Abstract
The implantable cardioverter-defibrillator (ICD) is the most effective therapy to prevent sudden cardiac death (SCD) in high-risk patients. To overcome infections and failure of transvenous leads, the most frightening complications of conventional ICDs, a completely subcutaneous ICD (S-ICD) has been developed and is currently adopted in routine clinical practice. In view of their long life-expectancy, low competitive risk of dying from non-arrhythmic causes, and high lifetime risk of lead-related complications requiring surgical revisions, young patients with cardiomyopathies and inherited arrhythmia syndromes have traditionally been considered ideal candidates for the S-ICD. However, as growing evidence supported S-ICD safety and efficacy, initial niche implant indications were abandoned in favor of a widespread use of this technology, that is currently adopted in common ICD candidates with severe left ventricular dysfunction. Indeed, guidelines recommend S-ICD implantation as an alternative to TV-ICDs in all ICD candidates, unless pacing is required. This review focuses on the contemporary experience with the S-ICD and explores future scenarios in which device-to-device communication will enable to combine leadless therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sudden cardiac death (SCD), defined as unexpected fatal event due to cardiac disease, is the most common cause of death, accounting for 20% of all deaths in Europe [1]. In young subjects, the predominant causes are channelopathies and cardiomyopathies, while in the adults, coronary artery disease (CAD) is more prevalent. The implantable cardioverter-defibrillator (ICD) is the most effective therapy to prevent SCD in high-risk patients [2, 3]. However, trans-venous ICD (TV-ICD) systems are associated with peri-implantation and long-term complications (systemic infections or endocarditis, lead failure, venous thrombosis) [4]. Particularly, the cumulative incidence of trans-venous lead failure reaches 40% eight years after implantation [5]. To overcome lead-related complications and morbidity associated with conventional ICDs, a non-transvenous ICD has been developed: the subcutaneous ICD (S-ICD).
2 Fundamentals of the S-ICD System
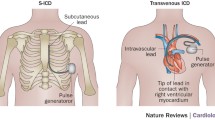
The S-ICD consists of a subcutaneous parasternal defibrillation lead and a pulse generator (PG) that is implanted in a pocket on the left lateral chest [6]. The S-ICD does not provide pacing for bradycardia or tachycardia (ATP), except for limited post-shock transcutaneous pacing [7]. The device is provided with remote monitoring, magnetic resonance compatibility and atrial fibrillation monitoring [8].
The S-ICD senses cardiac rhythm through three subcutaneous ECG signals (primary, secondary and alternate vectors) recorded by two sensing electrodes and the PG. It is crucial that ECG signals are correctly interpreted to avoid double QRS counting, T-wave or noise oversensing. Therefore, pre‐implant ECG screening is required to select patients with suitable subcutaneous signals. During screening, three electrodes are positioned on the chest in the same position of the S‐ICD distal and proximal lead sensing electrodes and the PG. ECG recordings are collected in supine and standing postures. In the early days, eligibility required manual screening, with QRS-T complexes superimposed on a plastic ruler provided with template boxes. Either the maximal R or S waves of the QRS complex and the T wave had to be contained within the profile of the template. More recently, an automated screening tool (AST) has been developed that shares digital filters with the sensing algorithm of the S-ICD (Fig. 1). AST predicts the actual performance of the sensing algorithm after implantation, facilitates screening, and reduces subjectivity associated with the manual procedure. When compared to manual screening, AST is associated with higher pass rate due to greater tolerance to high-amplitude T-waves [9].
Pre-implantation S-ICD screening with the automated screening tool (AST) for the primary, secondary, and alternate vectors. AST processes the signals by applying the digital filter that is also embedded in the sensing algorithm of the S-ICD. T waves from digitally filtered signals are lower in amplitude and smoothened as compared with unfiltered vectors
Of note, many patients implanted with an S-ICD in whom the screening predicts non-eligibility have normally working defibrillation systems, suggesting that inaccurate prediction of ineligibility may be a specific weakness of screening tools.
