical NM.
Results
The scFvD2B, which is derived from one of the most promising PSMA-specific mAbs, IgGD2B, has been recently investigated and labeled with Indium-111, Iodine-131, and Iodine-123. Overall, scFvD2B showed a great potential in the preclinical setting, demonstrating a promising pharmacokinetics, especially in terms of high stability and specificity, efficiently accumulating in PSMA-expressing PCa tumors.
Conclusion
scFvD2B seems to be a promising fragment as a molecular vector in NM applications. Nevertheless, further investigations, especially with radiometal-labeled scFvD2B, are necessary to better characterize and optimize the unique properties of this fragment, providing the basis for a rapid translation into the clinic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the second most frequent cancer and the third leading cause of cancer death for adult men worldwide [1]. Radical prostatectomy and local radiotherapy are largely successful for patients with localized cancer. About 50% of cases are diagnosed at a locally advanced stage, and about 30% have bone metastases at the time of diagnosis. Currently, the standard of care for metastatic PCa is androgen deprivation therapy (ADT), but despite initial responses, almost all patients progress to castration-resistant prostate cancer with a poor prognosis. Relapses after primary treatment of PCa occur, depending on initial tumor stages, from 10 to 53% [2]. A biochemical recurrence of PCa is currently evaluated by magnetic resonance imaging (MRI) and conventional imaging (i.e., computed tomography (CT) and bone scan). In the last 10 years, fluorodeoxyglucose and choline-based tracers have been used for positron emission tomography (PET) imaging for the detection of recurrence of PCa. However, all the abovementioned imaging modalities have showed some issues due to the limited diagnostic performance especially in the early stages, along with low specificity (Table 1) [3, 4]. Consequently, new target-specific tools for both early detection and therapy of PCa are imperatively needed.
One of the most outstanding biomarkers for early detection of PCa is the prostate-specific membrane antigen (PSMA), a type II membrane glycoprotein of 750 amino acids (MW = 120 kDa) whose overexpression in PCa is proportional to the stage and grade of the disease, regardless of androgen status. PSMA is also constitutively expressed by secretory cells within the prostatic epithelium, and found in proximal renal tubules and salivary glands. On the other hand, PSMA expression in cancer cells increases 100- to 1000-fold in all stages of PCa lesions, and in metastatic, recurrent, and hormone-refractory disease, rendering its expression level as a significant indicator for disease outcome [3, 11]. Therefore, PSMA is an ideal antigen for imaging and therapy based on monoclonal antibodies (mAbs) due to its large extracellular portion, which offers several available binding sites that enables specific targeting. In addition, it has been reported that PSMA, like the majority of membrane receptors, undergoes constitutive internalization after ligand binding [12], resulting in a very efficient uptake, deposit, and retention of the ligand into the cell, a prodromal characteristic for the success of imaging and therapeutic radiopharmaceuticals. Therefore, many efforts have been undertaken to develop high-affinity PSMA-specific ligands to be exploited in nuclear medicine (NM). The first agent to enter the clinic was the mAb ProstaScint®, a murine antibody labeled with indium-111 (111In; 111In-capromab pendetide, EUSA Pharma) for single photon emission tomography (SPECT) scan, approved by the US Food and Drug Administration (FDA) in 1996. ProstaScint recognizes an intracellular epitope of the receptor, and consequently it is able to visualize only apoptotic or necrotic PCa cells, leading to low sensitivity and poor clinical performances [13]. Subsequently, new mAbs targeting the extracellular portion of PSMA have been developed. Among them, one of the most promising is the humanized mAb J591, labeled with 111In for imaging purposes and Yttrium-90 (90Y) or Lutetium-177 (177Lu) for therapeutic applications. However, different cases of grade IV thrombocytopenia and neutropenia have been reported for 177Lu-J591, as probably the result of the slow renal clearance of the mAb, with consequent prolonged circulatory half-life and radiation exposure of the bone marrow [14]. Recently, Colombatti et al. developed, using conventional hybridoma technology, a new murine mAb (IgGD2B) targeting an extracellular epitope of the human PSMA [15]. However, IgGD2B presents the same drawbacks of all full mAbs employed in cancer imaging and therapy. Indeed, their large size is accountable for the slow blood clearance, high unspecific background activity, and poor tumor penetrability and accumulation. Therefore, peptides, small-molecule PSMA inhibitors (i.e., PSMA-11 and PSMA-617), and smaller mAb derivatives have been tested. Recently, the IgGD2B derivatives such as F(ab′)2, Fab [16], and single-chain variable fragment (scFvD2B) [17] have been investigated in preclinical models.
