Abstract
Introduction
Coffee is a complex brew that contains several bioactive compounds and some of them can influence blood pressure (BP) and endothelial function (EF), such as caffeine and chlorogenic acids (CGAs).
Aim
This study aimed to evaluate the acute effects of coffee on BP and EF in individuals with hypertension on drug treatment who were habitual coffee consumers.
Methods
This randomized crossover trial assigned 16 adults with hypertension to receive three test beverages one week apart: caffeinated coffee (CC; 135 mg caffeine, 61 mg CGAs), decaffeinated coffee (DC; 5 mg caffeine, 68 mg CGAs), and water. BP was continuously evaluated from 15 min before to 90 min after test beverages by digital photoplethysmography. Reactive hyperemia index (RHI) assessed by peripheral arterial tonometry evaluated EF before and at 90 min after test beverages. At the same time points, microvascular reactivity was assessed by laser speckle contrast imaging. Repeated-measures-ANOVA evaluated the effect of time, the effect of beverage, and the interaction between time and beverage (treatment effect).
Results
Although the intake of CC produced a significant increase in BP and a significant decrease in RHI, these changes were also observed after the intake of DC and were not significantly different from the modifications observed after the consumption of DC and water. Microvascular reactivity did not present significant changes after the 3 beverages.
Conclusion
CC in comparison with DC and water neither promoted an acute increase in BP nor produced an improvement or deleterious effect on EF in individuals with hypertension on drug treatment who were coffee consumers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hypertension affects almost one-third of the adult population [1], it is a major risk factor for cardiovascular diseases (CVDs) [2] and an important cause of death and disability-adjusted life years worldwide [3]. There is evidence that treatment to lower blood pressure (BP) is associated with a reduced risk for death and CVDs [4]. Lifestyle interventions, including dietary modifications, can lower BP. The most effective dietary changes are weight loss, reduced sodium intake, increased potassium intake, reduced consumption of alcohol and a healthy dietary pattern like the Dietary Approaches to Stop Hypertension (DASH) diet [5,6,7,8]. However, other dietary factors may also affect BP [7].
Caffeine consumption acutely increases BP. In a meta-analysis of crossover controlled clinical trials, single doses of caffeine (200–300 mg) induced in individuals with hypertension a mean increase of 8.1 mmHg in systolic BP and 5.7 mmHg in diastolic BP, which was observed in the first 60 min and persisted up to 180 min after caffeine intake [9]. Caffeine is a methylxanthine that is rapidly absorbed through the gastrointestinal tract, with blood levels peaking 15–120 min after ingestion. Once absorbed, caffeine spreads throughout the body, has a half-life typically of 2.5–4.5 h in adults, and is metabolized in the liver by cytochrome P450 (CYP) isoform CYP1A2 into paraxanthine, theobromine, and theophylline [10, 11]. Dietary sources of caffeine include coffee, tea, yerba mate, caffeinated soda (cola-type), and energy drinks [12]. According to the 2017 ACC/AHA High Blood Pressure Guideline, caffeine intake should generally be limited to 300 mg/day and patients with uncontrolled hypertension should avoid caffeine [8].
Coffee is a major dietary source of caffeine in Brazil [13] and other states [12, 14] and is one of the most consumed beverages worldwide [10]. The caffeine content in coffee is highly variable [15]. A cup of 8 oz (or 240 mL) of brewed coffee and instant coffee contain 96 mg and 62 mg of caffeine, respectively. Whereas a cup of 2 oz (or 60 mL) of espresso coffee contains 127 mg of caffeine [16].
In addition to being a major source of caffeine, coffee is rich in chlorogenic acids (CGAs), a family of polyphenolic compounds that are potent antioxidants and can favor BP reduction through improved endothelial function and nitric oxide bioavailability in the arterial vasculature [17, 18]. Therefore, the effect of coffee on BP may be lower than predicted based on its caffeine content [19]. Previous randomized controlled trials evaluating the acute effects of coffee on BP [20,21,22,23,24,25,26], and endothelial function [20, 22, 26,27,28] presented conflicting results. It is noteworthy that these studies were conducted in healthy individuals [20,21,22,23,24,25,26,27] and most were carried out in young subjects [21, 23,24,25,26,27]. The effects of caffeine on BP may be more pronounced in individuals with hypertension [29, 30] or the elderly [31], but to the best of our knowledge, there is a lack of randomized crossover clinical trials evaluating the acute effects of coffee on BP and endothelial function in individuals with hypertension on drug treatment. Therefore, this study aimed to evaluate the acute effects of coffee on BP and endothelial function in individuals with hypertension on antihypertensive drug treatment who were habitual coffee consumers.
2 Materials and Methods
This crossover randomized controlled trial was performed at the Laboratory of Clinical and Experimental Pathophysiology (CLINEX), located at the State University of Rio de Janeiro, Brazil. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the committee on ethics and research of the Pedro Ernesto University Hospital (CAAE: 87599418.3.0000.5259). Written informed consent was obtained from all participants. The present trial was registered at www.ensaiosclinicos.gov.br (ID no. RBR-964r2d). The recruitment and follow-up of the participants were conducted between November 2018 and February 2020. The study ended in March 2020 due to the COVID-19 pandemic.
