Abstract
Stains remain the first therapeutic approach in patients with dyslipidemia to control plasma lipids levels and cardiovascular risk. Multiple clinical trials have demonstrated the benefits of statins in reducing major cardiovascular adverse events in primary and secondary prevention. Moreover, in patients with coronary artery disease, statins decrease coronary atherosclerotic plaque volume and composition, inducing atheroma stabilization. Pitavastatin, is a new-generation lipophilic statin, indicated for the treatment of dyslipidemia and prevention of cardiovascular diseases. The purpose of this review, the first at our knowledge on this topic, is to summarize and examine the current knowledge about the effectiveness of pitavastatin in patients with coronary artery disease. The available data suggest that pitavastatin significantly, lowers the rate of adverse cardiovascular events, in patients at a high risk of atherosclerotic disease, with stable angina pectoris or with acute coronary syndrome. Moreover intravascular ultrasound have shown that pitavastatin induces favorable changes in plaque morphology, increasing the fibrous cap thickness, and decreasing both plaque and lipid volume indexes. Globally the efficacy of pitavastatin is greater or similar to other statins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Statins are still the mainstay for treatment of dyslipidemia and cardiovascular prevention [1]. Reduction cholesterol plasma levels is associated with a significant decrease of cardiovascular outcomes [2].This beneficial effect has been obtained with all available statins [3], particularly with high doses, to reach intensive reduction of LDL-C [1, 4].
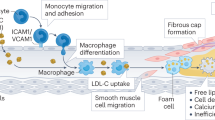
Coronary atherosclerosis is considered a chronic low-grade inflammatory disease, triggered by atherogenic lipoprotein (particularly oxLDL-C), reactive oxygen species, pro-inflammatory cytokines and macrophages infiltration into the arterial wall [5,6,7]. While most atherosclerotic plaques remain stable and silent for a long time, leading to slow progressive coronary narrowing, others become suddenly unstable or so called “vulnerable”. Intracoronary imaging have shown that the key aspect of a vulnerable plaque is the presence of a lipid rich core, (necrotic in some lesions) with an overlying thin fibrous cap < 65 microns [7,8,9,10]. It is now well established that thin-cap fibroatheroma (TCFA), plays a major role in plaque rupture, with subsequent coronary thrombosis [7,8,9,10,11,12]. Clinically these changes lead to acute coronary syndrome (ACS), including myocardial infarct, unstable angina, or cardiac arrest. Moreover vulnerable plaques are strongly and independently predictive of subsequent major acute cardiovascular events (MACE). This relationship has been well demonstrated in the PROMISE, PROSPECT, VIVA, and ATHEROREMO-IVUS studies [11, 13,14,15].
However, despite successful revascularization, there is evidence of a significant residual risk of future cardiovascular events, for different reasons: non-culprit thin cap fibroatheroma progression, incomplete revascularization, high plaque burden, persistent risk factors or increased inflammatory status [16,17,18].These findings emphasize the importance of an early identification and treatment of vulnerable plaques to avoid ACS.
Patients with coronary heart disease are recommended to use statins to reduce the risk of acute coronary syndrome. In addition to lower plasma lipids, there is growing evidence that statins, increasing fibrous cap thickness, have a beneficial effect in atheroma progression and in stabilizing high-risk coronary plaques [4, 19, 20].
Pitavastatin, a new-generation lipophilic statin, approved by the Food and Drugs Administration (FDA) and European Medicines Agency (EMA) is indicated for the treatment of dyslipidemia and prevention of cardiovascular diseases. Pitavastatin, 2 and 4 mg, is available in several countries, as brand or generics. The lipid lowering of the drug is either similar, or even greater [21,22,23] than that of other statins, with a high prevalence of patients achieving LDL-C target [21]. Globally pitavastatin, decreases total cholesterol (22–39%), LDL-C (40 to ~ 50%) and triglycerides (13–32%), according to approved dosage and increases HDL lipoproteins [21, 24, 25].