This review will provide a detailed account of scFvD2B potentiality as a new PSMA-specific molecular vector in NM applications.
scFvD2B production strategies and in vitro characterization
The scFvD2B was originally obtained by cloning the variable heavy (VH) and variable light (VL) chains genes of IgGD2B into a phagemidic vector, produced in a prokaryotic system (E. Coli) and opportunely purified. ScFvD2B is composed of a V segment of the VK1 family and a VH belonging to the VH3 family, which were covalently connected through a flexible (Gly4-Ser)3 peptide linker. The overall yield of scFvD2B with this method is high (12–14 mg/L), also probably due to a very stable pairing of the variable chains. The purified product consisted of a 28 kDa molecule principally in the form of monomers, with a very low tendency to aggregate, with dimers representing only 5–8% of the total amount [17].
The binding specificity of scFvD2B against the extracellular domain of PSMA was assessed qualitatively by both flow cytometry on PSMA-positive (LnCaP, PC3-PIP, MCF7-hPSMA, CHO-hPSMA) and PSMA-negative (PC3, MCF7, CHO) human cancer cell lines, and by immunohistochemistry on tumor surgical specimen. Of note, scFvD2B demonstrated to be highly specific for PSMA since it did not cross react with other proteins expressed on the cell surface of cancer cells or healthy tissues surrounding PSMA-positive tumors. The high specificity of scFvD2B for PSMA is also associated with a high affinity for the receptor, as attested in a comparative surface plasmon resonance (BIAcore 2000) assay using IgGD2B and J591 antibodies. Indeed, despite its monovalent binding, the fragment retains the majority of the parent antibody binding strength and it is characterized also by a slow dissociation constant (Koff = 2.24 mM) theoretically suitable for binding stability in vivo. The calculated affinity constant (Kd) for scFvD2B is 8.6 nM, which is about 20 times lower than that of IgGD2B, but it is similar to the Kd of the clinically investigated J591 mAb [18] as well as PSMA inhibitors, particularly all the precursors of PSMA-617 [11]. In addition, these data evidenced that the extracellular epitope of PSMA recognized by IgGD2B and scFvD2B differs from that of J591 [17]. Nevertheless, the authors did not report the exact sequence of this epitope.
Since, as discussed above, ideal vectors to be exploited in NM should be internalized by target cells, once demonstrated scFvD2B PSMA-binding specificity, its internalization by PSMA-expressing cancer cells has also been investigated in comparison with the full-length parent mAb. Indeed, the kinetic of internalization of IgGD2B and its fragment is comparable and characterized by a fast intracellular accumulation already detectable after 5 min of vector–receptor interaction, with a plateau at 2 h [17]. Nevertheless, the uptake of scFvD2B by PSMA-positive cells is lower than that of IgGD2B, but still quite high, with 40% of bound scFvD2B-PSMA internalized after 2 h.
ScFvs are, in general, relatively unstable molecules. However, both resistance to proteases, an essential characteristic for in vivo applications, and pH/storage stability have been assessed for scFvD2B, with excellent results. Moreover, neither incubation for 6 h in mouse sera and for 24 h in human sera, nor incubation up to 48 h at 37 °C in complete medium or storage for 18 months at – 20 °C affected scFvD2B integrity and binding activity [17].