3 Implantation and Defibrillation Threshold Testing
The original S-ICD implantation technique included three incisions: one on the left-lateral chest for the PG, and two on the left margin of the sternum for lead tunneling. More recently, an implantation technique that avoids the third superior parasternal incision (so-called “two-incision technique”) and uses an intermuscular pocket (between the serratus anterior and the latissimus dorsi muscles) for PG implantation has been developed [10]. The combination of these two techniques offers superior cosmetic results and likely reduces skin erosion and device infection [11]. Of note, intermuscular positioning of the PG prevents fat tissue interposition between the ICD and the thorax, thus reducing shock impedance and, possibly, defibrillation threshold (Fig. 2).
Chest radiograph of a patient implanted with a subcutaneous ICD. On lateral view (panel a), the amount of sub-coil fat and pulse generator placement in relation to the midline can be appreciated. On postero-anterior view (panel b) the amount of sub-generator fat can be determined. Sub-coil and sub-generator fat interposition is a well-known predictor of shock failure
After implantation, defibrillation threshold (DFT) is typically determined. VF is induced using 50 Hz current delivered by the device itself and a 65J shock is delivered. Independent studies have confirmed high rates of successful 65J DFT in clinical practice, ranging from 92 to 97% [12,13,14,15,16,17]. To predict the outcome of DFT testing, a chest radiograph-based score (the PRAETORIAN score) has been developed and validated [18]. With a three step approach, the PRAETORIAN score assesses (1) the amount of fat tissue between the sternum and the coil on the lateral chest X-ray, (2) the position of the S-ICD in relation to the mid-line on the lateral chest X-ray and (3) the amount of fat between the device and the thoracic wall on the postero-anterior chest X-ray. A low PRAETORIAN score is associated with a very low risk of conversion failure. Indeed, in a retrospective validation of two cohorts of S-ICD patients, a low PRAETORIAN score resulted in a negative predictive value of 99,8%, with sensitivity and specificity of 95% and 95%, respectively. A prospective randomized trial [19] is ongoing and will answer the question whether DFT testing should be performed when S-ICD implantation is optimized based on the PRAETORIAN score.
4 Clinical Experience with the S-ICD
4.1 General Considerations
According to current guidelines [20], all ICD candidates have a class IIa indication for an S-ICD as an alternative to the TV-ICD unless pacing for bradycardia, tachycardia, or resynchronization is needed or anticipated. Of note, patients who meet criteria for an ICD who have inadequate vascular access or are at high risk for infections, and in whom pacing is neither needed nor anticipated, have a class I indication [20]. The American Heart Association guidelines updated the indications for S-ICD implantation based of multiple non-randomized large prospective studies.
In the early S-ICD registries patients were younger, had a better left ventricular ejection fraction (LVEF) and less comorbidities than real-world traditional ICD patients. Indeed, in the IDE [21] and the EFFORTLESS [22] registries, only one third of patients had ischemic cardiomyopathy, mean age was 50 years, and LVEF 40%. The EFFORTLESS registry reported an appropriate shock rate of 5.8% and 13.5% at 1 and 5 years, respectively, while inappropriate shocks occurred in 8.1% at 1 year and 11.7% at 3.1 years. Overall, defibrillation success rate for spontaneous ventricular tachycardia or ventricular fibrillation (VT/VF) was 97.4%. In a smaller but long-term study [23], the 6-year follow up of a typical early S-ICD population showed 17% of appropriate shocks, 21% of inappropriate shocks, and 3% per year of complications, with no systemic infections or lead failures .
In the early S-ICD experience the rate of inappropriate shocks was in the range of 5–25%. The main reasons for inappropriate interventions were supraventricular tachycardia (SVT) and T-wave oversensing. Reducing inappropriate shocks requires a three steps approach. First, accurate patient selection and careful pre-implantation ECG screening. Second, programming two zones for arrhythmia detection (a conditional zone with discrimination algorithms for SVTs and a VF zone with detection based solely on heart rate). Third, activating specific algorithms for T wave filtering that reduce double sensing.