2.1 Subjects
Eligible participants were individuals with hypertension, aged 40–65 years, both sexes, presenting body mass index (BMI) ≥ 20 and < 40 Kg/m2 and on regular treatment with the same antihypertensive drugs for at least four weeks were selected from two hypertension outpatient clinics (State University of Rio de Janeiro, Rio de Janeiro city, Brazil). The exclusion criteria were: individuals with evidence of secondary hypertension or presenting BP ≥ 160/100 mmHg; non-habitual coffee consumers or excessive coffee consumers (> 4 cups of 240 mL/day); current or former smokers (quit < 12 months); subjects using nutritional supplements, hormone replacement therapy, antidiabetic drugs or lipid lowering medications; diagnosis of diabetes, cancer, chronic kidney or liver disease; clinically evident coronary disease; clinical signs of heart failure or arrhythmia; and pregnant or lactating women. Participants who engaged in physical activities, including light ones, such as walking, for at least 40 min three times a week were considered physically active.
2.2 Study Design
Potential participants, who met initial eligibility criteria (preliminary evaluation) and agree to take part in the study, were scheduled for a screening visit (V0). At this screening visit, they arrived at the laboratory after a 12-hour fasting period for clinical, nutritional, and laboratory evaluations. The eligible participants received the necessary instructions for the three study visits (V1, V2, and V3): 12-hour fasting; 36-h abstinence of alcohol and food items rich in caffeine, such as coffee, yerba mate, chocolate, caffeinated soda, and energy drinks; and avoidance of more than 2 servings of fruits and unusual physical exercises on the previous day.
Each participant attended three separate visits, one week apart. For the allocation of the participants to a sequence of interventions, a computer random number generator was used (http://www.random.org/sequences/). The random allocation sequence was generated by a researcher that did not enroll participants in the study and did not assign participants to the sequence of interventions. Participants and researchers were blinded concerning the caffeine content of coffee preparations (caffeinated or decaffeinated). At these visits, participants arrived at the Laboratory between 08:30 and 9:00 a.m. While fasting they were submitted to the baseline continuous evaluation of BP for 15 min. In sequence, participants ingested one of the three test beverages in no more than 10 min. After the test beverage intake, BP was continuously evaluated for 90 min.
2.3 Test Beverages
The coffee (Coffea arabica) was produced in São José do Vale do Rio Preto, Rio de Janeiro, Brazil. The obtained caffeinated and the decaffeinated roasted and ground coffees were inserted into capsules, compatible with a Nespresso coffee maker (Nespresso Inissia® C40; Brazil), which were sealed and stored until analysis and offer to patients. This procedure was chosen to provide uniformity of the coffee beverage and facility to offer coffee flavors, aromas, and convenience to volunteer patients in this study.
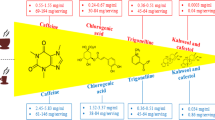
The caffeine and CGAs concentrations in the caffeinated and decaffeinated coffee were determined simultaneously using methanolic extraction in an ultrasound bath and quantification by high-performance liquid chromatographic (HPLC) method with diode-array detection (DAD) and reverse phase separation. A sample of 0.5 mL of the caffeinated or decaffeinated beverage was submitted to extraction with 5 mL of MeOH solution (containing 2 g/L of butylated hydroxytoluene—BHT)/10% acetic acid (85:15) with ultrasound for 30 min. After extraction, the sample was filtered in paper and transferred to a vial. The determination was performed by liquid chromatography with a Waters Alliance e2695 module using a Thermo BDS column (150 × 4.6 mm; 2.6 μm), elution was made with mobile phase A composed of 1.5 mL/L phosphoric acid solution, and mobile phase B composed of acetonitrile. The injection volume was 5 μL, the column temperature was 30 °C, and the injector temperature was 20 °C, with a running time of 30 min. Caffeine and CGAs were detected simultaneously in a Waters 2998 PDA detector, caffeine chromatogram was obtained at 270 nm and CGAs chromatograms were obtained at 345 nm (Fig. 1) [32].
The three test beverages were served in a 200 mL brown ceramic cup. No addition of sugar, milk, or other ingredients was allowed. The average content of CGAs in the caffeinated coffee was 0.349 mg/mL and in the decaffeinated coffee was 0.508 mg/mL. In other to offer a similar amount of CGAs in both coffees, the volume of caffeinated and decaffeinated espresso was 176 mL and 135 mL, respectively, and the volume of water was 175 mL. Therefore, caffeinated coffee contained 135 mg of caffeine and 61 mg of CGAs, while decaffeinated coffee contained 5 mg of caffeine and 68 mg CGAs.
2.4 Nutritional Assessment
To assess usual dietary intake over the last 6 months, a semiquantitative food frequency questionnaire (FFQ) containing eighty items and usual portions was used. This FFQ was developed for the Brazilian population based on commonly consumed foods and was validated against more accurate methods of dietary intake assessment [33]. Software SAS® and the Brazilian Table of Food Consumption [34] were used to process data from the FFQ. Alcohol intake was considered when the reported frequency was one or more times per week.
Height was measured using a stadiometer accurate to ± 0.5 cm, and weight was obtained using a digital scale accurate to ± 0.1 kg (Filizola S.A.), with participants wearing light clothing and no shoes after they attempted to empty their bladder. BMI was calculated using the standard equation (kg/m2) [35]. Waist circumference (WC) was measured in the standing position, midway between the lower margin of the last rib and the iliac crest, at mid-exhalation [36]. The waist-to-height ratio was obtained by dividing WC (cm) by height (cm). Anthropometric measurements were taken twice, and mean values were used.