Since scFvs are not glycosylated, they should be easily produced in prokaryotic cells and produced at affordable costs, as also in the case of scFvD2B. However, in the roadmap for characterization of a clinical-grade reagent, a good manufacturing practice (GMP) eukaryotic production is more suitable. Therefore, scFvD2B production in such conditions has also been investigated as an alternative to E. coli strategy [19]. GMP scFvD2B from the eukaryotic system (hereafter referred to scFvD2B*) was produced and then purified starting from a clone in which the Myc and His tags had been removed, with regard to the directions of the regulatory agency. The product was biologically and chemically characterized in comparison to the prokaryotic-produced scFvD2B. Interestingly, the two products differ in terms of molecular weight (scFvD2B = 29,095 Da and scFvD2B* = 26,361 Da) and isoelectric point (scFvD2B = 7.88 and scFvD2B* = 8.80), while instead scFvD2B* maintained similar binding characteristics, kinetics of internalization, and biochemical stability of the scFvD2B counterpart [19].
Radiolabeling of scFvD2B
First radiolabeling and in vivo biodistribution studies were performed using scFvD2B produced in prokaryotic systems. The fragment was initially labeled with 111In and Iodine-131 (131I) [17]. Labeling with 111In was achieved by conjugating the fragment to the bifunctional chelating agent 2-(pisothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triaceticacid (Bz-NOTA) prior to the labeling, whereas the 131I-radioiodination was accomplished using iodogen. The procedures followed to obtain and characterize the radioimmunoconjugates 111In-scFvD2B and 131I-scFvD2B were essentially as those previously described, as indicated by the authors [20, 21]. In both cases, results in terms of labeling efficiency, radiochemical purity, structural integrity after labeling, and immunoreactivity of the radiolabeled products on PSMA positive vs. PSMA negative cells (LNCaP and PC3-PIP vs. PC3 and A431) were excellent, with overall minor differences favoring the radioiodinated derivative [17, 22]. The stability of the two radiolabeled products was also assessed using the same conditions investigated for unlabeled scFvD2B, as reported above. In addition, it must be pointed out that the labeling methods investigated for scFvD2B did not affect its ability to recognize PSMA nor its internalization properties [17], suggesting that the fragment might be investigated also for other radiolabeling methods. Furthermore, since 131I (half-life-t1/2 of 8 days) is not an optimal radionuclide for imaging due to high-energy γ-photon and the concomitant β emission that are detrimental for image quality and radiation dosimetry, Iodine-123 (123I, t1/2 = 13.22 h and a predominant gamma emission of 159 keV) has been considered. 123I radiolabeling procedures were investigated for scFvD2B and scFvD2B* [19]. Interestingly, the radioiodination for scFvD2B* required some modifications as compared to the scFvD2B counterpart (e.g., protein concentration, radiolabeling buffer), resulting in a very variable radiolabeling efficiency (from 35.7 to 83.5%). Nevertheless, a radiochemical purity > 95% of the final product was achievable by size-exclusion chromatography [19].