Routine pre-implantation ECG screening during exercise is not recommended. However, it has been reported that disease-specific changes in subcutaneous signals may occur during exercise, such as in hypertrophic cardiomyopathy [24, 25]. These patients should therefore always undergo exercise screening. Heart rate-dependent bundle branch block is another condition that deserves special consideration. These patients can be safely managed by storing the wide QRS as the reference S-ICD ECG template.
As growing evidence supported S-ICD safety and efficacy, niche indications were abandoned in favor of a more extensive use of this technology, now adopted in patients with traditional ICD indications [3]. The prospective US registry S-ICD post-approval study [12] depicts a more contemporary scenario of clinical characteristics and acute outcomes of real-world patients implanted with an S-ICD, mostly affected by ischemic heart disease with low LVEF (32±15 %) and multiple comorbidities. In this setting, the S-ICD acute successful conversion rate was 98.7%, and the 30-day complication rate was 3.7%, supporting efficacy and safety of the system in the real-world setting.
4.2 Left Ventricular Dysfunction
Primary prevention patients with heart failure (HF) and reduced LVEF represent the vast majority of ICD candidates, including more than 70% of new ICD implants [13]. Two studies retrospectively evaluated the potential suitability for an S-ICD in independent cohorts of patients implanted with TV-ICDs who did not need bradycardia pacing at the time of implantation. At follow-up, 55 to 69% of these patients would have been clinically eligible for an S-ICD, with very low probability of developing a pacing indication for bradycardia or CRT during follow up [26, 27]. Indeed, the number of S-ICD recipients with LV dysfunction grew over years: as reported by a multicentre italian registry, the proportion of S-ICD patients with LVEF ≤35% increased from ≤2014 (33%) to 2017 (53%) [15]. This paradigm shift prompted a registry specifically designed to describe the outcomes of a contemporary S-ICD study population with reduced LVEF: the Understanding Outcomes with the S-ICD in Primary Prevention Patients with Low Ejection Fraction Study (UNTOUCHED) [28]. In the UNTOUCHED registry, that enrolled primary prevention patients with LVEF ≤35% and no pacing indication, 1.116 patients were implanted with an S-ICD and prospectively followed. S-ICD implantation success rate was 99.6%, with 93.5% of patients having a DFT ≤65 J. The rate of 30-day freedom from complications was 95.8%. These data reinforced the concept that S-ICD therapy has low perioperative complication rates and high conversion efficacy of induced VF also in a large higher-risk cohort with low LVEF. The two-year follow up of these patients is expected to assess mid-term safety and efficacy.
4.3 Cardiomyopathies
In view of their low competitive risk of dying from non-arrhythmic causes, young patients with cardiomyopathies [29] have traditionally been considered optimal candidates for the S-ICD [30]. Indeed, lead failure and infections are the most common complications in young patients implanted with an ICD [5]. Even excluding inappropriate shocks, the rate of transvenous ICD-related complications requiring surgical revision is as high as >2% per year, with an unacceptably high cumulative long-term incidence [31].
Due to the characteristic increase in left ventricular mass and profound ECG abnormalities, patients with hypertrophic cardiomyopathy (HCM) were initially considered poor candidates for the S-ICD. The main concerns were the risk of shock failure due to extreme left ventricular hypertrophy and of screening failure and/or inappropriate therapies due to T wave oversensing.
High DFT in HCM patients has been reported in some [32] but not all studies [33]. Concerning S-ICD, in an initial experience [34] HCM patients had a DFT well below the maximal 80J output. Moreover, data collected from a large unselected S-ICD population [35] showed that there is no association between inadequate ICD energy safety margin and HCM. Finally, two independent studies have shown that the S-ICD defibrillation success rate is comparable to that of TV-ICDs [36, 37]. Taken together, these studies suggest that S-ICDs are safe and effective in HCM patients (Fig. 3).
