Abstract
Nuclear medicine is a unique and valuable method that contributes to the diagnosis and assessment of many diseases in children. It is generally accepted that radiation exposures to children undergoing diagnostic nuclear medicine studies and the resulting risks are low. However, due to the lack of pediatric guidelines there has been a rather wide variation of pediatric radiopharmaceutical administered activities. As a result, pediatric radiation exposures have also varied over a broad range. Some practices have been able to obtain useful results with administered activities in the lowest ranges while other centers and practices have used considerably larger administered activities. This was dramatically highlighted by surveys of nuclear medicine departments in North America and beyond. Efforts in Europe and North America have resulted in the development and publication of pediatric guidelines. These were initially developed separately utilizing different models, but more recently were joined through harmonization activities; the two sets of guidelines are now further aligned. Dissemination of these guidelines is an ongoing activity. We believe that adhering to these standards can help assure that the most appropriate administered activity is employed. Along with this goal, it is essential that the image quality and their diagnostic value be assured. Beyond the application of the recent guidelines, radiation exposures in children can be reduced further by optimizing use, updating protocols, applying advanced image processing and potentially developing and introducing advanced imaging systems. Further improvements will likely result from increased communication and cooperation by several nuclear medicine organizations in addition to the dissemination of updated information to the clinic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction and background
Well-established nuclear medicine procedures have proven to be of great value in the diagnosis and assessment of pediatric oncologic and genitourinary tract diseases, as well as maladies of the musculoskeletal, cardiopulmonary, central nervous, lymphatic and endocrine systems.
Although most nuclear medicine procedures are performed in adults and children, pediatric aspects of nuclear medicine procedures require special considerations. Pediatric nuclear medicine manages patients of varying ages, sizes, behaviors and diseases that are unique to children. In order to obtain optimal results, the methodology employed must adapt to the characteristics of pediatric patients in ways to address specific diagnostic tasks [1].
Nuclear medicine procedures are considered safe and although some methods involve higher doses than common radiological procedures, many deliver lower doses. Because animal data, as well as some epidemiologic data, indicate the possibility for adverse health effects when subjects are exposed to radiation in this range, practitioners usually aim to use administered activities that are as low as possible while maintaining the diagnostic value of the images.
Until recently, there were not any guidelines to address administered activities in children. Some centers had not updated their levels of administered activities for children in years, perhaps decades. This lack of standards is evidenced in the previous paucity of pediatric guidelines in the radiopharmaceutical package inserts. In 2000, an informal review of radiopharmaceutical package inserts in the US showed a significant lack of information about pediatric radiopharmaceutical doses. Although all package inserts included administered activities recommendations for adult patients, only 4 out of a total of 22 package inserts reviewed contained pediatric dose guidelines.
In practice, pediatric administered activities have evolved due to a number of factors including local traditions, practitioners’ preference and experience, familiarity with children, anticipated radiation absorbed dose to the patient, type of study, available photon flux, instrumentation and the amount of time needed to perform the examination. The development of a number of methods including Clark’s rule, Webster’s rule, the Young rule, the body weight based rule, etc. have informed providers of the administered activities in children undergoing nuclear medicine procedures. These methods indicated a wide variability in absorbed dose amongst the pediatric population [2]. Therefore, the lack of common standards or guidelines had resulted in a wide range of administered activities and thus, variability of radiation exposures in children undergoing nuclear medicine procedures.
When employing nuclear medicine in children, it is considered good practice to achieve a reasonable balance between any potential risks and the real benefits to the patient accrued from these tests. In spite of this, there are instances when radiation exposures may be considered unnecessary or inappropriate. Such instances involve administered radiopharmaceutical activities that are higher than the appropriate standard dose, yet do not provide additional information, or administered activities that are too low such that they do not provide images of adequate quality. Overall, nuclear medicine procedures should be performed on the right patient, at the right time, with the correct dose and with the correct technique.
During the past decade or so, there has been increased concern in the public and scientific press about the potential risks to children that are exposed to ionizing radiation from diagnostic imaging, mainly from CT [3]. The timing of these publications coincided with independent efforts within the pediatric nuclear medicine community in Europe and North America, which focused on dose reduction and optimization.