In vivo preclinical biodistribution and imaging studies
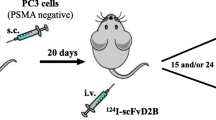
Preliminary biodistribution studies by Frigerio et al. were conducted using fluorophore-labeled IgGD2B highlighting the high specificity of scFvD2B for PSMA, with an emission peak at 3 h post-injection only in PSMA-expressing tumors. Thus, despite its rapid clearance from the blood pool, the strength of binding of the antibody fragment seemed to be sufficient to have a rapid localization in target tissues [17]. Such promising pharmacokinetics were maintained also in the case of radiolabeled scFvD2B, with some modifications [19, 22]. 111In- and 131I-labeled scFvD2B injected in male CD1 nude mice bearing PC3-PIP (PSMA positive) and PC3 (PSMA negative) tumors showed a favorable uptake in PSMA-positive tumors [22]. The maximum tumor uptakes of both radiolabeled scFvD2B were much lower than that observed for 111In-labeled IgGD2B and its Fab-derived fragments [16]. Nevertheless, they have a faster accumulation in the tumor with a maximum only after 3 h post-injection (p.i.) with 0.96 ± 0.28%ID/g (percentage of the injected dose per gram of tissue) and 2.09 ± 0.73%ID/g for 111In-scFvD2B and 131I-scFvD2B, respectively (Table 2), than 111In-labeled IgGD2B and its Fab-derived fragments (3–7 days and 24 h, respectively). Both the abovementioned characteristics (low tumor uptake and fast accumulation) were expected due to the smaller size of scFvD2B. The best tumor-to-blood ratio, it means the best condition for imaging scan, was observed at 24 h and 15 h p.i. for 111In-scFvD2B and 131I-scFvD2B, respectively [22]. Interestingly, while scFvD2B, regardless of the nuclide used for radiolabeling, localized preferentially in PSMA-expressing tumors, 111In-scFvD2B exhibited significantly higher background level in case of PSMA-negative or, better to say, low-expressing PSMA tumors [23]. The consequence of the higher background for 111In-scFvD2B is the highest accumulation also in the tumor. Indeed, the very interesting data from the comparison of two radiolabeled scFvD2B products concern the accumulation in the kidneys. Renal accumulation of the radiolabeled compounds may become a critical problem in therapy due to unsafe healthy organ dosimetry and in imaging, since a high accumulation in these organs can preclude the localization of closed metastases. 111In-labeled (Fab′)2 and Fab fragments of D2B showed a tremendously high accumulation in these organs, unlike the entire antibody 111In-IgGD2B [16]. Unfortunately, this behavior was observed also for 111In-scFvD2B, for which the renal uptake at 24 h p.i. was two orders of magnitude higher than the tumor uptake (Table 2). On the contrary, for 131I-scFvD2B, the renal accumulation was remarkably reduced with optimal tumor-to-kidney ratios from 15 h p.i. The authors hypothesize that this significant difference between the two compounds might be ascribed to a different intracellular metabolism that the two labeled fragments might undergo, as observed in other comparative studies [24, 25]. Thus, 131I-scFvD2B might be rapidly degraded to monoiodotyrosine and to other small catabolites at the lysosome level, and such catabolites should be then rapidly excreted from the renal cells; radiometal-labeled proteins, such as in this case 111In-scFvD2B, instead, might be degraded to small 111In-catabolites which remain trapped inside the renal cells. This occurrence may explain also the overall low backgrounds for liver, spleen, and the low-expressing PSMA PC3 tumor characterizing 131I-scFvD2B in comparison with the 111In-analogous. Thus, it seems that an iodine-labeled scFvD2B might be a promising agent for prostate cancer with NM techniques. However, it is suitable for therapy, but suboptimal for diagnostic imaging. Therefore, other iodine isotopes could be considered, such as 123I for SPECT or Iodine-124 for PET imaging. Frigerio and colleagues [19] evaluated the tumor-targeting properties of 123I-scFvD2B in preclinical study using different PSMA positive and negative tumor xenograft models. Clearance data were comparable for 123I- and 131I-scFvD2B. A preferential uptake was found for mice bearing a PSMA-positive tumor. The biodistribution studies were performed by blood and tissue samples, testing them with gamma counter and SPECT/CT imaging. Data showed a specific and significant uptake of the radiolabeled molecules, both for ex vivo and in vivo by SPECT/CT imaging biodistribution. The most favorable percentage of the injected dose per gram of tissue was found after 9 h p.i.; the uptake in PSMA positive tumors was significantly higher than in blood, kidney, and PSMA-negative tumors. Furthermore, after 24 h, no accumulation of the 123I-scFv2DB was reported in blood and in kidneys (Table 2), while it remained high in PSMA-positive tumors. Comparison of blood and tumor clearance of 123I-scFvD2B with previously obtained data of 131I-scFvD2B [22] suggested an improvement due to a clear trend toward a shorter circulatory half-life and a longer retention in PSMA-positive tumors.
