Abstract
Purpose
Lymphoscintigraphy is the imaging of choice in diagnosis of lymphedema. Diagnosis is made on the basis of qualitative assessment of tracer distribution at specified time points. A sound knowledge of the anatomy and function of the lymphatic system combined with understanding of tracer propagation and distribution through the lymphatic system is necessary for accurate diagnosis of lymphedema.
Technique and image findings
Lymphoscintigraphy at Aga Khan University Hospital (AKUH) is performed by intradermal injection of Tc 99m nanocolloid with planar imaging performed at 5, 15 min and 1½ h for reproducibility. Images are analyzed mainly by qualitative parameters that relate to tracer kinetics within the lymphatic system. We describe different image appearances which represent the various patterns of tracer distribution in normal lymphatics and with lymphedema.
Conclusion
Lymphoscintigraphy is a highly accurate and reproducible technique for the evaluation of the lymphedema. A thorough knowledge of the various imaging appearances on lymphoscintigraphy is necessary for proper interpretation of images. Addition of quantitative parameters would increase the accuracy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lymphedema is the painless progressive accumulation of protein-rich fluid in the interstitial spaces of the skin, resulting from an anatomic or functional obstruction of the lymphatic system [1, 2]. It is a chronic disease of the lymphatic system which can cause severe incapacitating swelling of the extremities [2, 3]. Lymphedema is most common in the lower limb, about 80% of cases, but can also occur in the arms, face, trunk, and external genitalia.
Pathology and clinical presentation
The primary pathology leading to lymphedema is dysfunction of the lymphatic transportation system [4]. Disruption of the lymphatic systems by pathological processes such as trauma, surgery and radiotherapy, infection, and congenital abnormalities can lead to lymphedema [5]. The disorder typically affects the dermis and spares the deeper compartments. The dermis has abundant lymphatic capillaries, forming superficial and deep plexuses [6,7,8].
Primary lymphedema is usually as a result of congenital abnormalities in the lymphatic system which can be either aplasia or hypoplasia [9, 10]. There are two main forms of primary lymphedema: lymphedema praecox (early onset, usually unilateral and before 35 years of age) and lymphedema tarda (late onset). Milroy disease is the autosomal dominant form of primary lymphedema with a very early age of onset. It is due to agenesis of the lymphatic system and is typically bilateral [11]. Valvular incompetence is a very rare cause of primary lymphedema [12].
Secondary lymphedema occurs as a result of obstruction or interruption of the normal lymphatic channels secondary to surgery and/or radiotherapy, trauma, or infection. In sub-Saharan Africa and Southeast Asia, filariasis is the most common cause of secondary lymphedema [13].
Lymphedema staging
Stage 1 is reversible lymphedema, with pitting edema and swelling that decreases when the limb is elevated, and stage 2 is non-pitting edema that does not decrease when the limb is elevated. Stage 3 is lymphostatic elephantiasis, with grossly swollen limb and hardened skin [14].
Clinical manifestations include persistent edema of > 3 months duration, minimal response to overnight elevation or diuretics, and the presence of skin changes such as thickened skin, hyperkeratosis and papillomatosis indicating early elephantiasis.
Lymphoscintigraphy technique and image interpretation
Lymphoscintigraphy is now one of the primary imaging modalities used in determining a diagnosis in patients with suspected extremity lymphedema [9, 10, 15], as it has been proved to be reliable and reproducible. The study is noninvasive with no known adverse effects [1, 16]. Careful attention to technical performance and image evaluation is essential to maximize the clinical utility of the investigation [5]. The radiation dose received during the examination is low (effective dose of 0.04–0.08 mSv after an injection of 20–40 MBq of 99mTc nanocolloid) and the study can be repeated after therapy [1].