The European effort: the development of the European Association of Nuclear Medicine (EANM) Dosage Card
The first international effort to harmonize the administered activities in pediatric Nuclear Medicine initiated by the Paediatric Task Group of the European Association of Nuclear Medicine (EANM) was started in the late 1980s. Piepsz et al. published the resulting recommendation (“A radiopharmaceuticals schedule for imaging in paediatrics”) which was endorsed by the EANM in 1990 [4]. The approach of Piepsz et al. [4] was to calculate the fraction of the administered activity as a function of the body surface area and to translate the resulting table into a weight-dependent table of fractions of activity to administer. A secondary aim was to provide a minimum amount of administered activities for 24 radiopharmaceuticals primarily labeled with Tc-99m.
In 2005, Jacobs et al. published a new study with the primary aim of determining if the 1990 version of the EANM dosage card resulted in weight-independent effective doses or count rates. Furthermore, the study intended to determine whether one dosage card was sufficient for 95 different radiopharmaceuticals, and, if not, how many cards were reasonably needed to take into account inter-tracer variability [5]. Jacobs et al. calculated normalization factors for count rate and effective doses as a function of body weight. The result was that normalization could be estimated accurately as a function of body weight by only one parameter. As a consequence, the 95 radiopharmaceuticals could be classified into three clusters A, B, C. Cluster A contained tracers for renal studies, cluster B contained all remaining tracers except iodine-labeled tracers for thyroid studies which belong to cluster C. Based on this work the authors suggested three tracer-dependent dosage cards to obtain weight-independent effective doses [5].
As a result, the EANM pediatrics and dosimetry committees developed and published a new version of the 1990 dosage card for 23 radiopharmaceuticals [6]. The radiopharmaceuticals included in the new dosage card were identified by the EANM committees to be of most relevance in pediatric Nuclear Medicine.
The formalism originally developed by Jacobs et al. [5] was revised so the new dosage card published in 2007 [6] calculates the administered activity by multiplying a baseline activity (a quantity valid only for calculation purposes) by different multiples for the three clusters A, B and C (the recommended radiopharmaceutical class).
Here, wt denotes the body weight (kg), AAdministered(wt) denotes the weight-dependent activity to be administered (in MBq), and Baseline Activity and Multiple (wt) are weight- and radiopharmaceutical-dependent factors to be used for the calculation of the activity to administer.
The baseline activity equals the activity to be administered to a child weighing 3 kg. The multiples have been derived by inverting the factors given by Jacobs et al., assuming recommended and, if there were no recommendations, reasonable activities for adults. In addition, Lassmann et al. [6] introduced a minimum recommended activity with a value which was determined based upon considerations concerning the limitations of conventional gamma cameras and PET scanners in terms of image quality at that time.
In 2008, an amendment to the 2007 EANM dosage card concerning a reduction of the recommended minimum dosage for F-18 and F-18-FDG to 26 MBq for 2D- and 14 MBq for 3D acquisitions was published [7]. Recently, Soares Machado et al. published a proposal for an extension of the EANM dosage card to Ga-68-labelled radiopharmaceuticals potentially administered to children [8].
The North American effort: the development of the North American Consensus Guidelines for Pediatric Radiopharmaceutical Administered Doses
Traditionally, there was a sense that nuclear medicine diagnostic studies carried very low radiation exposures and for quite some time, not much attention was paid to these large ranges of radiation exposures. This was dramatically revealed in a 2007 survey of administered radiopharmaceutical doses in premiere pediatric centers in North America. As suspected, the pediatric doses among the surveyed hospitals varied considerably. Radiopharmaceutical administered activities per body weight varied on average by a factor of 3 and, in one case, by a factor of 10 [9].
The greatest variability in administered activities was shown to be in newborns and infants. Publication of this survey elicited considerable interest among professionals dealing with pediatric imaging. The Image Gently campaign recommended the formation of an expert group to determine if a consensus could be achieved in order to address and hopefully reduce such large variability. The first meeting of this group took place in 2009 during the Annual Meeting of the Society for Pediatric Radiology (SPR) in Carlsbad, California. Also in 2009, there was a day-long Symposium on Dose Optimization in Pediatric Nuclear Medicine during the Annual Meeting of the Society of Nuclear Medicine (SNM) in Toronto, ON, Canada, where several aspects related to administered radiopharmaceutical activities in children were discussed. Since then, several Expert Consensus Workshops were held during the Annual Meetings of the Society of Nuclear Medicine, the Society for Pediatric Radiology and the International Society of Pediatric Radiology. Some of these early meetings also included experts from the European Association of Nuclear Medicine (EANM). Following several expert consensus workshops, the North American Guidelines for Administered Activities in Children and Adolescents was published in 2011 [10, 11]. Additional consensus workshops have been held jointly at the Annual Meetings of the SNMMI and EANM.