Discussion and conclusion
From the currently available literature, it emerges that the radiolabeled scFvD2B has different advantages. First, it has a high target specificity, an essential requirement for a radiopharmaceutical, since it minimizes the unnecessary radiation exposure to the body during imaging or therapy. The scFvD2B demonstrated both in vitro and in vivo in the preclinical setting a high specificity for PSMA, with a Kd comparable not only to other commercially available anti-PSMA mAbs [18], but also with the promising small molecular inhibitors PSMA-617, PSMA-11 [11] or iPSMA [23]. Second, the fragment demonstrated an excellent resistance to proteases and pH/storage stability, essential requirements for in vivo applications. Third, it is simple to produce both in prokaryotic and eukaryotic cells, with an acceptable cost. Preclinical studies are mainly focused on iodine-labeled based strategies. As pointed out by the authors themselves [22], radioiodination of scFvD2B seems to be more advantageous in terms of rapid clearance of the fragment, which avoids kidney accumulation and leads to an optimal tumor-to-kidney and tumor-to-background ratios. The same results were not obtainable when scFvD2B was labeled with a radiometal such as 111In [22]. Nevertheless, to our opinion, the bifunctional chelator chosen, Bz-NOTA, is not ideal for this radiometal [26], thus, the high uptake observed in mouse spleen, liver, and kidneys might be due to the circulating free/unchelated 111In rather than 111In-scFvD2B itself [27]. Nevertheless, accumulation in the kidneys was observable also for 111In-labeled F(ab′)2 and Fab fragments, but not for the entire IgGD2B, even when a more proper chelating system is employed (p-isothiocyanatobenzyl-diethylenetriaminepentaacetic acid, ITC-DTPA). This evidence, again, points out the inherent problems of kidney accumulation of radiometal-labeled proteins. Of note, labeling chemistry exerts a profound influence on the targeting and biodistribution properties of proteins, and especially the choice of chelators can alter radically their blood clearance, excretion pathways, and kidney accumulation [26, 28]. Thus, on one hand, it is possible to take advantage from the choice of a chelating system and a linker to maximize tumor uptake and minimize accumulation in non-target organs. On the other hand, various chemical and radiochemical factors must be considered, making this goal difficult to achieve [26, 29, 30]. The increase in the use of radiometals for labeling to mAbs, mAb-derived fragments, and peptides is largely due to their longer retention in tumors than iodinated counterparts. Therefore, different strategies for enhancing 111In-labeling procedures should be investigated, but also other radiometals for imaging, therapeutic, and theranostic purposes should be considered. Indeed, not only the labeling chemistry, as mentioned above, but also the radionuclide influences the pharmacokinetics and biodistribution properties of a protein/peptide [31]. Therefore, we suggest to further investigate the radiometals in the future. For example, on one hand, Copper-64 would be employed for its theranostic characteristics, able to evaluate both diagnostic and therapeutic properties of the fragment using the same radioisotope, but with different dosages. On the other hand, the employment of Gallium-68 (68Ga) and Technetium-99m for the diagnostic purpose would have the advantages of both a simple and a rapid synthesis in all NM departments. Finally, Zirconium-89, 90Y and Lutetium-177 (177Lu) for the radiolabeling of scFvD2B have the advantage to promote the therapeutic indication of the agent. By summarizing all the abovementioned considerations, kidney being the critical organ for the scFvD2B labeled with 123I, 131I, and 111In, probably the radiolabeling with 177Lu should require a specific premedication with amino acid, but in combination with 68Ga would be the simplest way to test the diagnostic and therapeutic properties of scFvD2B. Of note, we have recently designed a first-in-human trial to assess, in the foreseeable future, the biodistribution and safety profile of radiometal-labeled scFvD2B in cancer patients.
Overall, scFvD2B has been showing in the preclinical setting promising results for targeting PSMA-expressing PCa, due to its stability, high specificity, and favorable pharmacokinetics. In view of future imaging and therapeutic applications in NM, a careful attention should be paid especially on the chelate radiochemistry that is behind the labeling of scFvD2B with radiometals. In addition, besides PCa, PSMA is also expressed in the neovasculature of a wide variety of solid tumors, extending the range of future investigations of the applicability of scFvD2B in all this type of cancers.