The bioelectrical impedance analysis (BIA) procedure was performed using the Biodynamics BIA-450 body fat analyzer (Biodynamics Corp., Seattle, WA, USA) with the patient in the supine position according to the manufacturer’s instructions. Patients were advised to fast for at least 8 h before the analysis and not to perform strenuous physical activity on the day before the evaluation [37].
2.5 Laboratory Parameters
At the screening visit, blood samples were collected to evaluate serum levels of glucose, insulin, total cholesterol and fractions, triglycerides, creatinine, urea, and uric acid. These analyses were performed at the Central Laboratory and the Laboratory of Endocrine Physiology at Pedro Ernesto University Hospital. Glucose was determined by the enzymic method. Uric acid, total cholesterol, HDL cholesterol, and triglyceride concentrations were assessed by using the enzymatic colorimetric method. LDL-cholesterol was estimated by using the Friedewald formula [38]. Serum urea and creatinine were assessed by the kinetic method. Insulin was determined by the electrochemiluminescence immunoassay using a commercial kit (Roche Diagnostics). Insulin resistance status was assessed using the homeostasis model assessment for insulin resistance index [39]. The estimated glomerular filtration rate (eGFR) was obtained using CKD-Epidemiology Collaboration (CKD-EPI) equation [40].
2.6 Blood Pressure
BP and heart rate were continuously measured (beat-by-beat), in the lying state after a 20-min rest period, with a photoplethysmography finger cuff placed on the left middle finger using the equipment Finometer Pro (Finapres Medical System) [41]. A return-to-flow calibration was performed to provide adjustment of the finger arterial pressure with the brachial artery pressure [42]. During the evaluation, a digital cuff was automatically calibrated every seventy pulses to guarantee the physiological conditions of the digital artery. At baseline, before meals intake, records of BP were obtained for 15 min. Data from the first 10 min were discarded, and the mean of the values recorded in the final 5 min was considered as the baseline BP. To drink the three test beverages, participants remained seated in a semi-recumbent position. After completion of the beverages, they returned to a lying position, and values of BP were obtained for 90 min. In the statistical analyses, during the postprandial period, we included the mean values of each interval of 10 min.
2.7 Endothelial Function
Endothelial function was evaluated by peripheral arterial tonometry (PAT) method, using EndoPAT 2000 (Itamar Medical Ltd, Caesarea, Israel), a finger plethysmographic device. This is a non-invasive method that offers the possibility of an easy and rapid assessment of vascular function in which data are analyzed independently of the examiner. Alterations in pulsatile arterial volume detected by PAT have been associated with flow-mediated dilation measurements [43]. The measurements were performed through fingertip probes placed on the right and left index fingers. Measurement was started by an initial recording of a resting PAT signal for 5 min. Sequentially arterial flow on the non-dominant measurement arm was occluded by inflating the occlusion cuff approximately 50 mmHg above the previously determined systolic BP, but never below 200 mm Hg. The cuff was rapidly deflated after 5 min of occlusion to allow reactive hyperemia and the following 5 min were recorded. The other arm served as a control and the difference between the two arms was used by EndoPAT 2000 software to automatically calculate the reactive hyperemia index (RHI).
2.8 Cutaneous Microvascular Reactivity
Cutaneous microvascular reactivity was assessed with subjects lying down in the supine position in a room with temperature control (23 ± 1 °C) using the laser speckle contrast imaging technique. This technique provides a non-invasive evaluation of a wide area of tissue in real-time with very good spatial resolution and excellent reproducibility [44, 45]. The equipment used was PeriCam PSI-NR analyzer with a 780-nm wavelength (Perimed AB). Variations in the microvascular flow (endothelium-dependent and independent) were analyzed using post-occlusive reactive hyperemia (PORH). A cuff on the non-dominant arm of the participants was inflated to 50 mmHg above systolic BP for 5 min to occlude arterial flow and then induce PORH after it was deflated. Cutaneous blood flow on the forearm was evaluated before arterial occlusion (baseline period) and after fast cuff deflation. Images were analyzed by PIMSoft software (Perimed AB), and the measurements of cutaneous microvascular flow were expressed in arbitrary perfusion units (APU). Values of cutaneous vascular conductance (CVC) were obtained by dividing APU by medium BP, expressed as AUP/mmHg. To quantify skin microvascular reactivity, the following parameters were used: (1) hyperemia peak, which is the maximum value of CVC in the post-occlusion period; (2) amplitude of the PORH response, obtained by the difference between peak CVC and baseline CVC; and (3) AUC (area under the curve) in the post-occlusion period.
2.9 Statistical Methods
Categorical variables were expressed as absolute numbers and percentages. Continuous variables were presented as mean ± standard deviations. The Shapiro–Wilk test was used to test the normality of the continuous variables and skewed data were log-transformed to improve normality. Baseline comparisons among the three study visits (before the intake of the test beverages) were made using analysis of variance (ANOVA). Two-way repeated measures ANOVA was used to evaluate the effect of each beverage regardless of time (beverage effect), the effect of time, that is the change in the variable regardless of beverage (time effect), and the interaction between both factors (beverage and time), indicative of the difference of the response among the three beverages (beverage vs. time effect or treatment effect). The effect size was estimated as eta squared (ƞ2), and Cohen's definitions of small (0.01), medium (0.06), and large (0.14) effect sizes were applied [46]. One-way repeated measures ANOVA was performed to evaluate the effect of each beverage (separately) on BP and vascular function.