The North American Guidelines were based mainly on administered activities for adult corrected by body weight and including minimum and maximum recommended dosages for very small and large patients, respectively. The EANM Dosage Card was based on a different model as discussed.
Harmonization guidelines
During the EANM congress in 2012, a working group of the EANM and the SNMMI met to study the possibility of harmonizing the two guidelines. The purpose of this work was to identify differences between the guidelines and suggest changes to achieve a level of harmonization. Examination of the differences indicated that administered dose recommendations for the youngest patients were lower in the North American guidelines and conversely the recommended doses in older children were lower in the EANM Dosage Card [12]. These differences were discussed in joint consensus workshops between the two groups. Recommended doses were adjusted, so they would be less discrepant, leading to the development and publication of the Harmonized Guidelines and new versions of both the North American Guidelines and the EANM Dosage Card in 2014 [13, 14].
Web-based tools for determination of pediatric administered activities
In 2012, as an offshoot of a project by the European Union (www.peddose.net), a mobile version of the EANM Paediatric Dosage Card was created. This application, “PedDose” was initially available as an iAPP for iPhone/iPad (http://itunes.apple.com/us/app/peddose/id492680472?mt=8). In 2013, a version of the EANM dosage calculator for Android was released (https://play.google.com/store/apps/details?id=com.netkey.PedDose).
The App not only provides its users with the appropriate amount of radiopharmaceutical to inject, but it also provides an estimate of the effective dose delivered during a given paediatric examination. This development was considered necessary to increase nuclear medicine physicians’ awareness of the level of irradiation involved with paediatric procedures as the already existing paediatric dosage card only gives recommended activity to inject. The iApp is considered to be user-friendly in clinical practice, provides an estimate of the effective dose delivered according to ICRP references, and produces a printout of the summary of the procedure (radiopharmaceutical, patient’s weight, recommended injected activity and effective dose delivered for a reference patient) that can be appended to the patient’s file.
In North America, the Society of Nuclear Medicine and Molecular Imaging website includes the Pediatric Injected Activity Tool that provides recommended administered doses based on the patient’s body weight in kilograms. Once the appropriate radiopharmaceutical is selected and the patient weight entered, the tool provides the recommended administered activities (in MBq and mCi) for both the North American guidelines as well as for the EANM Dose Chart. (http://www.snmmi.org/pedactivitytool).
Follow up surveys of the effect of the NA guidelines in both pediatric and adult hospitals
A follow-up to the 2007 North American survey was performed in 2013 to evaluate the effect of the 2010 North American Guidelines on the same 13 pediatric institutions [15]. In general, the administered activities and the level of variation were reduced in the follow-up survey, especially for the procedures for which the North American Guidelines provided recommended parameters. All of the respondents were familiar with Image Gently and the 2010 North American Guidelines and 10 of the 13 institutions (77 %) modified their administered activities based upon the Guidelines.
Survey of general hospitals in the USA
In 2013, a survey was performed of 194 general hospitals in the US to evaluate the practice of pediatric nuclear medicine in the country and the impact of Image Gently and the 2010 North American Guidelines at these institutions. The sample considered general hospitals in the US with more than 300 beds excluding specialized hospitals such as dedicated pediatric, psychiatric, rehabilitation and veterans’ hospitals. All of the surveyed hospitals were contacted beforehand to obtain the email address of the nuclear medicine chief technologist or supervisor. Out of the 194 sites that received the emailed link of the online survey, 121 (62 %) responded. The high response rate was attributed to the fact that all of the sites were contacted prior to launching the survey.
Eighty-three of the responding sites indicated that they performed nuclear medicine studies in children. Of these sites, 55 % described themselves as community, teaching hospitals that hosted residents and/or medical students, 15 % as community, non-teaching hospitals and 30 % as large, academic hospitals. 50 % percent were located in urban areas, 48 % in suburban areas and 2 % in rural settings. Of those sites that performed studies in children, 83 % were familiar with Image Gently, 58 % were familiar with the 2010 North American Guidelines and 56 % said they altered imaging protocols because of the guidelines.