S-ICD eligibility on pre-implant ECG screening has been reported in the range of 85% to 90% [24, 38]. However, recent studies that assessed ECG changes occurring during exercise reported lower rates of eligibility [25, 39]. Overall, screening failure in HCM seems to be relatively low, provided that patients with more challenging QRS-T morphologies are carefully evaluated both at rest and during exercise. Of note, the annual risk of inappropriate therapies due to ECG abnormalities in S-ICD HCM patients is low [40] and similar to those without HCM [37].
The S-ICD is an attractive technology also for patients with arrhythmogenic right ventricular cardiomyopathy (ARVC). However, the peculiar substrate of ARVC, consisting in progressive loss of myocardium with fibrofatty replacement, may pose peculiar eligibility concerns. Indeed, loss of R wave voltage due to fibrofatty replacement forces the ICD to operate with higher sensitivity, thus entailing the risk of P- or T-wave oversensing and inappropriate shocks. Moreover, monomorphic VTs amenable for painless ATP (unavailable on the S-ICD) are relatively frequent in ARVC patients with stable myocardial scars. Despite these concerns, the worldwide experience in ARVC patients with the S-ICD showed relatively low rate of inappropriate shocks, appropriate detection and treatment of true VT/VF, and no need for ICD extraction because of the need for bradycardia support or anti-tachycardia pacing [41]. This latter aspect seems to be of utmost importance, as the S-ICD has traditionally been considered unsuitable for ARVC patients due to the lack of ATP. Recent evidence suggest that older patients with advanced disease more often show re-entrant VTs around stable myocardial scars, while young patients commonly experience sudden onset of VF, reflecting the acute electrical instability of the early stages of the disease [41, 42]. Therefore, in primary prevention patients, the risk of ICD shocks triggered by monomorphic VTs should be balanced against the long-term complications of transvenous defibrillation leads. Moreover, catheter ablation should be considered as a strategy for eliminating frequent monomorphic sustained VTs.
4.4 Inherited Arrhythmia Syndromes
Inherited arrhythmia syndromes (IAS), including long QT syndrome, Brugada syndrome (BrS) and catecholaminergic polymorphic ventricular tachycardia, mostly affect young subjects with long life expectancy. Moreover, in patients with IAS the index arrhythmia is usually VF or polymorphic VT, which is unresponsive to ATP. Therefore, life-saving non-transvenous technologies have clear advantages in these patients. Overall, data from large prospective registries [22] and smaller study cohorts [43] demonstrated that the S‐ICD is safe and effective in terminating VF in IAS patients and has similar rates of inappropriate shocks as compared to the traditional TV-ICD. However, disease-specific concerns have been raised for patients with BrS, in whom the peculiar QRS-T abnormalities suggest the need for accurate pre-implant screening in order to avoid post-implant inappropriate shocks. Indeed, spontaneous dynamic ST-segment elevation and T wave morphology changes have been described during sleep, following meals, or after exercise in BrS. In this view, the basal resting screening may theoretically be inadequate to predict the risk of future T-wave oversensing [44], and repeating the test after ajmaline or flecainide drug challenge has been suggested to qualify BrS patients for S-ICD [45]. Despite these concerns, there is currently no evidence from large prospective registries that patients with BrS have an increased risk of inappropriate shocks with the S-ICD. This might be due to the specific algorithms and digital filters for sensing and rhythm discrimination that work actively on the device and not on the screening tool. Alternatively, the false-positive prediction of ineligibility may be a specific weakness of screening tools. Indeed, a substantial fraction of S-ICD recipients in whom the AST predicts non-eligibility have perfectly working S-ICDs [46].