Recently, a large meta-analysis has reported, that pitavastatin (1 mg to 16 mg/day), is more potent than atorvastatin, rosuvastatin, and fluvastatin in reducing LDL-C [26]. However 16 mg/day is out of the approved and common therapeutic dosage. Considering the relationship between LDL-C and risk of major cardiovascular events ESC/EAS and ACC/AHA guidelines [1, 27] recommend statins not only for secondary, but also for primary cardiovascular prevention, according to LDL-C level and total cardiovascular risk.
The aim of this review, the first at our knowledge on this topic, is to summarize the relevant literature on the role of pitavastatin on primary and secondary cardiovascular prevention and, particularly, on quantitative and qualitative aspect of coronary atherosclerotic plaques.
A literature search was conducted in PubMed and EMBASE, using the keywords pitavastatin, coronary artery disease, cardiovascular prevention, atherosclerotic coronary plaques, to identify relevant scientific articles. We did not consider short communications, editorials and posters. Moreover we did not report changes in lipids, because beyond the scope of this review. The resulting articles were evaluated by the authors for suitability for the review.
A total of 42 publications were identified and evaluated, 29 were excluded and 13 were eligible and included in the review (Fig. 1). Overall a total of 8515 patients (age 61.4–68.1 years), were treated with pitavastatin during 2 to 240 weeks (Table 1). Pitavastatin (2–4 mg/day) was compared with low dose (1 mg/day), with placebo/diet, atorvastatin, (10–20 mg/day), pravastatin (10 mg/day), fluvastatin (30 mg/day). Most of the data available with pitavastatin and reported in our review have been obtained in Asian population.
2 Piatavastatin: Primary and Secondary Cardiovascular Prevention
In primary cardiovascular prevention, pitavastatin has been compared with atorvastatin in the TOHO-LIP, a multicenter open trial, with blinded endpoints [23]. This study included mainly patients (75.3%) with hypercholesterolemia and concomitant high cardiovascular risk factors (advanced age, diabetes, hypertension). The cumulative 5-year incidence of the primary endpoint (composite of cardiovascular death, sudden death, acute myocardial infarction, ischemic stroke, transient ischemic attack, or heart failure) was significantly lower in pitavastatin than in atorvastatin group (2.9% vs 8.1%, p = 0.006). Particularly repeated coronary revascularization, in non-culprit lesion, for stable angina, was needed in 4.5% and in 12.9%, (p < 0.001) of patients assigned to pitavastatin and atorvastatin respectively.

Several trials have also investigated the efficacy of pitavastatin in secondary cardiovascular prevention, in patients with stable coronary artery disease or with ACS.
The REAL-CAD [28], a multicenter, prospective study, with blinded end points, randomized 13,054 patients with stable coronary artery disease (CAD), either to 1 mg/day or 4 mg/day of pitavastatin for an average of 3.9 years. The primary end point was a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, or unstable angina, while the secondary end point also included coronary revascularization. Differently from the low dose, the high-dose of pitavastatin, significantly improved the cumulative incidence of the primary (4.6% vs 5.6%; p = 0.01) and secondary end point (8.5% vs 10.4%; p = 0.002). A significantly more cardiovascular relative risk reduction (RRR) was obtained in patients with diabetes (− 25%), or aged < 65 years (− 33%).

Differently from these studies that have been performed in patients at high cardiovascular risk or with stable coronary disease, the CIRCLE and LAMIS trials [29, 30] evaluated patients with acute myocardial infarct (AMI), undergoing percutaneous coronary intervention (PCI).