All statistical analyses were performed using STATA version 13 (StataCorp LP) software. p < 0.05 was considered statistically significant. It was not possible to determine the desired sample size to achieve a significant change in BP based on previous studies, because of the non-existence of a prior clinical trial with a similar intervention in individuals with hypertension.
3 Results
One hundred and fifteen individuals were interviewed, of which 98 agreed to participate in the study. Among them, in the preliminary evaluation, twenty-three subjects met the eligibility criteria and were scheduled for the screening visit (the most common reasons for exclusion of the 75 individuals were age above 65 years, diagnosis of diabetes, use of lipid lowering medication or nutritional supplements, body mass index < 20 or ≥ 40 kg/m2 and current smoking). After the screening visit, sixteen hypertensive individuals met all the eligibility criteria and were randomized into the study. The reasons for exclusion of the seven individuals in the screening visit were fasting plasma glucose > 126 mg/dL (n = 2), BP ≥ 160/100 mmHg (n = 1), changes in work schedule (n = 2) and loss of interest (n = 2). Fourteen participants completed the three study visits (Fig. 2) and were included in the statistical analyses.
The characteristics of the participants, their habitual dietary intake, and laboratory parameters are presented in Table 1. None of the participants was using aspirin or other antiplatelet agents. At baseline (before the intake of test beverages), there was no significant difference in BP, endothelial function, and microvascular reactivity among caffeinated coffee, decaffeinated coffee, and water.
Modifications in BP and heart rate from baseline to 90 min after each test beverage, evaluated by photoplethysmography, are presented in Figure 3. Two-way repeated measures ANOVA revealed a significant effect of time (time effect p < 0.0001; ƞ2 ≥ 0.12) for systolic, diastolic and mean BP, with no significant difference between beverages and no significant time vs. beverage interaction. One-way ANOVA for repeated measurements evaluated the effects of each beverage separately and showed a significant increase in systolic, diastolic, and mean BP after the consumption of caffeinated coffee and decaffeinated coffee (p < 0.05), but not after water. The increase in systolic/diastolic BP (mmHg) (90 min—baseline) was 10 ± 14/8 ± 7 after caffeinated coffee, 8 ± 15/7 ± 7 after decaffeinated coffee, and 6 ± 16/4 ± 6 after water.
Mean values of (a) systolic blood pressure, (b) diastolic blood pressure, (c) mean blood pressure and (d) heart rate at baseline (0 min) and during 90 min after the consumption of caffeinated coffee, decaffeinated coffee and water. BP = blood pressure; a p < 0.05 in one-way repeated measures analysis of variance (ANOVA) evaluating the effect of time (time effect) in each beverage: for systolic, diastolic and mean BP after caffeinated coffee and decaffeinated coffee; *p values for two-way repeated measures ANOVA; ƞ2 = eta squared (effect size)
Table 2 contains the mean values of endothelial function and microvascular reactivity at baseline and 90 minutes after each test beverage. For changes in endothelial function (RHI), according to two-way repeated measures ANOVA, there was a significant effect of time (p = 0.0009; ƞ2 = 0.273), but a non-significant effect of beverage and a non-significant beverage vs. time interaction. Considering separately each beverage, one-way repeated measures ANOVA identified a significant decrease in RHI after caffeinated coffee and decaffeinated coffee, but not after water. Considering the variables related to microvascular reactivity, two-way repeated measures ANOVA identified no significant effect of time, beverage, and beverage vs. time interaction. Changes in these variables, considering separately each beverage, were not significant after the intake of caffeinated coffee, decaffeinated coffee, and water.
4 Discussion
In the present crossover randomized controlled clinical trial, based on a sample of individuals with hypertension on antihypertensive drug treatment who were habitual coffee consumers, there was no significant difference in the acute response of BP and endothelial function to the intake of caffeinated coffee, decaffeinated coffee, and water.
As mentioned above, almost all previous randomized clinical trials evaluating the acute effects of caffeinated coffee on BP included young healthy individuals [21, 23,24,25,26,27]. Some of these studies did not observe a significant increase in BP in comparison with decaffeinated coffee or water [20,21,22], while others observed [23,24,25,26]. The caffeine content in coffee is probably one important factor that influences the acute changes in BP and theoretically can help explain the divergence between the results of the studies. In the present study, the amount of caffeine in the caffeinated coffee was 135 mg. In previous randomized trials, that also did not find a significant increase in BP after caffeinated coffee in comparison with decaffeinated coffee and/or water [20,21,22], the amount of caffeine in caffeinated coffee was similar (130 mg) [20], lower (80 mg) [21] and higher (270 mg) [22] than in the present study. Moreover, in previous studies that observed a significant acute increase in BP after caffeinated coffee compared to decaffeinated coffee, the caffeine content in the caffeinated coffee was similar (150 mg) [23, 24] or lower (80 mg [25] and 54 mg [26]) than the caffeine content in the present study. Therefore, based on the results of the present study and on the results of these studies conducted with healthy individuals we may infer that the content of caffeine may not be the only determinant of the acute effects of coffee on BP.