The current protocol in Aga Khan University Hospital (AKUH) utilizes 20–40 MBq 99mTc-nanocolloid, injected intradermally at the first interdigital space of both limbs, regardless of whether edema is unilateral or bilateral, with static image acquisition at 5, 15 min and 1.5 h intervals. 99mTc-nanocolloid has an optimal particle size and neutral pH, which following intradermal injection allows better and faster visualization of the superficial compartment lymphatic pathways and does not cause a burning sensation [2, 7]. Other radiotracers which can be used include macromolecules such as Dextran-99mTc, which is less optimal for lymph node imaging. On the other hand, other colloids such as 99mTc-(human serum albumin) HSA microcolloid and 99mTc-sulfur colloid have larger particle size which results in slower transit. Field of view is up to the lower abdomen for the 15 min and 1.5 h images. The liver and spleen are included in the field of view in the 15 min image to detect any intravasation. The patients walk for 5 min before the 15 min image. Two minutes of muscular exercise has been shown to counteract the increased gradient pressure of the lymphatic system, accelerating lymph drainage [7].
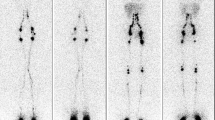
In patients with normal lymphatic anatomy and function, a predictable sequence is seen on lymphoscintigraphy. Symmetric migration of the radionuclide is seen through discrete lymph vessels (three to five lymph vessels per calf and one to two per thigh) [1, 13] (Figs. 1, 2). Bilateral visualization of ilioinguinal lymph nodes occurs within 1 h [2].
43-Year-old female. Bilateral lower limb swelling for more than 10 years. Normal passage of tracer was demonstrated. Some tracer in the bladder is noted present small amount of unbound Tc99m. Some crossover lymphatics also thought to exist with communication of the left and right ilioinguinal nodal groups
On lymphoscintigrams with abnormal findings, a variety of findings can be identified including interruption of lymphatic flow, collateral lymph vessels, progressive dermal and flow, delayed flow, delayed visualization or non-visualization of lymph nodes, a reduced number of lymph nodes, dilated lymphatics and in severe cases non-visualization of the lymphatic system at all [1] (Figs. 3, 4, 5, 6, 7, 8). Dermal backflow, also referred to as dermal flow or dermal forward-flow, reflects the increased numbers of abnormally dilated lymphatic capillaries. Visualization of popliteal nodes in the setting of lymphedema occurs as a result of recruitment of the deep lymphatics due to insufficiency of the superficial lymphatics. Interruption of the lymphatic channels with or without formation of lymphocoele implies a direct trauma to the lymphatic channels. Purely qualitative analysis has been reported to be very accurate for confirming or excluding the diagnosis of lymphedema, with a sensitivity as high as 92% and a specificity as high as 100% [4].
14-Year-old female with an 8-month history of left lower limb swelling; noted history of treatment for a parasitic infection with improvement, but persistent swelling. Tracer migration to the left inguinal lymph nodes was clearly reduced and delayed in this patient. Visualization of a popliteal lymph node in the later images implies recruitment of drainage through the deep lymphatics due to impaired drainage through the superficial system
36-Year-old male with a 6-week history of right lower limb swelling. This is a case of late onset unilateral right-sided primary lymphedema (lymphedema tarda). No lymphatic channels visualized on the right in keeping with aplasia. The minimal tracer is seen in a right inguinal lymph node which likely represents crossover from the left inguinal nodes
Between August 2014 and July 2015, a total of 55 lymphoscintigraphy examinations were performed in the part of evaluation of patients with long-standing lower limb swelling in the absence of other known causes of lower limb swelling (Tables 1, 2, 3). Three examinations were excluded from review: one due to lack of 1.5 h imaging and two due to inadequate field of view. We present some of the imaging appearances including a suggested model of quantitative assessment. All procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments.
Quantitative and semi-quantitative analysis parameters can be used complementary to visual analysis, to better characterize discrete changes or for monitoring therapeutic assessment in sequential studies [17]. One of the easier and reproducible quantitative parameters is estimating the amount of tracer at the ilioinguinal nodes at a specified time point (Fig. 9).
Computation of ilioinguinal lymph nodes uptake as a quantitative index in lymphedema diagnosis. Region of interest was drawn at the injection site and the inguinal nodes at the 1½ hour images and percentage of the amount of tracer in the ilioinguinal nodes computed. This is correlated well with the findings from the visual analysis
Kramer et al. observed that recognition of milder degrees of lymphatic abnormality is highly dependent on the standardization of technique and on quantitative analysis [18].