The CIRCLE study [29], compared pitavastatin, with pravastatin, atorvastatin and no statin (control group). After 70 months of treatment, the rate of MACE occurred in 8.3%, of patients treated with pitavastatin, in 27.2%, 19.3% and 35.1% of subjects assigned to pravastatin, atorvastatin and control group, respectively (p < 0.001). Multivariate analysis, after adjusting for different clinical factors, revealed that, compared with the controls, the relative risk of cardiovascular events was reduced by 51% with pitavastatin, by 21% and 27% with pravastatin and atorvastatin (p < 0.001). Particularly pitavastatin, differently from the other statins, significantly decreased the rate of recurrent PCI, in both, new coronary lesions (4.4% vs 12.6% and 9.9% p = 0.003) and target lesion (3.3% vs 12.6% and 7.5%, p < 0.01). The low rate of MACE observed in the CIRCLE study [29] has been confirmed by the LAMIS trial (7.3%), performed in patients with AMI, treated with pitavastatin before revascularization and followed for 12 months [30].
Overall these findings clearly demonstrate a significant benefit of pitavastatin (2–4 mg/day), in reducing the rate of major cardiovascular events, in patients at high risk for atherosclerotic disease and in those with stable CAD, or recent AMI.
3 Pitavastatin: Effect on Quantitative and Qualitative Aspects of Atherosclerotic Plaque
Many studies, carried out in patients with ACS or stable coronary artery disease, have evaluated the changes induced by pitavastatin on quantitative and qualitative aspects of non-culprit coronary atherosclerotic plaque, using a variety of intracoronary imaging techniques, [Intravascular ultrasound (IVUS), virtual histology intravascular ultrasound (VH-IVUS), integrated backscatter intravascular ultrasound (IB-IVUS), angioscopy or optical coherence tomography (OCT)].
These effects have been investigated in comparison with placebo [31], atorvastatin [32,33,34], pravastatin [34, 35] and fluvastatin [34], as well in open-label trials.
In a trial performed in patients with scheduled or primary PCI [31], pitavastatin administered within 48 h from revascularization, significantly lowered by 10,6% plaque volume index that, instead, was increased by 8.1% in the placebo group (p < 0.001).
Similar effect has been obtained comparing pitavastatin with atorvastatin [32] in patients with ACS. Patients treated with pitavastatin showed a significantly reduction of atheroma volume index (− 2.6%, p < 0.001), which instead increased by + 0.2% in atorvastatin treated subjects. It is noteworthy that this result was achieved after a very short time of statins treatment (2–3 weeks) and, particularly, in patients with dense calcium plaque ratios < 10%.
Conversely in the JAPAN-ACS [33], atorvastatin and pitavastatin, started within 72 h after successful revascularization, led to a similar changes in coronary plaque volume, (pitavastatin − 16.9%, atorvastatin − 18.1%, p = 0.5), indicating a non-inferiority of pitavastatin, compared with atorvastatin.
Further beneficial effects of pitavastatin have been evaluated using virtual histology intravascular ultrasound (VH-IVUS), a method for accurate analysis of coronary atherosclerotic plaques composition. In patients with angina pectoris [34, 35], pitavastatin, differently from pravastatin, decreased coronary plaque volume index (− 5.0% vs + 1.1%) and the amount of fibro-fatty component (− 25.7% vs − 20.9%). This finding has also been obtained in the YOKOHAMA-ACS study performed in patients with ACS [36], treated 72 h after revascularization. At 10 months follow-up, atheroma volume was significantly lowered in pitavastatin, but not in pravastatin (− 8.1% vs + 0.4%) and also in fluvastatin (30 mg/day) treated patients (+ 3.1%), while the effect of pitavastatin and atorvastatin were comparable (− 8.1% and − 11.1%). However the dose of fluvastatin in this study have been very low, because high dose (60 mg/day) has been reported to be associated with a significant regression of plaque volume [37].