Some factors can modify the effect of caffeine on BP, and one of them is the tolerance to caffeine that is observed after repeated and regular administration [30, 47]. The present study included only habitual coffee/caffeine consumers, which can induce tolerance, diminishing its acute effect on BP [10, 48]. For example, in a crossover randomized clinical trial healthy subjects were stratified as habitual and non-habitual coffee consumers. Participants consumed in three laboratory sessions: espresso coffee, decaffeinated espresso, and warm water. Differences in systolic BP among the three interventions were observed only in non-habitual consumers [49]. Some studies that have evaluated the acute effects of coffee on BP included only habitual coffee/caffeine consumers [22, 25]. Considering that there is evidence that the tolerance to the pressor effect of caffeine may not be complete (daily caffeine intake may produce only a partial tolerance with a persistent BP response to caffeine) [50,51,52] and that not all studies included only habitual caffeine consumers, tolerance to caffeine may have contributed to the inconsistent results of the studies that have evaluated the acute effects of coffee on BP.
Other components of coffee could also explain the non-significant acute increase in BP after caffeinated coffee compared to decaffeinated coffee and water observed in the present study and other clinical trials conducted with healthy individuals [20,21,22]. Coffee is a complex brew with several components, many of which are biologically active, including CGAs, diterpenes, potassium, magnesium, melanoidins, quinines, lignans, and trigonelline [15, 53]. Some of these components may favor the decrease of BP, such as the minerals potassium and magnesium and the CGAs [8, 17]. CGAs are a family of polyphenolic compounds, which are esters between quinic acid and especially the following acids: caffeic, ferulic, and coumaric. The principal CGA in coffee is 5-O-caffeoyl-quinic acid. The content of CGAs in coffee is widely variable, a regular cup of coffee contains 70–300 mg of CGAs. CGAs are potent dietary antioxidants and can lead to BP reduction through improved endothelial function and nitric oxide bioavailability in the arterial vasculature [17]. The content of CGAs in the caffeinated and decaffeinated coffee of the present study was similar: 61 and 68 mg, respectively. So, in the present study we were able to evaluate: (1) the effects of caffeine on BP when we compared caffeinated coffee vs. decaffeinated coffee, and (2) the effects of coffee (considering all components) on BP in the comparison of caffeinated coffee vs. water. The differences in the content of CGAs in the caffeinated coffee used in the studies evaluating the acute effects on BP may also contribute to the inconsistent results of these studies.
In the present study, caffeinated coffee did not improve or worsen endothelial function in comparison with decaffeinated coffee and water. Some previous randomized controlled trials observed not only beneficial [22, 26, 28] but also deleterious effects [20, 27] of caffeinated coffee compared to decaffeinated coffee and/or water. For example, recently Pavão et al. [28] evaluated the effects of regular caffeinated coffee (133 mg caffeine; ~ 140 mg CGA) and decaffeinated coffee (~ 178 mg CGA) in 18 subjects with overweight or obesity and found a better effect on FMD in the caffeinated coffee intake group (p = 0.014).
The potential effects of coffee on endothelium may be attributed not only to CGAs but also to hydroxy-hydroquinone (HHQ) and caffeine. During coffee roasting, there is a loss of CGAs and generation of HHQ, an oxidative substance that may impair vascular function and may cancel the beneficial effect of CGAs on endothelial function [54]. In a study conducted by Kajikawa et al. [55] a single intake of coffee with a high content of CGAs and low content of HHQ, but not coffee with a high content of CGAs and high content of HHQ or placebo coffee, significantly improved postprandial flow-mediated vasodilation. As most of the studies evaluating the effects of coffee on endothelial function do not report the content of HHQ in coffee, this substance may also contribute to the diverging results.
The strength of this study is the evaluation, in a randomized clinical trial, of the acute effects of coffee on BP and endothelial function in individuals with hypertension on antihypertensive treatment. Another strength is the method used to evaluate BP: the digital photoplethysmography technique provides BP values non-invasively and continuously, beat to beat [41]. The main limitation of the present study is the non-existence of a prior clinical trial with a similar intervention in hypertensive individuals, which prevented the estimation of the sample size. However, most of the previous studies evaluating the acute effects of coffee or caffeine on BP and endothelial function had similar or lower sample sizes [22,23,24,25, 27, 28, 56]. The sample size of this crossover study was sufficiently powered to detect a relatively small difference in BP of 5 ± 6 mmHg between beverages (alpha = 0.05, power = 80%). A key advantage of the crossover design is that, for a given significance level, power, and effect size, a smaller sample size is required compared with a parallel design, because each participant acts as his or her own control, so the within-participant variability is removed [57]. Moreover, in the present study, the effect size was also estimated (as eta squared), indicating a small effect size (ƞ2 < 0.04) in all the statistical analyses that presented non-significant p-values (≥ 0.05). As effect size indices are not dependent upon the sample size, they can help inform about the potentially meaningful effects of the intervention. They provide a description of the size of observed effects that is independent of the possibly misleading influences of sample size [58, 59].
5 Conclusions
The findings of this study pointed out that caffeinated coffee compared to decaffeinated coffee and water neither caused an acute increase in BP nor produced an acute improvement or deleterious effect on endothelial function in individuals with hypertension on antihypertensive drug treatment who were habitual coffee consumers.