4.5 Congenital Heart Disease (CHD)
Patients with CHD represent a minority of ICD candidates. Due to limited access to the right ventricle (e.g., extracardiac Fontan, anomalous or occluded veins) and intracardiac shunts with potential thromboembolic risks, TV-ICDs may present specific disadvantages in these patients. Moreover, most CHD patients at risk for SD are young. Therefore, the long-term risk of device infection or lead failure is unacceptably high.
While these considerations could prompt the use of the S-ICD in most CHD patients, it is important to consider the potential risk of developing pacing indication during follow-up and the need for combining S-ICD with conventional pacemakers [47].
4.6 Future Directions
The S-ICD was designed to provide shock-only therapy in patients without pacing indications. However, the need for bradycardia or anti-tachycardia pacing may occur either at first evaluation or during follow-up. This prompted to investigate a leadless pacemaker provided with communication capabilities combined with an S-ICD. The first proof-of-concept study of a leadless pacemaker communicating with an S-ICD yielded interesting preliminary results [48]. A second pre-clinical study described the acute and chronic performance of a modular leadless pacemaker/S-ICD system and provided evidence, for first the first time, that an S-ICD can consistently command a leadless pacemaker to deliver ATP automatically when VT is detected within the programmed therapy zone [49]. Clinical trials with a validated modular system are planned and will be the first to address the question whether combined leadless pacing and defibrillation therapy will provide the forthcoming standard of care for most ICD candidates.
References
Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Bloma N, Borggrefe M, Camm J, Elliott PM, et al. ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European. Eur Heart J. 2015;2015:2793–867.
Moss AJ, Hall WJ, Cannom D, Daubert J, Higgins S, Klein H, Levine J. Improved survival with an implanted defibrillator in patients. N Engl J Med. 1996;335(26):1933–40.
Adduci C, Ali H, Francia P, Mantovani R, Palano F, Lupo P, Foresti S, et al. The subcutaneous implantable cardioverter-defibrillator: current trends in clinical practice between guidelines and technology progress. Eur J Intern Med. 2019.
Van Rees JB, De Bie MK, Thijssen J, Borleffs CJW, Schalij MJ, Van Erven L. Implantation-related complications of implantable cardioverter-defibrillators and cardiac resynchronization therapy devices: a systematic review of randomized clinical trials. J Am Coll Cardiol. 2011;58:995–1000.
Kleemann T, Becker T, Doenges K, Vater M, Senges J, Schneider S, Saggau W, et al. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of >10 years. Circulation. 2007;115(19):2474–80.
Adduci C, Palano F, Francia P. Safety, efficacy and evidence base for use of the subcutaneous implantable cardioverter defibrillator. J Clin Med (internet). 2018;7(3):53. https://www.mdpi.com/2077-0383/7/3/53.
Migliore F, Mattesi G, Zorzi A, Viani S, Bongiorni MG, Francia P, Curcio A, et al. The subcutaneous implantable cardioverter- defibrillator in clinical practice. Giornale Ital Cardiol. 2019. 641–550.
Ali H, Lupo P, Cappato R. The entirely subcutaneous defibrillator—a new generation and future expectations. Arrhythmia Electrophysiol Rev (internet). 2015;04(2):116. https://www.radcliffecardiology.com/articles/entirely-subcutaneous-defibrillator-new-generation-and-future-expectations.
Francia P, Ziacchi M, De Filippo P, Viani S, D’Onofrio A, Russo V, Adduci C, et al. Subcutaneous implantable cardioverter defibrillator eligibility according to a novel automated screening tool and agreement with the standard manual electrocardiographic morphology tool. J Interv Card Electrophysiol. 2018;52(1):61–7.
Knops RE, Olde Nordkamp LRA, De Groot JR, Wilde AAM. Two-incision technique for implantation of the subcutaneous implantable cardioverter-defibrillator. Hear Rhythm. 2013;10(8):1240–3.