Angioscopy is an interesting tool to detect the lipid core of coronary atherosclerosis [8, 38, 39]. This imaging modality has been used in the TOGETHAR [40] trial, that assessed the effectiveness of pitavastatin in coronary plaques with large lipid core (yellow grade ≥ 2). After 52 weeks of treatment yellow grade intensity was significantly lowered from 2.9 to 2.6, (p < 0.04), and was correlated with the maximum yellow grade at baseline. Therefore, by decreasing yellow grade, pitavastatin stabilized coronary plaque vulnerability, given the negative relationship between high lipid-rich core and fibrous cap thickness [8, 41].
A complete overview of atheroma changes induced by pitavastatin has been evaluated in a study that used, in the same patients and simultaneously, angiography, serial optical coherence tomography (OCT), grayscale IVUS and integrated backscatter-IVUS imaging [42]. Patients with stable CAD, undergoing elective PCI, were treated with pitavastatin or with diet (control group) for 9 months. Differently from controls, pitavastatin significantly decreased plaque volume and lipid volume indexes (− 6.5%, p = 0.03 and − 6.7%, p = 0.02 respectively) and significantly increased by 35% (p = 0.001) fibrous cap thickness.

Coronary Angiographic, Grayscale IVUS, IB-IVUS, and OCT at Baseline (A) and at Follow-Up (B)
Taken all together, the results of these studies highlight that pitavastatin (2–4 mg/day) decreases the incidence of MACE in primary and secondary prevention, suppresses atherosclerotic plaques progression and leads to stabilization of vulnerable plaques, an important hallmark correlated with reduced risk of acute cardiovascular events [10, 11, 13,14,15].
4 Potential Pharmacological Mechanism Involved in the Effects on Coronary Plaques
While the favourable effect of statins, in primary and secondary cardiovascular prevention, is well documented, the mechanism by which statins slow the progression of coronary atherosclerotic lesions and, particularly, modify atheroma components, is not precisely understood [19, 20]. However there is evidence that patients with ACS, treated with statins, before PCI, have an improvement of cardiovascular outcomes [43,44,45]. Likewise, it has been shown, that the risk of PCI-related peri-procedural complications and MACE [20, 46,47,48] can be significantly prevented by statins preloading, (before PCI), both in patients with ACS or with stable coronary artery disease [1, 49,50,51]. The cardiovascular protection of statins pretreatment, both in subjects already in treatment or in naïve-statins patients, does not appear to be due to the significant reduction of plasma lipids levels, but rather to two differential time-related effects, on one side an early increase of fibrous cap thickness and on the other the reduction of coronary plaque volume [19, 44, 52].
The effect of early statin therapy on the atheroma components has been highlighted by the ESCORT study [53], performed in two groups of patients with ACS, which started pitavastatin either within 24 h (early statin group), of intravascular optical coherence tomography (OCT), or 3 weeks after (late statin group). After 3 weeks, minimum fibrous-cap thickness was significantly greater only in the early treated group (+ 20 μm, p < 0.05), while this parameter was decreased in the late treated group, (− 5 μm, p < 0.05), without any change on plaque lipid volume in both groups. Conversely, after a long period (36 months), fibrous-cap thickness significantly increased further, while plaque lipid volume significantly decreased in all patients. Therefore pitavastatin, after a short period, increased only fibrous-cap thickness, lowering plaque vulnerability, while after a long period it also decreased plaque volume. These results confirm the finding of a previous study which has shown an increase in fibrous-cap thickness with simultaneously, angiography, serial optical coherence tomography (OCT), grayscale IVUS and integrated backscatter-IVUS imaging [42].
The potential pharmacological mechanism for the early impact of statins on atherosclerotic plaque vulnerability, might be related, besides the lipid-lowering, with the so-called “pleitropic effects” [54] and, particularly, with the antithrombotic and anti-inflammatory properties [50, 55].
Pitavastatin, has been reported, improves endothelial function, decreases some markers of platelet activation, reduces oxidative stress and modulates vascular inflammatory pathway [23, 56]. These effects have been evaluated in “vitro” [57], in animal models [58], as well in patients with atherosclerotic lesions [59].