Additional studies evaluating the effects of caffeinated coffee containing different amounts of caffeine and/or CGAs during different conditions and periods of time are necessary for a comprehensive knowledge about the effects of coffee on BP and vascular function. These studies will help to establish more precise recommendations for coffee consumption by hypertensive patients.
References
Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–37. https://doi.org/10.1038/s41581-019-0244-2.
Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, Hemingway H. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet. 2014;383(9932):1899–911. https://doi.org/10.1016/S0140-6736(14)60685-1.
GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–94. https://doi.org/10.1016/S0140-6736(18)32225-6.
Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178(1):28–36. https://doi.org/10.1001/jamainternmed.2017.6015.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I. 2018 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(33):3021–104. https://doi.org/10.1093/eurheartj/ehy339.
Oparil S, Acelajado MC, Bakris GL, Berlowitz DR, Cífková R, Dominiczak AF, Grassi G, Jordan J, Poulter NR, Rodgers A, Whelton PK. Hypertension. Nat Rev Dis Primers. 2018;4:18014. https://doi.org/10.1038/nrdp.2018.14.
Appel LJ. The effects of dietary factors on blood pressure. Cardiol Clin. 2017;35(2):197–212. https://doi.org/10.1016/j.ccl.2016.12.002.
Paul KW, Robert MC, Wilbert SA, Donald EC, Karen JC, Cheryl DH, Sondra MD, Samuel G, Kenneth AJ, Daniel WJ, Eric JML, Paul M, Bruce O, Sidney CS, Crystal CS, Randall SS, Sandra JT, Randal JT, Kim AW, Jeff DW, Jackson TW. Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–248. https://doi.org/10.1016/j.jacc.2017.11.006.
Mesas AE, Leon-Muñoz LM, Rodriguez-Artalejo F, Lopez-Garcia E. The effect of coffee on blood pressure and cardiovascular disease in hypertensive individuals: a systematic review and meta-analysis. Am J Clin Nutr. 2011;94(4):1113–26. https://doi.org/10.3945/ajcn.111.016667.
van Dam RM, Hu FB, Willett WC. Coffee, caffeine, and health. N Engl J Med. 2020;383(4):369–78. https://doi.org/10.1056/NEJMra1816604.
Chrysant SG. The impact of coffee consumption on blood pressure, cardiovascular disease and diabetes mellitus. Expert Rev Cardiovasc Ther. 2017;15(3):151–6. https://doi.org/10.1080/14779072.2017.1287563.
Reyes CM, Cornelis MC. Caffeine in the diet: country-level consumption and guidelines. Nutrients. 2018;10(11):1772. https://doi.org/10.3390/nu10111772.
Sartori GO, Silva MV. Caffeine in Brazil: intake, socioeconomic and demographic determinants, and major dietary sources. Nutrire. 2016;41:11. https://doi.org/10.1186/s41110-016-0014-x.
Fulgoni VL 3rd, Keast DR, Lieberman HR. Trends in intake and sources of caffeine in the diets of US adults: 2001–2010. Am J Clin Nutr. 2015;101(5):1081–7. https://doi.org/10.3945/ajcn.113.080077.
O’Keefe JH, DiNicolantonio JJ, Lavie CJ. Coffee for cardioprotection and longevity. Prog Cardiovasc Dis. 2018;61(1):38–42. https://doi.org/10.1016/j.pcad.2018.02.002.
Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory. Food Data Central. 2022. https://fdc.nal.usda.gov/.
Zhao Y, Wang J, Ballevre O, Luo H, Zhang W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens Res. 2012;35(4):370–4. https://doi.org/10.1038/hr.2011.195.
Liang N, Kitts DD. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients. 2015;8(1):16. https://doi.org/10.3390/nu8010016.
Rebello SA, Van Dam RM. Coffee consumption and cardiovascular health: getting to the heart of the matter topical collection on ischemic heart disease. Curr Cardiol Rep. 2013;15(10):403. https://doi.org/10.1007/s11886-013-0403-1.
Buscemi S, Verga S, Batsis JA, Donatelli M, Tranchina MR, Belmonte S, Mattina A, Re A, Cerasola G. Acute effects of coffee on endothelial function in healthy subjects. Eur J Clin Nutr. 2010;64(5):483–9. https://doi.org/10.1038/ejcn.2010.9.
Teng CL, Lim WY, Chua CZ, Teo RS, Lin KT, Yeo JC. Does a single cup of caffeinated drink significantly increase blood pressure in young adults? A randomised controlled trial. Aust Fam Phys. 2016;45(1):65–8.
Boon EAJ, Croft KD, Shinde S, Hodgson JM, Ward NC. The acute effect of coffee on endothelial function and glucose metabolism following a glucose load in healthy human volunteers. Food Funct. 2017;8(9):3366–73. https://doi.org/10.1039/c7fo00926g.
Washio T, Sasaki H, Ogoh S. Acute impact of drinking coffee on the cerebral and systemic vasculature. Physiol Rep. 2017;5(10):e13288. https://doi.org/10.14814/phy2.13288.
Mahmud A, Feely J. Acute effect of caffeine on arterial stiffness and aortic pressure waveform. Hypertension. 2001;38(2):227–31. https://doi.org/10.1161/01.hyp.38.2.227.