Migliore F, Allocca G, Calzolari V, Crosato M, Facchin D, Daleffe E, Zecchin M, et al. Intermuscular two-incision technique for subcutaneous implantable cardioverter defibrillator implantation: results from a multicenter registry. PACE Pacing Clin Electrophysiol. 2017;40:278–85.
Gold MR, Aasbo JD, El-Chami MF, Niebauer M, Herre J, Prutkin JM, Knight BP, et al. Subcutaneous implantable cardioverter-defibrillator post-approval study: clinical characteristics and perioperative results. Hear Rhythm. 2017;14(10):1456–63.
Friedman DJ, Parzynski CS, Varosy PD, Prutkin JM, Patton KK, Mithani A, Russo AM, et al. Trends and in-hospital outcomes associated with adoption of the subcutaneous implantable cardioverter defibrillator in the United States. JAMA Cardiol. 2016;1(8):900–11.
Boersma L, Barr C, Knops R, Theuns D, Eckardt L, Neuzil P, Scholten M, et al. Implant and midterm outcomes of the subcutaneous implantable cardioverter-defibrillator registry: the EFFORTLESS study. J Am Coll Cardiol. 2017;70(7):830–41.
D’Onofrio A, Pieragnoli P, Biffi M, Nigro G, Migliore F, Francia P, De Filippo P, et al. Subcutaneous implantable cardioverter defibrillator implantation: an analysis of Italian clinical practice and its evolution. Int J Cardiol. 2018;162–7.
Diemberger I, Migliore F, Ricciardi G, Ottaviano L, Tavoletta V, Francia P, Viani S, et al. Time to therapy delivery and effectiveness of the subcutaneous implantable cardioverter-defibrillator. Hear Rhythm. 2019;16(10):1531–7.
Adduci C, Spadoni L, Palano F, Francia P. Ventricular fibrillation undersensing due to air entrapment in a patient implanted with a subcutaneous cardioverter defibrillator. J Cardiovasc Electrophysiol. 2019;30(8):1373–4.
Quast AFBE, Baalman SWE, Brouwer TF, Smeding L, Wilde AAM, Burke MC, Knops RE. A novel tool to evaluate the implant position and predict defibrillation success of the subcutaneous implantable cardioverter-defibrillator: the PRAETORIAN score. Hear Rhythm. 2019;16:403–10.
Quast AFBE, Baalman SWE, Betts TR, Boersma LVA, Bonnemeier H, Boveda S, Brouwer TF, et al. Rationale and design of the PRAETORIAN-DFT trial: a prospective randomized comparative trial of subcutaneous implantable cardioverter-defibrillator implantation with and without defibrillation testing. Am Heart J. 2019;214:167–74.
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary. Circulation. 2018;138:e272–391.
Weiss R, Knight BP, Gold MR, Leon AR, Herre JM, Hood M, Rashtian M, et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation. 2013;128(9):944–53.
Lambiase PD, Barr C, Theuns DAMJ, Knops R, Neuzil P, Johansen JB, Hood M, et al. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD registry. Eur Heart J. 2014;35(25):1657–65.
Quast AFBE, van Dijk VF, Yap SC, Maass AH, Boersma LVA, Theuns DA, Knops RE. Six-year follow-up of the initial Dutch subcutaneous implantable cardioverter-defibrillator cohort: long-term complications, replacements, and battery longevity. J Cardiovasc Electrophysiol. 2018;29(7):1010–6.
Francia P, Adduci C, Palano F, Semprini L, Serdoz A, Montesanti D, Santini D, et al. Eligibility for the subcutaneous implantable cardioverter-defibrillator in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. 2015;26(8):893–9.
Srinivasan NT, Patel KH, Qamar K, Taylor A, Bacà M, Providência R, Tome-Esteban M, et al. Disease severity and exercise testing reduce subcutaneous implantable cardioverter-defibrillator left sternal ECG screening success in hypertrophic cardiomyopathy. Circ Arrhythmia Electrophysiol. 2017;10(4).