5 Appropriate Dosage
The dosage of pitavastatin in cardiovascular prevention deserves some comments. Although, in most studies, high dose (4 mg/day), significantly protected patients from recurrent cardiovascular events, the LAMIS II trial [60], performed in subjects with AMI, did not show significant difference between 2 and 4 mg/day of pitavastatin. The primary efficacy endpoint (composite of cardiac death, nonfatal myocardial infarction, target-lesion revascularization, hospitalization for unstable angina, heart failure or arrhythmic events) and the secondary efficacy endpoint (target vessel revascularization + MACE) occurred in 9.1–9.1% and in 9.5–9.8% respectively, confirming the results of LAMIS, TOGETHAR and TOHO-LIP studies [23, 29, 30, 40]. However, as the lipids lowering of pitavastatin is dose dependent [61, 62], 4 mg/day, would be the suitable dosage for secondary CV prevention, as reported, particularly in the REAL-CAD and ESCORT trials [28].
6 Cost-Effectiveness
There are several reports that support the cost-effectiveness of pitavastatin treatment, although this item may be a minor problem since the drug is available as generic in an increasing number of countries all over the world. Jeong et al. [63] performed a comparison of the cost-effectiveness of statins according to the baseline low-density lipoprotein cholesterol level in Korea: cost of pitavastatin was lower than pravastatina, atorvastatin or simvastatin after getting the same LDL cholesterol reduction. Sansanayudh et al. [64] Comparative Efficacy and Safety of Low-Dose Pitavastatin Versus Atorvastatin in Patients with Hypercholesterolemia. Pitavastatin 1 mg once daily was associated with very low monthly cost per percent LDL-C reduction ($0.77) compared with atorvastatin 10 mg once daily ($1.56).
7 Conclusions
Our review, the first, to our knowledge on this topic, provides the evidence that pitavastatin decreases the rate of cardiovascular outcomes, in primary and secondary cardiovascular prevention, both in patients with ACS or stable CAD. Moreover the impact of pitavastatin on atheroma volume and composition has been demonstrated in several studies by using different methods of imaging. Globally the effectiveness of pitavastatin is better or not inferior to that of other statins. The 2019 ESC/EAS guidelines [1] consider pitavastatin, together with rosuvastatin and atorvastatin, a suitable choice, for cardiovascular prevention in patients with dyslipidemia, particularly for the absence of metabolic drug–drug interactions. Moreover early therapy with pitavastatin in patients with ACS increases fibrous-cap thickness and decreases the volume and lipid content of vulnerable coronary lesions. This aspect is underlined by the ESC/EAS guidelines [1], which recommend to initiate soon statin treatment, before PCI, in patients with ACS, to lower the risk of peri-procedural or future MACE. The beneficial effect of statins in patients with CAD is the result on one side of the lipids lowering activity and on the other of the cholesterol-independend “pleiotropic effects”. Cardiologists can consider pitavastatin as an alternative therapeutic choice, bearing in mind the cost-effectiveness, in comparison with other statins with similar efficacy.
References
Mach F, Baigent C, Catapano AL, et al. ESC/EAS Guidelines for the management of dyslipidaemia: lipids modification to reduce cardiovascular risk. Eur Heart J. 2019;2019(00):1–78.
Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–97.
Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81.
Wang S, Xiu J, Liao W, et al. Relative effect of current intensive lipid-lowering drugs on cardiovascular outcomes in secondary prevention—a meta-analysis of 12 randomized trials. Circ J. 2019;83:1356–67.
Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–51.
Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci (Lond). 2018;132:1243–52.
Boren J, Chapman MJ, Ronald M, Krauss RM, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41:2313–30.
Garcia-Garcia HM, Costa MA, Serruys PW. Imaging of coronary atherosclerosis: intravascular ultrasound. Eur Heart J. 2010;3:2456–69.
Batty JA, Subba S, Luke P, et al. Intracoronary imaging in the detection of vulnerable plaques. Curr Cardiol Rep. 2016;18:1–12.