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Han M, Partin AW (2001) Current clinical applications of the In-capromab Pendetide scan (ProstaScint(R) scan, Cyt-356). Rev Urol 3:165–171
von Eyben FE, Roviello G, Kiljunen T et al (2018) Third-line treatment and 177Lu-PSMA radioligand therapy of metastatic castration-resistant prostate cancer: a systematic review. Eur J Nucl Med Mol Imaging 45:496–508. https://doi.org/10.1007/s00259-017-3895-x
Evangelista L, Cuppari L, Zattoni F et al (2019) The future of choline PET in the era of prostate specific membrane antigen. Q J Nucl Med Mol Imaging 63:19–28. https://doi.org/10.23736/S1824-4785.18.03062-5
Evangelista L, Guttilla A, Zattoni F et al (2013) Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur Urol 63:1040–1048. https://doi.org/10.1016/j.eururo.2012.09.039
Fanti S, Minozzi S, Castellucci P et al (2016) PET/CT with (11)C-choline for evaluation of prostate cancer patients with biochemical recurrence: meta-analysis and critical review of available data. Eur J Nucl Med Mol Imaging 43:55–69. https://doi.org/10.1007/s00259-015-3202-7
Evangelista L, Zattoni F, Gutilla A et al (2013) Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clin Nucl Med 38:305–314. https://doi.org/10.1097/RLU.0b013e3182867f3c
Ren J, Yuan L, Wen G et al (2016) The value of anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT in the diagnosis of recurrent prostate carcinoma: a meta-analysis. Acta Radiol 57:487–493. https://doi.org/10.1177/0284185115581541
Kim SJ, Lee SW, Ha HK (2019) Diagnostic performance of radiolabeled prostate-specific membrane antigen positron emission tomography/computed tomography for primary lymph node staging in newly diagnosed intermediate to high-risk prostate cancer patients: a systematic review and meta-analysis. Urol Int 102:27–36. https://doi.org/10.1159/000493169
Perera M, Papa N, Christidis D et al (2016) Sensitivity, specificity, and predictors of positive 68Ga–prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol 70:926–937. https://doi.org/10.1016/j.eururo.2016.06.021
Gourni E, Henriksen G (2017) Metal-based PSMA radioligands. Molecules 22:523. https://doi.org/10.3390/molecules22040523
Liu H, Rajasekaran AK, Moy P et al (1998) Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res 58:4055–4060
Wilkinson S, Chodak G (2004) The role of 111indium-capromab pendetide imaging for assessing biochemical failure after radical prostatectomy. J Urol 172:133–136. https://doi.org/10.1097/01.ju.0000132138.02846.08
Calopedos RJS, Chalasani V, Asher R et al (2017) Lutetium-177-labelled anti-prostate-specific membrane antigen antibody and ligands for the treatment of metastatic castrate-resistant prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 20:352–360. https://doi.org/10.1038/pcan.2017.23
Colombatti M, Grasso S, Porzia A et al (2009) The prostate specific membrane antigen regulates the expression of IL-6 and CCL5 in prostate tumour cells by activating the MAPK pathways. PLoS One 4:e4608. https://doi.org/10.1371/journal.pone.0004608
Lütje S, van Rij CM, Franssen GM et al (2015) Targeting human prostate cancer with 111In-labeled D2B IgG, F(ab′)2 and Fab fragments in nude mice with PSMA-expressing xenografts. Contrast Media Mol Imaging 10:28–36. https://doi.org/10.1002/cmmi.1596
Frigerio B, Fracasso G, Luison E et al (2013) A single-chain fragment against prostate specific membrane antigen as a tool to build theranostic reagents for prostate cancer. Eur J Cancer 49:2223–2232. https://doi.org/10.1016/j.ejca.2013.01.024
Smith-Jones PM, Vallabahajosula S, Goldsmith SJ et al (2000) In vitro characterization of radiolabeled monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen. Cancer Res 60:5237–5243