Karatzis E, Papaioannou TG, Aznaouridis K, Karatzi K, Stamatelopoulos K, Zampelas A, Papamichael C, Lekakis J, Mavrikakis M. Acute effects of caffeine on blood pressure and wave reflections in healthy subjects: should we consider monitoring central blood pressure? Int J Cardiol. 2005;98(3):425–30. https://doi.org/10.1016/j.ijcard.2003.11.013.
Noguchi K, Matsuzaki T, Sakanashi M, Hamadate N, Uchida T, Kina-Tanada M, Kubota H, Nakasone J, Sakanashi M, Ueda S, Masuzaki H, Ishiuchi S, Ohya Y, Tsutsui M. Effect of caffeine contained in a cup of coffee on microvascular function in healthy subjects. J Pharmacol Sci. 2015;127(2):217–22. https://doi.org/10.1016/j.jphs.2015.01.003.
Papamichael CM, Aznaouridis KA, Karatzis EN, Karatzi KN, Stamatelopoulos KS, Vamvakou G, Lekakis JP, Mavrikakis ME. Effect of coffee on endothelial function in healthy subjects: the role of caffeine. Clin Sci (Lond). 2005;109(1):55–60. https://doi.org/10.1042/CS20040358.
Pavão TP, Chemello D, Ferigollo A, Saffi MAL, Moresco RN, Stein CDS, Emanuelli T, Somacal S, Moriguchi EH, Badimon L, Chagas P. Acute effect of coffee on arterial stiffness and endothelial function in overweight and obese individuals: A randomized clinical trial. Clin Nutr ESPEN. 2022;50:33–40. https://doi.org/10.1016/j.clnesp.2022.05.003.
Hartley TR, Sung BH, Pincomb GA, Whitsett TL, Wilson MF, Lovallo WR. Hypertension risk status and effect of caffeine on blood pressure. Hypertension. 2000;36(1):137–41. https://doi.org/10.1161/01.hyp.36.1.137.
Turnbull D, Rodricks JV, Mariano GF, Chowdhury F. Caffeine and cardiovascular health. Regul Toxicol Pharmacol. 2017;89:165–85. https://doi.org/10.1016/j.yrtph.2017.07.025.
Izzo JL, Ghosal A, Kwong T, Freeman RB, Jaenike JR. Age and prior caffeine use alter the cardiovascular and adrenomedullary responses to oral caffeine. Am J Cardiol. 1983;52(7):769–73. https://doi.org/10.1016/0002-9149(83)90413-7.
Corrêa CLO, Merces-Penha E, Dos Anjos MR, Pacheco S, Freitas-Silva O, Luna AS, Gottschalk LMF. Use of asparaginase for acrylamide mitigation in coffee and its influence on the content of caffeine, chlorogenic acid, and caffeic acid. Food Chem. 2021;338:128045. https://doi.org/10.1016/j.foodchem.2020.128045.
Sichieri R, Everhart JE. Validity of a Brazilian frequency questionnaire against dietary recalls and estimated energy intake. Nutr Res. 1998;8:1649–59.
University of Campinas (UNICAMP). Center for Studies and Research in Food. In: Brazilian Table of Food Composition, 4th ed. Sao Paulo: Campinas (2011).
World Health Organization (2000) Obesity: Preventing and Managing the Global Epidemic. In: Report of a WHO Consultation. WHO Technical Report Series no. 894. Geneva: WHO.
World Health Organization (WHO). WHO Waist Circumference and Waist-Hip Ratio. Report of a WHO Expert Consultation. Geneva: World Health Organization; 2008. https://www.who.int/publications/i/item/9789241501491.
Kyle UG, Bosaeus I, De-Lorenzo AD, Deurenberg P, Elia M, Manuel-Gómez J, Lilienthal-Heitmann B, Kent-Smith L, Melchior JC, Pirlich M, Scharfetter H, Schols A, Pichard C, ESPEN. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23(6):1430–53. https://doi.org/10.1016/j.clnu.2004.09.012.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. https://doi.org/10.1093/clinchem/18.6.499.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF. Turner RC Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412–9. https://doi.org/10.1007/BF00280883.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. Clinical Practice Guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2012;3:1–150.
Langewouters GJ, Settels JJ, Roelandt R, Wesseling KH. Why use Finapres or Portapres rather than intra-arterial or intermittent non-invasive techniques of blood pressure measurement? J Med Eng Technol. 1998;22(1):37–43.
Bos WJ, van Goudoever J, van Montfrans GA, van den Meiracker AH, Wesseling KH. Reconstruction of brachial artery pressure from noninvasive finger pressure measurements. Circulation. 1996;94(8):1870–5.
Brant LC, Barreto SM, Passos VM, Ribeiro AL. Reproducibility of peripheral arterial tonometry for the assessment of endothelial function in adults. J Hypertens. 2013;31:1984–1890. https://doi.org/10.1097/HJH.0b013e328362d913.
Roustit M, Millet C, Blaise S, Dufournet B, Cracowski JL. Excellent reproducibility of laser speckle contrast imaging to assess skin microvascular reactivity. Microvasc Res. 2010;80(3):505–11. https://doi.org/10.1016/j.mvr.2010.05.012.
Hellmann M, Roustit M, Cracowski JL. Skin microvascular endothelial function as a biomarker in cardiovascular diseases? Pharmacol Rep. 2015;67:803–10. https://doi.org/10.1016/j.pharep.2015.05.008.