Conclusion
Because many institutions are establishing lymphedema centers, lymphatic imaging will become more prevalent. As this occurs, it will be important to develop standardized procedures and radiopharmaceuticals to perform these examinations and standardized criteria to interpret the results. This pictorial essay provides insight into the imaging appearances of normal and abnormal lymphoscintigrams.
References
Moshiri M, Katz DS, Boris M, Yung E (2002) Using lymphoscintigraphy to evaluate suspected lymphedema of the extremities. Am J Roentgenol 178(2):405–412
Keeley V (2006) The use of lymphoscintigraphy in the management of chronic oedema. J Lymphoed 1(1):42–57
Moffatt C, Franks P, Doherty D, Williams A, Badger C, Jeffs E et al (2003) Lymphoedema: an underestimated health problem. QJM 96(10):731–738
Weissleder H, Weissleder R (1988) Lymphedema: evaluation of qualitative and quantitative lymphoscintigraphy in 238 patients. Radiology 167(3):729–735
Scarsbrook A, Ganeshan A, Bradley K (2007) Pearls and pitfalls of radionuclide imaging of the lymphatic system. Part 2: evaluation of extremity lymphoedema. Br J Radiol 80(951):219–226
Mohler ER, Mondry TE (2011) Lymphedema: prevention and treatment. UpToDate. http://www.uptodate.com/contents/lymphedema-prevention-and-treatment?source=see_link. Accessed 10 Sept 2016
Tartaglione G, Pagan M, Morese R, Cappellini GA, Zappalà AR, Sebastiani C et al (2010) Intradermal lymphoscintigraphy at rest and after exercise: a new technique for the functional assessment of the lymphatic system in patients with lymphoedema. Nucl Med Commun 31(6):547–551
Williams WH, Witte CL, Witte MH, McNEILL GC (2000) Radionuclide lymphangioscintigraphy in the evaluation of peripheral lymphedema. Clin Nucl Med 25(6):451–464
Szuba A, Rockson SG (1998) Lymphedema: classification, diagnosis and therapy. Vasc Med 3(2):145–156
Ter S-E, Alavi A, Kim CK, Merli G (1993) Lymphoscintigraphy A reliable test for the diagnosis of lymphedema. Clin Nucl Med 18(8):646–654
Andersson HC, Parry DM, Mulvihill JJ (1995) Lymphangiosarcoma in late-onset hereditary lymphedema: case report and nosological implications. Am J Med Genet 56(1):72–75
Szczesny G, Olszewski WL, Gorecki A (2005) Lymphoscintigraphic monitoring of the lower limb lymphatic system response to bone fracture and healing. Lymph Res Biol 3(3):137–145
Shenoy R (2008) Clinical and pathological aspects of filarial lymphedema and its management. Korean J Parasitol 46(3):119–125
Mohler ER et al (2013) Clinical manifestations and diagnosis of lymphedema. http://www.uptodate.com/contents/clinical-features-and-diagnosis-of-peripheral-lymphedema. Accessed 12 Dec 2016
Warren AG, Brorson H, Borud LJ, Slavin SA (2007) Lymphedema: a comprehensive review. Ann Plast Surg 59(4):464–472
Rijke AM, Croft BY, Johnson RA, de Jongste AB, Camps J (1990) Lymphoscintigraphy and lymphedema of the lower extremities. J Nucl Med Off Publ Soc Nucl Med 31(6):990
Sapienza MT, Endo IS, Ferraro GC, Tavares MG, Neto C, de Carvalho G, Guedes Neto HJ, Lewin S, Marone MM (2006) Criteria for semi-quantitative analysis of lymphoscintigraphy in lower limb lymphedema. J Vasc Bras 5(4):288–294
Kramer EL (2004) Lymphoscintigraphy: defining a clinical role. Lymph Res Biol 2(1):32–37
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
Verbal and written informed consent was obtained from the patients or next of kin. The standard protocol including dosage, route of radiotracer injection and time to imaging were not altered. Potential patient identifiers were not used.
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Nganga, E.C., Gitau, S. & Makhdomi, K. Lower limb lymphoscintigraphy patterns among patients with lower limb lymphedema: a pictorial essay. Clin Transl Imaging 6, 135–143 (2018). https://doi.org/10.1007/s40336-018-0266-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-018-0266-y