Vergallo R, Porto I, D’Amario D, et al. Coronary atherosclerotic phenotype and plaque healing in patients with recurrent acute coronary syndromes compared with patients with long-term clinical stability: an in vivo optical coherence tomography study. JAMA Cardiol. 2019;4:321–9.
Ferencik M, Mayrhofer T, Bittner DO, et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol. 2018;3:144–52.
Iannaccone M, Quadri G, Taha S, et al. Prevalence and predictors of culprit plaque rupture at OCT in patients with coronary artery disease: a meta-analysis. Eur Heart J Cardiovasc Imaging. 2016;17:1128–37.
Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35.
Calvert PA, Obaid DR, O’Sullivan M, et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in Vulnerable Atherosclerosis) Study. JACC Cardiovasc Imaging. 2011;4:894–901.
Cheng JM, Garcia-Garcia HM, de Boer SP, et al. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Eur Heart J. 2014;35:639–47.
Guedeney P, Claessen BE, Kalkman DN, et al. Residual inflammatory risk in patients with low LDL cholesterol levels undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2019;73(19):2401–9.
McPherson JA, Maehara A, Weisz G, et al. Residual plaque burden in patients with acute coronary syndromes after successful percutaneous coronary intervention. JACC Cardiovasc Imaging. 2012;5:S76-85.
Bangalore S, Guo Y, Samadashvili Z, et al. Outcomes with complete versus incomplete revascularization in patients with multivessel coronary disease undergoing percutaneous coronary intervention with everolimus eluting stents. Am J Cardiol. 2020;125:362–9.
Ozaki Y, Garcia-Garcia HM, Solomon S, Beyene SS, et al. Effect of statin therapy on fibrous cap thickness in coronary plaque on optical coherence tomography—review and meta-analysis. Circ J. 2019;83:1480–8.
Xiao Y, He S, Zhang Z, et al. Effect of high-dose statin pretreatment for myocardial perfusion in patients receiving percutaneous coronary intervention (PCI): a meta-analysis of 15 randomized studies. Med Sci Monit. 2018;24:9166–76.
Saito Y. Treatment options for hypercholesterolemia and combined dyslipidemia: focus on pitavastatin. Clin Med Insights Ther. 2011;3:517–25.
Hoy SM. Pitavastatin: a review in hypercholesterolemia. Am J Cardiovasc Drugs. 2017;17:157–68.
Moroi M, Nagayama D, Hara F, et al. Outcome of pitavastatin versus atorvastatin therapy in patients with hypercholesterolemia at high risk for atherosclerotic cardiovascular disease. Int J Cardiol. 2020;305:139–46.
Chan P, Shao L, Tomlinson B, et al. An evaluation of pitavastatin for the treatment of hypercholesterolemia. Expert Opin Pharmacother. 2019;20:103–13.
Tokgözoglu L, Zamorano JL. Current perspectives on the use of statins in the treatment of dyslipidaemic patients: focus on pitavastatin. Drugs Context. 2020;9:1–11.
Adams SP, Alaeiilkhchi N, Wright JM. Pitavastatin for lowering lipids. Cochrane Database Syst Rev. 2020;6: CD012735.
Arnett DK, Blumenthal RS, Albert MA, et al. ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:563–95.
Taguchi I, Iimuro S, Iwata H. High-dose versus low-dose pitavastatin in japanese patients with stable coronary artery disease (REAL-CAD) A randomized superiority trial. Circulation. 2018;137:1997–2009.
Maruyama T, Takada M, Nishibori Y, et al. Comparison of preventive effect on cardiovascular events with different statins—The CIRCLE Study. Circ J. 2011;75:1951–9.
Suh SY, Rha S-W, Ahn TH, et al. Long-term safety and efficacy of Pitavastatin in patients with acute myocardial infarction (from the Livalo Acute Myocardial Infarction Study [LAMIS]). Am J Cardiol. 2011;108:1530–5.