Richardson JTE. Eta squared and partial eta squared as measures of effect size in educational research. Educ Res Rev. 2011;6(2):135–47. https://doi.org/10.1016/j.edurev.2010.12.001.
EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2015. Scientific opinion on the safety of caffeine. EFSA J. 2015;13(5):4102. https://doi.org/10.2903/j.efsa.2015.4102.
Mort JR, Kruse HR. Timing of blood pressure measurement related to caffeine consumption. Ann Pharmacother. 2008;42(1):105–10. https://doi.org/10.1345/aph.1K337.
Zimmermann-Viehoff F, Thayer J, Koenig J, Herrmann C, Weber CS, Deter HC. Short-term effects of espresso coffee on heart rate variability and blood pressure in habitual and non-habitual coffee consumers-a randomized crossover study. Nutr Neurosci. 2016;19(4):169–75. https://doi.org/10.1179/1476830515Y.0000000018.
Lovallo WR, Wilson MF, Vincent AS, Sung BH, McKey BS, Whitsett TL. Blood pressure response to caffeine shows incomplete tolerance after short-term regular consumption. Hypertension. 2004;43(4):760–5. https://doi.org/10.1161/01.HYP.0000120965.63962.93.
Farag NH, Vincent AS, Sung BH, Whitsett TL, Wilson MF, Lovallo WR. Caffeine tolerance is incomplete: persistent blood pressure responses in the ambulatory setting. Am J Hypertens. 2005;18(5 Pt 1):714–9. https://doi.org/10.1016/j.amjhyper.2005.03.738.
McMullen MK, Whitehouse JM, Shine G, Towell A. Habitual coffee and tea drinkers experienced increases in blood pressure after consuming low to moderate doses of caffeine; these increases were larger upright than in the supine posture. Food Funct. 2011;2(3–4):197–203. https://doi.org/10.1039/c0fo00166j.
Hu GL, Wang X, Zhang L, Qiu MH. The sources and mechanisms of bioactive ingredients in coffee. Food Funct. 2019;10(6):3113–26. https://doi.org/10.1039/c9fo00288j.
Higashi Y. Coffee and endothelial function: a coffee paradox? Nutrients. 2019;11(9):2104. https://doi.org/10.3390/nu11092104.
Kajikawa M, Maruhashi T, Hidaka T, Nakano Y, Kurisu S, Matsumoto T, Iwamoto Y, Kishimoto S, Matsui S, Aibara Y, Yusoff FM, Kihara Y, Chayama K, Goto C, Noma K, Nakashima A, Watanabe T, Tone H, Hibi M, Osaki N, Katsuragi Y, Higashi Y. Coffee with a high content of chlorogenic acids and low content of hydroxyhydroquinone improves postprandial endothelial dysfunction in patients with borderline and stage 1 hypertension. Eur J Nutr. 2019;58(3):989–96. https://doi.org/10.1007/s00394-018-1611-7.
Sasaki H, Hirasawa A, Washio T, Ogoh S. Acute effect of coffee drinking on dynamic cerebral autoregulation. Eur J Appl Physiol. 2016;116(5):879–84. https://doi.org/10.1007/s00421-016-3345-7.
Dwan K, Li T, Altman DG, Elbourne D. CONSORT 2010 statement: extension to randomised crossover trials. BMJ. 2019;366:l4378. https://doi.org/10.1136/bmj.l4378.
Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141(1):2–18. https://doi.org/10.1037/a0024338.
Sullivan GM, Feinn R. Using effect size-or why the P value is not enough. J Grad Med Educ. 2012;4(3):279–82. https://doi.org/10.4300/JGME-D-12-00156.1.
Acknowledgments
The authors express their sincere gratitude to Maria de Lourdes Guimarães Rodrigues, Débora Cristina Torres Valença, Wille Oigman and Sergio Emanuel Kaiser.
Funding
This research was funded by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author Contributions
Conceptualization, F.B.A.L.C., F.G.C, O.F., M.F.N. and M.R.S.T.K.; methodology, F.B.A.L.C., F.G.C., M.R.C, O.F., M.F.N. and M.R.S.T.K.; formal analysis, M.R.S.T.K.; investigation, F.B.A.L.C, F.G.C., S.P., O.F.; resources, O.F., M.N., M.K.; data curation, S.P., O.F., M.R.S.T.K.; writing—original draft preparation, F.B.A.L.C., F.G.C., S.P., O.F., M.R.S.T.K.; writing—review and editing, O.F., M.F.N., M.R.S.T.K.; visualization, M.R.S.T.K.; supervision, O.F., M.F.N., M.R.S.T.K.; project administration, O.F., M.F.N., M.R.S.T.K.; funding acquisition, O.F., M.R.S.T.K.. All authors have read and agreed to the published version of the manuscript.”
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Pedro Ernesto University Hospital (CAAE: 87599418.3.0000.5259, 06.06.2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lima de Castro, F.B., Castro, F.G., da Cunha, M.R. et al. Acute Effects of Coffee Consumption on Blood Pressure and Endothelial Function in Individuals with Hypertension on Antihypertensive Drug Treatment: A Randomized Crossover Trial. High Blood Press Cardiovasc Prev 31, 65–76 (2024). https://doi.org/10.1007/s40292-024-00622-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-024-00622-8