The sites were asked about 5 commonly performed pediatric nuclear medicine procedures: Tc-99m MDP bone scans, Tc-99m MAG3 renograms, Tc-99m DMSA renal scans, Tc-99m based hepatobiliary scans, and F-18 FDG PET whole body scans. They were asked about their administered activity by weight (MBq/kg), the minimum and maximum dosages and the activities that they would administer to 2 hypothetical patients: a 5-year-old boy (20 kg, 110 cm tall) and a 10 year old girl (30 kg, 140 cm tall). The median value for all reported parameters (dosage by weight, maximum or minimum dosages) was equal to the recommended value of the North American Guidelines in all cases. More than 50 % of the sites were compliant with the Guidelines with respect to both the acquisition parameters and the administered activities for the two hypothetical patients, particularly those sites indicating that they were familiar with the Guidelines. However, there remained a wide variation in the reported data; as much as a factor of 16, for those sites that were not familiar with the Guidelines.
Nuclear medicine global initiative
In 2012, a collection of international organizations involved in nuclear medicine decided to engage in a project of common interest worldwide. The underlying objectives of the endeavor (called the Nuclear medicine global initiative or NMGI) were to promote human health by advancing the field of nuclear medicine and molecular imaging, encourage global collaboration in education, and harmonize procedure guidelines and other policies that ultimately lead to improvements in quality and safety in the field throughout the world.
It was decided that the first NMGI project would consider the issues involved in the standardization of administered activities in pediatric nuclear medicine. Table 1 lists the organizations that participated in the project. The findings of the project were presented in a “white paper” that was divided into 2 parts. The part 1 paper discussed why this particular project was chosen [15]. In addition, it discussed the value of pediatric nuclear medicine, current understanding of the risk of cancer associated with ionizing radiation as it applies to pediatric nuclear medicine, and internal radiation dosimetry as it pertains to children. Gaps in the current state of knowledge on these topics were also discussed. A comprehensive listing of pertinent educational and reference materials available in print and online was also provided. The part 2 paper, which was recently submitted for publication, described the current standards for administered activities in children and adolescents that have been developed by various organizations. These include the North American Consensus Guidelines and the EANM paediatric dosage card that are described above, as well as the standards recently published by the Japanese Society of Nuclear Medicine [16].
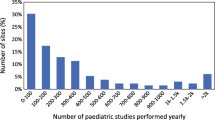
The results of an investigation into current practice of pediatric nuclear medicine across the globe, specifically with regards to administered activities, as determined by an international survey of nuclear medicine clinics and centers, are also presented. There were 313 validated responses from 29 countries to the survey. The responders were organized into 7 regions: Asia (not including Japan), Australia/New Zealand, EANM (member nations of the EANM), Japan, Latin America, North America and South Africa. It was decided to consider Japan as a region separate from Asia since 98 of the 313 entries were from this country. Sites listed the 5 general nuclear medicine procedures that they most commonly performed in children. The 3 most common procedures were bone scans using Tc-99m, radionuclide renogram using Tc-99m and renal scarring and differential function imaging using Tc-99m DMSA. In addition, 51 % of the responding sites performed F-18 FDG PET/CT in children. Similar to the general hospital survey previously described, the sites were asked about the amount of activity they would administer to 2 hypothetical pediatric patients (5-year-old boy weighing 20 kg and 110 cm tall; 10-year-old girl weighing 30 kg and 140 cm tall) for these procedures. The data showed considerable variation in these reported values across regions, but also within regions. Except for South Africa, whose sites reported values consistent with the EANM paediatric dosage card, regions with applicable standards (EANM, Japan and North America) tended to have less variation than those without standards.
Based on their discussion and the survey findings, NMGI put forth the following recommendations:
-
The value of pediatric nuclear medicine is clearly recognized. However, care must be taken to assure that these studies are applied appropriately in those patients who can best benefit.
-
Much information is available both in print and online regarding the appropriate application of nuclear medicine in children as well as our current understanding of radiation dosimetry in these patients. Nuclear medicine professionals who image children should take advantage of these materials in order to be better informed and thus better serve our pediatric patients.
-
Gaps remain in our knowledge of the biokinetics and radiation dosimetry associated with the application of nuclear medicine in children. There is also limited information regarding the potential risk of adverse health effects from ionizing radiation in children at the dose levels most pertinent to nuclear medicine. More complete understanding of these issues would allow for better optimization of pediatric nuclear medicine.
-
There remains a wide variability in the practice of pediatric nuclear medicine across the globe. Clinical sites in those regions that have developed guidelines for administered activities in children tend to be consistent with those guidelines although some wide variations still exist.