Takashima H, Ozaki Y, Yasukawa T, et al. Impact of lipid-lowering therapy with pitavastatin, a new HMG-CoA reductase inhibitor, on regression of coronary atherosclerotic plaque. Circ J. 2007;71:1678–84.
Toi T, Taguchi I, Yoneda S, et al. Early effect of lipid-lowering therapy with pitavastatin on regression of coronary atherosclerotic plaque—comparison with atorvastatin. Circ J. 2009;73:1466–72.
Hiro T, Kimura T. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol. 2009;54:293–302.
Nozue T, Yamamoto S, Tohyama S, et al. Statin treatment for coronary artery plaque composition based on intravascular ultrasound radiofrequency data analysis. Am Heart J. 2012;163:191–9.
Nozue T, Yamamoto S, Tohyama S, et al. Comparison of the effects of pitavastatin versus pravastatin on coronary artery plaque phenotype assessed by tissue characterization using serial virtual histology intravascular ultrasound. Heart Vessels. 2015;30:36–44.
Matsushita K, Hibi K, Komura N, et al. Effects of 4 statins on regression of coronary plaque in acute coronary syndrome. Circ J. 2016;80:1634–43.
Nasu K, Tsuchikane E, Katoh O, et al. Effect of fluvastatin on progression of coronary atherosclerotic plaque evaluated by virtual histology intracascular ultrasound. JACC Cardiovasc Interv. 2009;2:689–96.
Ueda Y, Ohtani T, Shimizu M, Hirayama A, Kodama K. Assessment of plaque vulnerability by angioscopic classification of plaque color. Am Heart J. 2004;148:333–5.
Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–72.
Kodama K, Komatsu S, Ueda Y, et al. Stabilization and regression of coronary plaques treated with pitavastatin proven by angioscopy and intravascular ultrasound: The TOGETHAR trial. Circ J. 2010;74:1922–8.
Kubo T, Imanishi T, Takarada S, et al. Implication of plaque color classification for assessing plaque vulnerability: a coronary angioscopy and optical coherence tomography investigation. JACC Cardiovasc Interv. 2008;1:74–80.
Hattori K, Ozaki Y, Ismail TF, et al. Impact of statin therapy on plaque characteristics as assessed by serial OCT, grayscale and integrated backscatter–IVUS. Am Coll Cardiol Imaging. 2012;5:169–77.
Navarese EP, Kowalewski M, Andreotti F, et al. Meta-analysis of time-related benefits of statin therapy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am J Cardiol. 2014;113:1753–6.
Gili S, Iannaccone M, Colombo F, et al. Effects of statins on plaque rupture assessed by optical coherence tomography in patients presenting with acute coronary syndromes: insights from the optical coherence tomography (OCT)-FORMIDABLE registry. Eur Heart J Cardiovasc Imaging. 2018;19:524–31.
Vervueren PL, Elbaz M, Dallongeville J, Arveiler D, Ruidavets JB, Montaye M, et al. Relationships between chronic use of statin therapy, presentation of acute coronary syndromes and one-year mortality after an incident acute coronary event. Int J Cardiol. 2013;163:102–4.
Neumann F-J, Sousa-Uva M, Ahlsson A, et al. ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2018;2019(40):87–165.
Goliasch G, Winter MP, Ayoub M, et al. A contemporary definition of periprocedural myocardial injury after percutaneous coronary intervention of chronic total occlusions. JACC Cardiovasc Interv. 2019;12:1915–23.
Cavallini C, Savonitto S, Violini R, et al. Impact of the elevation of biochemical markers of myocardial damage on long-term mortality after percutaneous coronary interventions: results of the CK-MB and PCI study. Eur Heart J. 2005;26:1494–8.