Frigerio B, Franssen G, Luison E et al (2017) Full preclinical validation of the 123I-labeled anti-PSMA antibody fragment scFvD2B for prostate cancer imaging. Oncotarget 8:10919–10930. https://doi.org/10.18632/oncotarget.14229
Coliva A, Zacchetti A, Luison E et al (2005) 90Y Labeling of monoclonal antibody MOv18 and preclinical validation for radioimmunotherapy of human ovarian carcinomas. Cancer Immunol Immunother 54:1200–1213. https://doi.org/10.1007/s00262-005-0693-2
Figini M, Martin F, Ferri R et al (2009) Conversion of murine antibodies to human antibodies and their optimization for ovarian cancer therapy targeted to the folate receptor. Cancer Immunol Immunother 58:531–546. https://doi.org/10.1007/s00262-008-0575-5
Frigerio B, Benigni F, Luison E et al (2015) Effect of radiochemical modification on biodistribution of scFvD2B antibody fragment recognising prostate specific membrane antigen. Immunol Lett 168:105–110. https://doi.org/10.1016/j.imlet.2015.09.012.105-110
Hernández-Jiménez T, Ferro-Flores G, Ocampo-Garcia B et al (2018) 177Lu-DOTA-HYNIC-Lys(Nal)-Urea-Glu: synthesis and assessment of the ability to target the prostate specific membrane antigen. J Radioanal Nucl Chem 318:2059–2066. https://doi.org/10.1007/s10967-018-6239-9
Press OW, Shan D, Howell-Clark J et al (1996) Comparative metabolism and retention of iodine-125, yttrium-90, and indium-111 radioimmunoconjugates by cancer cells. Cancer Res 56:2123–2129
Geissler F, Anderson SK, Venkatesan P et al (1992) Intracellular catabolism of radiolabeled anti-µ antibodies by malignant B-cells. Cancer Res 52:2907–2915
Price EW, Orvig C (2014) Matching chelators to radiometals for radiopharmaceuticals. Chem Soc Rev 43:260–290. https://doi.org/10.1039/c3cs60304k
Watanabe N, Oriuchi N, Endo K et al (1999) Localization of indium-111 in human malignant tumor xenografts and control by chelators. Nucl Med Biol 26(7):853–858. https://doi.org/10.1016/S0969-8051(99)00057-8
Strand J, Honarvar H, Perols A et al (2013) Influence of macrocyclic chelators on the targeting properties of 68Ga-labeled synthetic affibody molecules: comparison with 111In-labeled counterparts. PLoS One 8:e70028. https://doi.org/10.1371/journal.pone.0070028
Akizawa H, Uehara T, Arano Y (2008) Renal uptake and metabolism of radiopharmaceuticals derived from peptides and proteins. Adv Drug Deliv Rev 60:1319–1328. https://doi.org/10.1016/j.addr.2008.04.005
Li L, Olafsen T, Anderson AL et al (2002) Reduction of kidney uptake in radiometal labeled peptide linkers conjugated to recombinant antibody fragments. Site-specific conjugation of DOTA-peptides to a Cys-diabody. Bioconjug Chem 13:985–995. https://doi.org/10.1021/bc025565u
Tolmachev V, Stone-Elander S (2010) Radiolabelled proteins for positron emission tomography: pros and cons of labelling methods. Biochim Biophys Acta 1800:487–510. https://doi.org/10.1016/j.bbagen.2010.02.002
Funding
No funds were used for this review manuscript.
Author information
Authors and Affiliations
Contributions
DC: content planning, literature search and review, manuscript writing, and editing. AZ: manuscript writing. LE: manuscript writing and content planning. NS: content planning, manuscript writing, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
No animals or humans were involved.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Carpanese, D., Zorz, A., Evangelista, L. et al. Targeting prostate cancer with the anti-PSMA scFvD2B: a theranostic promise for nuclear medicine. Clin Transl Imaging 7, 295–301 (2019). https://doi.org/10.1007/s40336-019-00337-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-019-00337-0