-
Countries and regions that do not currently have pediatric guidelines for administered activities should either develop their own or officially adopt currently existing guidelines.
-
Those regions that currently have guidelines should expand these to all nuclear medicine procedures practiced in children and should continue to strive for harmonization among these guidelines.
-
Administered activity for paediatric patients should be incorporated into audit processes for Nuclear Medicine sites, whether by local/country based programs, or other audit methods (e.g. IAEA QUANUM program).
-
Paediatric dose recommendations should be incorporated in formal training curriculum, and recertification programs, for all Nuclear Medicine professionals.
-
All organizations involved in nuclear medicine should disseminate the findings and recommendations of this endeavor to their members and constituents. The appropriate use of pediatric nuclear medicine and the adherence to guidelines for administered activities for children and adolescents should be actively promoted to a wide audience.
Additional approaches beyond the guidelines aimed at reducing pediatric administered radiopharmaceutical activities
Beyond the adherence to the pediatric radiopharmaceutical guidelines, there are opportunities to achieve even lower radiation exposures in children undergoing nuclear medicine examinations. These include the following: (1) appropriate use and sequencing of diagnostic imaging procedures that can properly address the specific diagnostic task at the lowest radiation exposure, cost and risk, (2) adjusting routine acquisition and display protocols that can be adapted to the diagnostic question being asked, (3) application of advanced image processing for planar, SPECT and PET, (4) use of advanced instrumentation, and (5) education and communication of best practices.
While delivering the lowest radiation exposures possible, with the application of advanced image processing it is possible to reduce radiation doses even further below current standards while maintaining and even dramatically improving image quality. For example, in DMSA renal SPECT and MDP skeletal SPECT radiation administered activities can be reduced by at least 1/2 utilizing OSEM 3D with resolution recovery [17, 18]. Similarly significant reductions in radiation exposures can be achieved in planar imaging, both static and dynamic, for example in 99mTc- renography and hepatobiliary studies [19, 20].
Summary
Nuclear medicine is a unique and valuable methodology that contributes to the diagnosis and assessment of many diseases in children. It is generally accepted that radiation exposures in children undergoing diagnostic nuclear medicine studies are low. However, due to the lack of pediatric guidelines there has been a wide variation in pediatric radiopharmaceutical administered activities leading to a broad range of radiation exposures. Some practices have been able to obtain valuable results with administered activities in the lowest ranges, while other centers and practices have used considerably larger administered activities in order to obtain relevant information. Surveys performed so far highlighted the spectrum of administered doses reported by nuclear medicine departments worldwide.
Recognizing that establishing pediatric guidelines is essential to dose reduction, efforts in Europe and North America have been made to develop and publish a protocol. These groups initially developed guidelines separately by utilizing different models. More recently, however, joint harmonization activities have reduced discrepancies. Recently, Japan has also published guidelines of administered activities in children.
Dissemination of these guidelines is an ongoing activity and we believe that adhering to such standards for pediatric radiopharmaceutical administered doses can help assure that the lowest administered activity is employed. Along with this goal, it is essential that the image quality and diagnostic value are assured.
Beyond the application of recent guidelines, radiation exposures in children can be reduced further by optimizing use, updating protocols, applying advanced image processing and potentially, developing and introducing advanced imaging systems. Moving forward, increased communication and cooperation by several nuclear medicine organizations would likely result in further improvements.