De Bie MK, Thijssen J, Van Rees JB, Putter H, Van Der Velde ET, Schalij MJ, Van Erven L. Suitability for subcutaneous defibrillator implantation: results based on data from routine clinical practice. Heart. 2013;99(14):1018–23.
Melles MC, Yap SC, Bhagwandien RE, Sakhi R, Szili-Torok T, Theuns DAMJ. Frequency of need for antitachycardia or antibradycardia pacing or cardiac resynchronization therapy in patients with a single-chamber implantable cardioverter-defibrillator. Am J Cardiol. 2018;122(12):2068–74.
Boersma LV, El-Chami MF, Bongiorni MG, Burke MC, Knops RE, Aasbo JD, Lambiase PD, et al. Understanding outcomes with the EMBLEM S-ICD in primary prevention patients with low EF study (UNTOUCHED): clinical characteristics and perioperative results. Hear Rhythm. 2019;16(11):1636–44.
Maron BJ, Casey SA, Olivotto I, Sherrid M V., Semsarian C, Autore C, Ahmed A, et al. Clinical course and quality of life in high-risk patients with hypertrophic cardiomyopathy and implantable cardioverter-defibrillators. Circ Arrhythmia Electrophysiol. 2018;11(4).
Migliore F, Pelliccia F, Autore C, Bertaglia E, Cecchi F, Curcio A, Bontempi L, et al. Subcutaneous implantable cardioverter defibrillator in cardiomyopathies and channelopathies. J Cardiovasc Med. 2018. 633–42.
Olde Nordkamp LRA, Postema PG, Knops RE, Van Dijk N, Limpens J, Wilde AAM, De Groot JR. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: a systematic review and meta-analysis of inappropriate shocks and complications. Hear Rhythm. 2016;13(2):443–54.
Roberts BD, Hood RE, Saba MM, Dickfeld TM, Saliaris AP, Shorofsky SR. Defibrillation threshold testing in patients with hypertrophic cardiomyopathy. PACE Pacing Clin Electrophysiol. 2010;33(11):1342–6.
Francia P, Adduci C, Semprini L, Palano F, Santini D, Musumeci B, Santolamazza C, et al. Prognostic implications of defibrillation threshold testing in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. 2017;28(1):103–8.
Weinstock J, Bader YH, Maron MS, Rowin EJ, Link MS. Subcutaneous implantable cardioverter defibrillator in patients with hypertrophic cardiomyopathy: an initial experience. J Am Heart Assoc. 2016;5(2):1–6.
Friedman DJ, Parzynski CS, Heist EK, Russo AM, Akar JG, Freeman JV, Curtis JP, et al. Ventricular fibrillation conversion testing after implantation of a subcutaneous implantable cardioverter defibrillator report from the national cardiovascular data registry. Circulation. 2018;137:2463–77.
Maurizi N, Tanini I, Olivotto I, Amendola E, Limongelli G, Losi MA, Allocca G, et al. Effectiveness of subcutaneous implantable cardioverter-defibrillator testing in patients with hypertrophic cardiomyopathy. Int J Cardiol. 2017;231:115–9.
Lambiase PD, Gold MR, Hood M, Boersma L, Theuns DAMJ, Burke MC, Weiss R, et al. Evaluation of subcutaneous ICD early performance in hypertrophic cardiomyopathy from the pooled EFFORTLESS and IDE cohorts. Hear Rhythm. 2016;13(5):1066–74.
Maurizi N, Olivotto I, Olde Nordkamp LRA, Baldini K, Fumagalli C, Brouwer TF, Knops RE, et al. Prevalence of subcutaneous implantable cardioverter-defibrillator candidacy based on template ECG screening in patients with hypertrophic cardiomyopathy. Hear Rhythm. 2016;13(2):457–63.