Soud M, Ho G, Kuku KO, et al. Impact of statins preloading before PCI on periprocedural myocardial infarction among stable angina pectoris patients undergoing percutaneous coronary intervention: a meta-analysis of randomized controlled trials. Cardiovasc Revasc Med. 2018;19:971–5.
Patti G, Cannon CP, Murphy SA, et al. Clinical benefit of statin pretreatment in patients undergoing percutaneous coronary intervention. Circulation. 2011;123:1622–32.
Zhai C, Cong H, Liu Y, et al. Effect of high-dose statin pretreatment on the incidence of periprocedural myocardial infarction in patients undergoing percutaneous coronary intervention: grading the evidence through a cumulative meta-analysis. Clin Cardiol. 2015;38:668–78.
Hou J, Xing L, Jia H, et al. Comparison of intensive versus moderate lipid-lowering therapy on fibrous cap and atheroma volume of coronary lipid-rich plaque using serial optical coherence tomography and intravascular ultrasound imaging. Am J Cardiol. 2016;117:800–6.
Nishiguchi T, Kubo T, Tanimoto T, et al. Effect of early pitavastatin therapy on coronary fibrous-cap thickness assessed by optical coherence tomography in patients with acute coronary syndrome. The ESCORT study. JACC Cardiovasc Imaging. 2018;11:829–38.
Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017;120:229–43.
Diamantis E, Kyriakos G, Quiles-Sanchez L. The anti-inflammatory effects of statins on coronary artery disease: an updated review of the literature. Curr Cardiol Rev. 2017;13:209–16.
Davignon J. Pleiotropic effects of pitavastatin. Br J Clin Pharmacol. 2011;73:518–35.
Chen LW, Lin CS, Tsai MC, et al. Pitavastatin exerts potent anti-inflammatory and immunomodulatory effects via the suppression of AP-1 signal transduction in human T cells. Int J Mol Sci. 2019;20:2–15.
Qadir F, Alam SM, Siddiqi AQ, Kamran A. Pitavastatin is a potent anti-inflammatory agent in the rat paw model of acute inflammation. Pak J Pharm Sci. 2014;27:2169–75.
Watanabe T, Kawasaki M, Tanaka R, et al. Anti-inflammatory and morphologic effects of pitavastatin on carotid arteries and thoracic aorta evaluated by integrated backscatter trans-esophageal ultrasound and PET/CT: a prospective randomized comparative study with pravastatin (EPICENTRE study). Cardiovasc Ultrasound. 2015;2(13):17.
Hong YJ, Jeong MH, Bae JH, et al. Efficacy and safety of pitavastatins in patients with acute myocardial infarction: Livalo in Acute Myocardial Infarction Study (LAMIS) II. Korean J Intern Med. 2017;32:656–67.
Catapano AL. Pitavastatin: a different pharmacological profile. Clin Lipid Suppl. 2012;7:3–9.
Luo Z, Zhang Y, Gu J, et al. Pharmacokinetic properties of single- and multiple-dose pitavastatin calcium tablets in healthy Chinese volunteers pharmacokinetic properties of single- and multiple-dose. Curr Ther Res. 2015;77:52–7.
Jeong YJ, Kim H, Baik SJ, Kim TM, Yang SJ, Lee SH, et al. Analysis and comparison of the cost-effectiveness of statins according to the baseline low-density lipoprotein cholesterol level in Korea. J Clin Pharm Ther. 2017;42:292–300.
Sansanayudh N, Wongwiwatthananukit S, Putwai P, Dhumma-Upakorn R. Comparative efficacy and safety of low-dose pitavastatin versus atorvastatin in patients with hypercholesterolemia. Ann Pharmacother. 2010;44:415–23.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fici, F., Faikoglu, G., Tarim, B.A. et al. Pitavastatin: Coronary Atherosclerotic Plaques Changes and Cardiovascular Prevention. High Blood Press Cardiovasc Prev 29, 137–144 (2022). https://doi.org/10.1007/s40292-021-00496-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-021-00496-0