References
Treves ST (2014) Pediatric nuclear medicine/PET, 4th edn. Springer, New York
Treves ST, Baker A, Fahey FH, Cao X, Davis RT, Drubach LA, Grant FD, Zukotynski K (2011) Nuclear medicine in the first year of life. J Nucl Med 52(6):905–925
Brenner DJ (2010) Medical imaging in the 21st century—getting the best bang for the rad. N Engl J Med 362(10):943–945
Piepsz A, Hahn K, Roca I, Ciofetta G, Toth G, Gordon I, Kolinska J, Gwidlet J (1990) A radiopharmaceuticals schedule for imaging in paediatrics. Eur J Nucl Med 17(3–4):127–129
Jacobs F, Thierens H, Piepsz A, Bacher K, Van De Wiele C, Ham H, Dierckx R (2005) Optimised tracer-dependent dosage cards to obtain weight-independent effective doses. Eur J Nucl Med Mol Imaging 32(5):581–588
Lassmann M, Biassoni L, Monsieurs M, Franzius C, Jacobs F (2007) The new EANM paediatric dosage card. Eur J Nucl Med Mol Imaging 34(5):796–798
Lassmann M, Biassoni L, Monsieurs M, Franzius C, Dosimetry E (2008) The new EANM paediatric dosage card: additional notes with respect to F-18. Eur J Nucl Med Mol Imaging 35(9):1666–1668
Machado JS, Beykan S, Herrmann K, Lassmann M (2016) Recommended administered activities for 68 Ga-labelled peptides in paediatric nuclear medicine. Eur J Nucl Med Mol Imaging:1–4
Treves ST, Davis RT, Fahey FH (2008) Administered radiopharmaceutical doses in children: a survey of 13 pediatric hospitals in North America. J Nucl Med 49(6):1024–1027
Gelfand MJ, Parisi MT, Treves ST (2011) Pediatric radiopharmaceutical administered doses: 2010 North American consensus guidelines. J Nucl Med 52(2):318–322
Treves ST, Parisi MT, Gelfand MJ (2011) Pediatric radiopharmaceutical doses: new guidelines. Radiology 261(2):347–349
Grant FD, Gelfand MJ, Drubach LA, Treves ST, Fahey FH (2015) Radiation doses for pediatric nuclear medicine studies: comparing the North American consensus guidelines and the pediatric dosage card of the European Association of Nuclear Medicine. Pediatr Radiol 45(5):706–713
Lassmann M, Treves ST (2014) Pediatric radiopharmaceutical administration: harmonization of the 2007 EANM paediatric dosage card (Version 1.5. 2008) and the 2010 North American consensus guideline. Eur J Nucl Med Mol Imaging 41(8):1636
Treves ST, Lassmann M, Group ESPDHW (2014) International guidelines for pediatric radiopharmaceutical administered activities. J Nucl Med 55(6):869–870
Fahey FH, Bom HH-S, Chiti A, Choi YY, Huang G, Lassmann M, Laurin N, Mut F, Nunez-Miller R, O’Keeffe D (2015) Standardization of administered activities in pediatric nuclear medicine: a report of the first nuclear medicine global initiative project, part 1—statement of the issue and a review of available resources. J Nucl Med 56(4):646–651
Koizumi K, Masaki H, Matsuda H, Uchiyama M, Okuno M, Oguma E, Onuma H, Kanegawa K, Kanaya S, Kamiyama H (2014) Japanese consensus guidelines for pediatric nuclear medicine. Ann Nucl Med 28(5):498–503
Sheehy N, Tetrault TA, Zurakowski D, Vija AH, Fahey FH, Treves ST (2009) Pediatric 99mTc-DMSA SPECT performed by using iterative reconstruction with isotropic resolution recovery: improved image quality and reduced radiopharmaceutical activity 1. Radiology 251(2):511–516
Stansfield EC, Sheehy N, Zurakowski D, Vija AH, Fahey FH, Treves ST (2010) Pediatric 99mTc-MDP bone SPECT with ordered subset expectation maximization iterative reconstruction with isotropic 3D resolution recovery 1. Radiology 257(3):793–801
Hsiao EM, Cao X, Zurakowski D, Zukotynski KA, Drubach LA, Grant FD, Yahil A, Vija AH, Davis RT, Fahey FH (2011) Reduction in radiation dose in mercaptoacetyltriglycerine renography with enhanced planar processing. Radiology 261(3):907–915
Fahey F, Zukotynski K, Zurakowski D, Markelewicz R, Falone A, Vitello M, Cao X, Grant F, Drubach L, Vija AH (2014) Beyond current guidelines: reduction in minimum administered radiopharmaceutical activity with preserved diagnostic image quality in pediatric hepatobiliary scintigraphy. Eur J Nucl Med Mol Imaging 41(12):2346–2353
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Ted Treves, Michael J. Gelfand, Alison Goodkind, Frederic H. Fahey and Michael Lassmann declare that they have no conflict of interest.
Ethical standards
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Rights and permissions
About this article
Cite this article
Treves, S.T., Gelfand, M.J., Goodkind, A. et al. Standardization of pediatric nuclear medicine administered radiopharmaceutical activities: the SNMMI/EANM Joint Working Group. Clin Transl Imaging 4, 203–209 (2016). https://doi.org/10.1007/s40336-016-0170-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-016-0170-2