Sakhi R, Yap SC, Michels M, Schinkel AFL, Kauling RM, Roos-Hesselink JW, Theuns DAMJ. Evaluation of a novel automatic screening tool for determining eligibility for a subcutaneous implantable cardioverter-defibrillator. Int J Cardiol. 2018;272(1):97–101.
Nazer B, Dale Z, Carrassa G, Reza N, Ustunkaya T, Papoutsidakis N, Gray A, et al. Appropriate and inappropriate shocks in hypertrophic cardiomyopathy patients with subcutaneous implantable cardioverter-defibrillators: an international multicenter study. Hear Rhythm. 2020;18.
Migliore F, Viani S, Bongiorni MG, Zorzi A, Silvetti MS, Francia P, D’Onofrio A, et al. Subcutaneous implantable cardioverter defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy: results from an Italian multicenter registry. Int J Cardiol. 2019;1(280):74–9.
Corrado D, Zorzi A, Cerrone M, Rigato I, Mongillo M, Bauce B, Delmar M. Relationship between arrhythmogenic right ventricular cardiomyopathy and brugada syndrome. Circ Arrhythmia Electrophysiol. 2016;9(4).
Rudic B, Tülümen E, Berlin V, Röger S, Stach K, Liebe V, El-Battrawy I, et al. Low Prevalence of inappropriate shocks in patients with inherited arrhythmia syndromes with the subcutaneous implantable defibrillator single center experience and long-term follow-up. J Am Heart Assoc. 2017;6(10).
Olde Nordkamp LRA, Conte G, Rosenmöller BRAM, Warnaars JLF, Tan HL, Caputo ML, Regoli F, et al. Brugada syndrome and the subcutaneous implantable cardioverter-defibrillator. J Am Coll Cardiol. 2016. 665–6.
Conte G, Kawabata M, De Asmundis C, Taravelli E, Petracca F, Ruggiero D, Caputo ML, et al. High rate of subcutaneous implantable cardioverter-defibrillator sensing screening failure in patients with Brugada syndrome: a comparison with other inherited primary arrhythmia syndromes. Europace. 2018;20(7):1188–93.
Bögeholz N, Pauls P, Güner F, Bode N, Fischer A, Dechering D, Frommeyer G, et al. Direct comparison of the novel automated screening tool (AST) versus the manual screening tool (MST) in patients with already implanted subcutaneous ICD. Int J Cardiol. 2018;265:90–6.
Ferrero P, Ali H, Barman P, Foresti S, Lupo P, D’Elia E, Cappato R, et al. Entirely subcutaneous defibrillator and complex congenital heart disease: Data on long-term clinical follow-up. World J Cardiol (internet). 2017;9(6):547. https://www.wjgnet.com/1949-8462/full/v9/i6/547.htm.
Tjong FVY, Brouwer TF, Kooiman KM, Smeding L, Koop B, Soltis B, Shuros A, et al. Communicating antitachycardia pacing-enabled leadless pacemaker and subcutaneous implantable defibrillator. J Am Coll Cardiol. 2016. 1865–6.
Tjong FVY, Brouwer TF, Koop B, Soltis B, Shuros A, Schmidt B, Swackhamer B, et al. Acute and 3-month performance of a communicating leadless antitachycardia pacemaker and subcutaneous implantable defibrillator. JACC Clin Electrophysiol. 2017;3(13):1487–98.
Funding
There are no funding sources relevant to this manuscript to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
P.F. received speaker fees and fellowship support from Boston Scientific, and research grants from Abbott.
Electronic supplementary material
Below is the link to the electronic supplementary material.



Rights and permissions
About this article
Cite this article
Adduci, C., Palano, F., Silvetti, G. et al. Prevention of Sudden Cardiac Death: Focus on the Subcutaneous Implantable Cardioverter-Defibrillator. High Blood Press Cardiovasc Prev 27, 291–297 (2020). https://doi.org/10.1007/s40292-020-00394-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-020-00394-x